Abstract
This report shows the results of stereotactic radiation therapy for progressive residual pilocytic astrocytomas. Medical records of patients who had undergone stereotactic radiation therapy for a progressive residual pilocytic astrocytoma were reviewed. Between 1995 and 2010, 12 patients with progression of a residual pilocytic astrocytoma underwent stereotactic radiation therapy at UCLA. Presentation was headache (4), visual defects (3), hormonal disturbances (2), gelastic seizures (2) and ataxia (1). MRI showed a cystic (9), mixed solid/cystic (2) or solid tumor (1); located in the hypothalamus (5), midbrain (3), thalamus (2), optic chiasm (1) or deep cerebellum (1). Median age was 21 years (range 5–41). Nine tumors received stereotactic radiotherapy (SRT). Three tumors received stereotactic radiosurgery (SRS), two of them to their choline positive regions. SRT median total dose was 50.4 Gy (40–50.4 Gy) in a median of 28 fractions (20–28), using a median fraction dose of 1.8 Gy (1.8–2 Gy) to a median target volume of 6.5 cm3. (2.4–33.57 cm3) SRS median dose was 18.75 Gy (16.66–20 Gy) to a median target volume of 1.69 cm3 (0.74–2.22 cm3). Median follow-up time was 37.5 months. Actuarial long-term progression-free and disease-specific survival probabilities were 73.3 and 91.7 %, respectively. No radiation-induced complications were observed. Stereotactic radiation therapy is a safe and effective modality to control progressive residual pilocytic astrocytomas. Better outcomes are obtained with SRT to entire tumor volumes than with SRS targeting choline positive tumor regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization classification, pilocytic astrocytomas are low grade astrocytomas [1]. They are usually well circumscribed and slow growing cystic tumors with minimum infiltration growth outside post-contrast magnetic resonance imaging (MRI) volumes. They most commonly affect children and young adults. These tumors may be stable for prolonged periods of time with or without intervention [2]. Once the tumor becomes symptomatic or progresses, complete surgical removal is desirable as the first line treatment option [3, 4]. Thus, they are potentially curable by surgery and have been associated with 10-year survival rates of >90 % in children and 63–83 % in adults [2–7].
Nevertheless, complete resection is not always feasible depending on the location of the tumor (e.g. hypothalamus). In these cases, surgical options are limited to partial resection or biopsy. After this initial approach, patients are observed or offered adjuvant radiotherapy or chemotherapy [3, 4, 8–11]. Conventional radiation therapy with relatively wide target volumes has been used but is undesirable in children because of its possible side effects on cognition [12, 13] and the increased risk for the development of a secondary malignancy [14, 15].
Radiation therapy via stereotactic tumor localization minimizes these potential radiation-related side effects by targeting the tumor precisely and using focused radiation beams. Furthermore, it offers the feasibility of treatment delivery in a single procedure (i.e. stereotactic radiosurgery: SRS) [16–23]. Another choice is to deliver radiation therapy in a conventionally fractionated fashion, albeit still utilizing a stereotactic approach (i.e. stereotactic radiotherapy: SRT) [24]. Moreover, selective targeting of the spectroscopic choline positive areas of the tumor could theoretically further reduce these risks.
Patients with progressive residual pilocytic astrocytomas would be ideal candidates for radiosurgery. However, no randomized controlled trial comparing therapies for these patients has been done, and few prospective data is available.
This report shows the results of stereotactic radiation therapy for partially resected progressive pilocytic astrocytomas treated at the University of California, Los Angeles (UCLA) between 1995 and 2010.
Methods
After approval by our Institutional Review Board, medical records of the patients who had undergone stereotactic radiation therapy (i.e. SRS or SRT) for a progressive pilocytic astrocytoma after its initial surgical resection were reviewed. Tumor progression was defined as a ≥25 % increase in the MRI tumor volume during follow-up [22, 23].
