Abstract
Epilepsy in glioblastoma multiforme (GBM) patients is common. Hematological toxicity is a potential side effect of antiepileptic drugs (AEDs) and a frequent limiting-dose effect of temozolomide (TMZ). The aim of the study was to investigate the impact of AEDs on thrombocytopenia in GBM patients treated with radiotherapy and TMZ. A cohort of 101 newly diagnosed GBM patients treated with radiotherapy and TMZ was reviewed. Clinical data, presence of seizures, AEDs use, platelet count, and accumulated TMZ dose were analyzed at each cycle. Thrombocytopenia was operationalized as a continuous platelet count and a dichotomic variable (cut-off <100.000/mm3). This cut-off represents the threshold beyond which TMZ treatment is modified. A linear and a probit pooled cross-sectional regression analysis were used to study the impact of age, gender, AEDs, and accumulated TMZ on thrombocytopenia. Impact of AEDs on survival was also analyzed. Thirty-five patients (35%) presented seizures at onset and 18 (27%) during follow-up. Seven (13%) needed two or more AEDs for seizure control. Grade 3–4 thrombocytopenia was found in 8%. Decrease in platelet count was related to accumulated TMZ (p < 0.001), age (p < 0.001), and valproate (p = 0.004). Platelet count <100.000/mm3 was only associated with accumulated TMZ (p = 0.001). Recursive Partitioning Analysis prognostic class was the only variable with significant impact on survival. Valproate and age had an independent negative effect on total platelet count, although neither had an effect on critical thrombocytopenia (<100.000/mm3). Therefore, the systematic withhold of valproate in GBM patients might not be justified. Nevertheless, this negative effect may be taken into account especially in elderly patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma multiforme (GBM) is the most frequent malignant primary brain tumor in adults [1]. Standard therapy includes temozolomide (TMZ) administered concomitantly with radiation therapy (RT) followed by adjuvant TMZ. This regimen has represented the most relevant therapeutic advance in the last two decades [2]. The main dose-limiting adverse effect of TMZ is hematological toxicity, particularly thrombocytopenia [2–4].
Seizures, either at presentation or during the follow-up, are common in high-grade gliomas with a frequency that ranges from 30 to 50% [5–7]. Medical management of brain tumor-related epilepsy is complicated by interactions between antiepileptic (AEDs) and antineoplastic drugs [8–12]. Among classical AEDs, phenytoin, carbamazepine, and phenobarbital have cytochrome-P450 (CYP450)-inducing properties (EIAEDs), which may reduce the blood levels of antineoplastic drugs, with a potential negative effect on antitumoral activity [13, 14]. However, recently published studies showed controversial unexpected results in terms of survival with regard to the use of classical AEDs in the GBM population [15–17]. Conversely, valproic acid (VPA) has enzyme-inhibiting properties leading to an increase in serum concentration of some chemotherapy drugs and a potential higher risk of toxicity as suggested in several studies of chemotherapy regimens without TMZ [16, 18], and in just one retrospective study with TMZ [17]. TMZ is a prodrug that undergoes spontaneous conversion under physiological conditions to the active alkylating agent, with a minor hepatic metabolism [19]. Therefore, we should not expect a significant interaction between TMZ and AEDs.
Hematological toxicity is a well-recognized side effect of classical and new generation AEDs. Classical AEDs have a rate of blood dyscrasias of 3–4 per 100.000 prescriptions [20]. Specifically, VPA-induced thrombocytopenia may be as high as 17% [21]. In contrast, only isolated cases have been associated with levetiracetam (LEV) [22–27].
The main purpose of this study was to investigate the potential impact of AEDs on thrombocytopenia in a cohort of GBM patients treated with the standard chemoradiotherapy protocol. Additionally, we evaluated the impact of AEDs on progression-free survival (PFS) and overall survival (OS).
Methods
Patients
We reviewed 101 patients with newly histologically confirmed GBM, diagnosed between June 2004 and July 2009, except for 2 patients diagnosed in 2001 who were included in the EORTC/NCIC TMZ trial. Clinical data were prospectively included in the databases of two Spanish University Hospitals; 77 from Hospital Universitari de Bellvitge-ICO Duran i Reynals and 24 from Hospital Clínic of Barcelona. We included all patients treated with the standard regimen: RT (total dose of 60 Gy; fraction dose of 2 Gy) with concomitant TMZ (75 mg/m2/day) over 6 weeks, followed by a 4-week break, and TMZ (150–200 mg/m2/day for 5 days each 28 days) for 6 months. TMZ doses in adjuvant therapy were 150 and 200 mg/m2 in subsequent cycles [2]. Complete blood count was done before each TMZ cycle. Hematological toxic effects were graded according the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI-CTCAE), version 3.0 (http: www.cancer.org, 2006). The protocol was approved by the Ethical Committee of Hospital Universitari de Bellvitge–ICO Duran i Reynals.
Analysis of predictors of thrombocytopenia
To study the effect of AEDs on hematological toxicity, four categories of AEDs were identified based on their effect on the CYP450 isoenzymes system (Table 1). These categories were updated in each TMZ cycle. Specifically, patients who needed a replacement of AED therapy or patients who needed an add-on AED were switched to the corresponding category. Likewise, patients initially classified as non-AEDs at diagnosis who suffered seizures during TMZ therapy, were included in the corresponding category. Time of seizure appearance and use of prophylactic AEDs were also registered. Decisions on AED preferences were taken by the attending physician.
Other variables analyzed included age, gender, Karnosfky Performance Status (KPS), brain tumor-related characteristics (location, surgical management), and Recursive Partitioning Analysis (RPA) prognostic class of the Radiation Therapy Oncology Group (RTOG) [28, 29]. The extent of surgical resection was classified as biopsy, subtotal, and gross total resection [30]. Treatment-related characteristics such as total dose of administered RT and accumulated TMZ dose (mg/m2) were also evaluated. Accumulated TMZ dose was updated in each cycle of treatment.
Analysis of PFS and OS
PFS was recorded from the date of surgical diagnosis to the date of tumor progression and OS was measured from the diagnosis date to the last follow-up or death. Progression criteria were established by the multidisciplinary Neuro-oncology Committee of each center based on Macdonald response criteria [31]. Brain magnetic resonance imaging (MRI) was performed every 3 months or when patients presented a clinical worsening. For these purposes, AED category was reclassified as follows: patients who developed seizures during the course of TMZ therapy were categorized in the corresponding AEDs group depending on the chosen drug; patients initially classified in the LEV group who required, during the course of treatment, the adding-on of EIAEDs or VPA to control seizures were censored at this point; patients initially