For SRS, the stereotactic frame was attached to the skull under local anesthesia. Patients treated with SRT had the frame attached to the thermoplastic mask. High resolution MRI imaging which included axial, coronal and sagittal images with and without contrast material for lesion visualization and a computerized tomography (CT) for stereotactic localization were acquired. The target volume included enhanced and non-enhanced tumor regions. The contrast enhancing and the cystic portion of the tumor defined the clinical target volume (CTV) for patients treated with SRT. The planned target volume (PTV) for these lesions was the CTV plus a margin (added in order to ensure adequate dose coverage for the identified CTV and account for the theoretical positioning uncertainty). Patients treated with SRS had specifically the choline positive regions of the tumor defined as the PTV, in an attempt to minimize possible adverse effects of radiation for children and spare close eloquent structures. All procedures were performed using a LINAC-based stereotactic system. Initially, treatments were performed using a Philips SRS 200 system adapted into a Clinac 18 LINAC (Varian, Palo Alto, CA). A dedicated LINAC for radiosurgery was first used in 1996, the X-Knife system (Radionics, Burlington, MA). From 1998 to 2009, the Novalis® system (Brainlab, Feldkirchen, Germany) was used and, since then, the Novalis TX® (Brainlab, Feldkirchen, Germany) has been used. The radiation delivery technique varied from single/multiple isocenters to shaped beam (dynamic arcs or static beams) depending on the size of the lesion and the time period of treatment delivery. All patients were treated as outpatients. No specific protocol for adjuvant medication during treatment was used.
Progression-free and disease-specific survival times were calculated in months from the date of radiation treatment start to the date of event (i.e., tumor progression or patient death due to the tumor) or last follow-up. Statistical evaluation was performed with commercially available software (SPSS version 18.0). Progression-free and disease-specific survival probabilities were calculated with the Kaplan–Meier method.
Results
Between 1995 and 2010, 12 patients with a progressive residual pilocytic astrocytoma received stereotactic radiation therapy at UCLA. Because of the tumor location, a partial surgical debulking had initially been performed for all cases, with histological confirmation. None of these patients received adjuvant chemotherapy or radiotherapy after this initial procedure. The tumors were closely monitored and showed local progression during subsequent follow-up. In an attempt to control their growth, they received salvage radiation therapy with either SRS or SRT.
There were eight female and four male patients. The median age at the start of radiation treatment was of 21 years (range 5–41 years). Clinical presentation of the tumor had been of headache (4 patients), visual defects (3 patients), hormonal disturbances (diabetes insipidus and failure to thrive) (2 patients), gelastic seizures (2 patients) and ataxia (1 patient). Initial MRI studies showed the primary tumor to be a cystic (n = 9), mixed solid/cystic (n = 2) or solid mass (n = 1); located in the hypothalamus (n = 5), midbrain (n = 3), thalamus (n = 2), optic chiasm (n = 1) or deep cerebellum (n = 1). (Table 1).
Nine of the tumors received SRT (Patients 1–9) and three received SRS (Patients 10–12). Two of these tumors were treated with SRS to their spectroscopic choline positive regions only (Patients 10 and 11). The SRT median total dose was 50.4 Gy (range 40–50.4 Gy) in a median of 28 fractions (range: 20–28 fractions), using a median fraction dose of 1.8 Gy (range: 1.8–2 Gy) to a median target volume of 6.5 cm3 (range: 2.4–33.57 cm3). Margins of 8mm were used in all the SRT cases for the planned target volume (PTV) (Table 2). The SRS median dose was 18.75 Gy (range: 16.66–20 Gy) to a median target volume of 1.69 cm3 (range 0.74–2.22 cm3) (Table 3). After the radiation treatment was done, each patient was followed every 3–6 months clinically and by means of MRI studies. If they presented symptoms, interval MRI was also obtained.
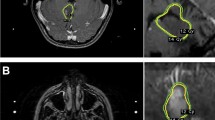
The median follow-up time after radiation treatment was of 37.5 months (range 3–168 months). The outcomes of the 12 patients analyzed in this series are described in Table 4. The actuarial long-term progression-free and disease-specific survival probabilities were of 73.3 and 91.7 %, respectively. A case of local tumor control two years after treatment (Patient 9) is illustrated in Fig. 1. The three tumor progression events as well as the single disease-specific death (Fig. 2) occurred within the first year. No complications attributable to radiation therapy were observed in this series.
The disease-specific death (Patient 3) occurred in a 41 year-old female, who had presented with diabetes insipidus and partial visual loss and had a malignant transformation of her primary hypothalamic pilocytic tumor in the first month after receiving the corresponding radiation therapy. Tumor progression started as a rapid local cystic growth into the third ventricle, which required emergency placement of a ventriculoperitoneal shunt. Afterwards, she was found to have a leptomeningeal spread of the tumor. Pathology from the cerebrospinal fluid cytology revealed high grade malignant gliomatous cells compatible with meningeal carcinomatosis. She died 2 months later (Fig. 2).
The other two tumor progression events happened in Patients 10 and 11. Both tumors progressed at the ninth month after the initial SRS treatment, which had focused on spectroscopic choline positive areas of the tumor. Both were cystic tumors and the progression also started with growth of their cystic components. These patients had a prolonged survival time after radiation, in spite of the local tumor control failure.
Patient 10 presented with visual defects and was found to have a cystic pilocytic tumor originating in the optic chiasm when she was 10 year-old. twelve months after the initial craniotomy, she had a local asymptomatic progression of the residual tumor. SRS to spectroscopic choline positive areas of the tumor was performed, in an attempt to minimize the radiation exposure of the optic chiasm, hypothalamus and pituitary gland. A dose of 18.75 Gy was administered to 3 choline positive targets of 15 mm of radius each (PTV = 2.22 cm3). Nine months after this therapy, the tumor progressed and surgery was required once more. The anatomopathological findings were again consistent with pilocytic astrocytoma.
Patient 11 was a 5 year-old male who presented with failure to thrive and progressive headaches in the morning. In this case, he was found to have a hypothalamic cystic pilocytic astrocytoma. After two debulking surgeries, the tumor continued to show local progression and therefore radiation therapy was delivered. Because of its proximity to the optic apparatus, it was decided to perform SRS, again targeting the choline positive regions of the tumor only. A dose of 20 Gy was administered to a 1.69 cm3 area. Nine months after treatment, MRI showed tumor progression. The patient required another surgical debulking. Pathology corroborated the diagnosis of pilocytic astrocytoma.
Discussion
The natural history of pilocytic astrocytomas is very benign. It is characterized by high survival rates [2–7]. Therefore, it has been suggested that the best initial therapeutic approach is observation after gross total resection [25, 26]. However, after subtotal resection or biopsy of an otherwise unresectable tumor, there is controversy regarding the use of adjuvant therapy (radiotherapy or chemotherapy) versus observation. When there is evidence of residual tumor progression, adjuvant treatment is needed [6–9]. Up to date, few prospective data have been published regarding this particular issue [27].
We report a series of 12 patients who presented with a pilocytic astrocytoma, which progressed after its initial surgery, justifying the need of salvage radiation treatment. With the techniques of SRS and SRT, we have achieved actuarial long-term progression-free and disease-specific survival probabilities of 73.3 and 91.7 %, respectively. These outcomes are compatible with other reports. The University of Pittsburg published a tumor control rate of 68 %, having most of their patients with either recurrent or unresectable disease [18, 21]. A series from Japan had a tumor control rate of 91.7 % [28]. This good control is expected considering the well-known impact of radiation on low-grade astrocytomas [29]. Pilocytic astrocytomas represent an even more benign tumor, known also to be sensitive to radiation [25, 29].
One of our patients had an early cystic progression, which turned out to be a malignant transformation of the primary pilocytic astrocytoma in the first month after radiation, causing her demise 2 months later. This is probably not a case of malignant transformation induced by radiation, since it usually occurs several years after treatment. It is known that the likelihood of malignant transformation is related to cellularity of the residual tumor (i.e. volume of the solid portion). Patients who underwent surgery alone presented a higher incidence of malignant transformation when compared to patients who received postoperative radiotherapy for residual lesion [30, 31]. This case could also have represented a separate disease entity not appreciated in the initial surgical anatomopathological specimen (i.e. a sampling error).
The cases treated with SRS to the choline positive area were two of the youngest patients of this series. Children at this age require general sedation for radiation treatment delivery. Both of them had a cystic lesion in the diencephalic region. Due to the eloquence of areas where the tumor was located, we attempted to limit the PTV to the solid and metabolic active areas of the lesion. Our rationale was that radiation of these portions of the lesion would lead to local tumor control. The limited tumor volume allowed for a safe treatment using single dose radiation (SRS). The third patient treated with SRS in our series had a very small tumor (0.74 cm3), which was safely treated with single dose to its entire volume. However, the two cases treated with SRS focused to spectroscopic choline positive areas failed local control. After these two failures, the approach was abandoned. This outcome highlights the need to irradiate the entire visible tumor (contrast enhancing and cystic components on the MRI studies). Our three cases of tumor progression show that the solid lesions are more likely to respond while the cystic lesions are the more likely to progress after radiation, in accordance to other series [16, 22, 23].
The pilocytic tumors requiring radiation treatment are often the ones located in areas where total surgical resection is not feasible. Moreover, these patients are usually very young. Considering that the entire lesion should be irradiated, our experience shows that SRT would be a safer alternative for the great majority of the cases. The SRT, with tight margins as well as utilizing the well-established radiobiological advantages of fractionation [24], would offer protection to the surrounding brain in young patients while allowing a good coverage of the entire tumor. Very often, what is considered tolerable for eloquent structures, as far as the single dose constraint is concerned (e.g. 12 Gy to the 10 cm3 volume, according to accepted dose-volume guidelines), would have hardly been achievable in view of the characteristics of the lesions treated in this series.
There are several limitations to this report. Like other previous published series, the current cohort is small, therefore limiting the ability to perform more elaborated statistical analysis of the data. Only five patients completed a minimum of 5 years follow-up period, which is less than desirable when considering lesions with a slow progression rate. Also, our patients were imaged and treated during a long period of time between 1995 and 2010, which implies that a variety of neurosurgical, radiation and imaging techniques were used for each of them. Conclusions drawn from this series might be different if the currently available diagnostic and therapeutic technologies are used.
Finally, we did not observe any radiation induced side effect in our series. Other reports have documented the appearance of cerebral edema and radiation necrosis [16, 17, 19, 22, 23]. Of course, there might be a chance of additional delayed radiation effects up to 10 or 20 years later in these patients, such as neurocognitive decline or secondary malignancy [12–15, 32]. As an attempt to minimize the risks of radiation therapy, the stereotactic radiation treatment approach was used. The volumes treated with SRS, having been focused on choline positive areas, were extremely small. We thus did not expect significant long-term neurocognitive consequences. With further advances in imaging and treatment delivery technologies, these potential risks are expected to be minimized.
Conclusions
In patients with pilocytic astrocytoma, surgery is the treatment of choice. However, in patients with partially resected or unresectable progressive tumors, stereotactic irradiation with either SRS or SRT is a promising alternative.
Stereotactic radiation therapy is safe and effective to control progression of residual progressive pilocytic astrocytomas. Stereotactic radiosurgery focusing on the spectroscopic choline positive areas of the tumor is not sufficient to provide local tumor control and therefore should not be recommended.
References
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Forsyth PA, Shaw EG, Scheithauer BW et al (1993) Supratentorial pilocytic astrocytomas. A clinicopathologic, prognostic, and flow cytometric study of 51 patients. Cancer 72:1335–1342
Wallner KE, Gonzales MF, Edwards MS et al (1988) Treatment results of juvenile pilocytic astrocytoma. J Neurosurg 69:171–176
Kehler U, Arnold H, Muller H (1990) Long-term follow-up of infratentorial pilocytic astrocytomas. Neurosurg Rev 13:315–320
Gjerris F, Klinken L (1978) Long term prognosis in children with benign cerebellar astrocytoma. J Neurosurg 49:179–184
Hayostek CJ, Shaw EG, Scheithauer B et al (1993) Astrocytomas of the cerebellum. A comparative clinicopathologic study of pilocytic and diffuse astrocytomas. Cancer 72:856–869
Sgouros S, Fineron PW, Hockley AD (1995) Cerebellar astrocytoma of childhood: long-term follow-up. Childs Nerv Syst 11:89–96
Burger PC, Breiter SN, Fisher PG (1996) Pilocytic and fibrillary astrocytomas of the brainstem—a comparative clinical, radiological and pathological study. J Neuropathol Exp Neurol 55:640
Rodriguez HA, Edwards MS, Levin VA (1990) Management of hypothalamic gliomas in children: an analysis of 33 cases. Neurosurgery 26:242–246
Packer RJ, Ater J, Allen J et al (1997) Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg 86:747–754
Prados MD, Edwards MS, Rabbitt J et al (1997) Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol 32:235–241
Li J, Bentzen S, Renschler M, Mehta M (2008) Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 71:64–70
Welzel G, Fleckenstein K, Schaefer J et al (2008) Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 72:1311–1318
Smith MA, Seibel NL, Altekruse SF et al (2010) Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28:2625–2634
Friedman DL, Whitton J, Leisenring W et al (2010) Subsequent neoplasms in 5-year survivors of childhood cancer: the childhood cancer survivor study. J Natl Cancer Inst 102:1083–1095
Henderson MA, Achilles JF, Timmerman RD et al (2009) Gamma knife stereotactic radiosurgery for low-grade astrocytomas. Stereotact Funct Neurosurg 87:161–167
Boethius J, Ulfarsson E, Rahn T, Lippittz B (2002) Gamma knife radiosurgery for pilocytic astrocytomas. J Neurosurg 97(Suppl 5):677–680
Hadjipanayis CG, Kondziolka D, Gardner P, Niranjan A, Dagam S, Flickinger JC et al (2002) Stereotactic radiosurgery for pilocytic astrocytomas when multimodal therapy is necessary. J Neurosurg 97:56–64
Kondziolka D, Lunsford LD, Flickinger JC (1992) Stereotactic radiosurgery in children and adolescents. Pediatr Neurosurg 16:219–221
Somaza SC, Kondziolka D, Lunsford LD, Flickinger JC, Bissonette DJ, Albright AL (1996) Early outcomes after stereotactic radiosurgery for growing pilocytic astrocytomas in children. Pediatr Neurosurg 25:109–115
Hadjipanayis CG, Kondziolka D, Flickinger JC, Lunsford LD (2003) The role of stereotactic radiosurgery for low-grade astrocytomas. Neurosurg Focus 14(5):15
Kano H, Kondziolka D, Nirankan A, Flickinger JC, Lunsford LD (2009) Stereotactic radiosurgery for pilocytic astrocytomas part 1: outcomes in adult patients. J Neurooncol 95:211–218
Kano H, Nirankan A, Kondziolka D, Flickinger JC, Pollack IF et al (2009) Stereotactic radiosurgery for pilocytic astrocytomas part 2: outcomes in pediatric patients. J Neurooncol 95:219–229
Lee SP, Withers HR, Fowler JF (2006) Radiobiological considerations. In: Slotman BJ, Solberg T, Wurm R (eds) Extracranial stereotactic radiotherapy and radiosurgery. Taylor & Francis, New York, pp 131–176
Brown PD, Buckner JC, O’Fallon JR et al (2004) Adult patients with supratentorial pilocytic astrocytomas: a prospective multicenter clinical trial. Int J Radiat Oncol Biol Phys 58:1153–1160
Karim AB, Afra D, Cornu P et al (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52:316–324
Ishkanian A, Laperriere NJ, Xu W et al (2011) Upfront observation versus radiation for adult pilocytic astrocytoma. Cancer 117(17):4070–4079. doi:10.1002/cncr.25988
Kida Y, Kobayashi T, Mori Y (2000) Gamma knife radiosurgery for low-grade astrocytomas: results of long-term follow-up. J Neurosurg 93(suppl 3):42–46
Van Den Bent MJ, Afra D, de Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomized trial. Lancet 366:985–990
Johannsen TB, Lien HH, Hole KH et al (2003) Radiological and clinical assessment of long-term brain tumor survivors after radiotherapy. Radiother Oncol 69:169–176
Ellis JA, Waziri A, Balmaceda C, Canoll P, Bruce JN, Sisti MB (2009) Rapid recurrence and malignant transformation of pilocytic astrocytoma in adult patients. J Neurooncol 95:377–382
Laack NN, Brown PD, Ivnik RJ et al (2005) Cognitive function after radiotherapy for supratentorial low-grade gliomas: a north central cancer treatment group prospective study. Int J Radiat Oncol Biol Phys 63:1175–1183
Disclosure
The authors report neither conflicts of interest nor ethical issues regarding this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lizarraga, K.J., Gorgulho, A., Lee, S.P. et al. Stereotactic radiation therapy for progressive residual pilocytic astrocytomas. J Neurooncol 109, 129–135 (2012). https://doi.org/10.1007/s11060-012-0877-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0877-5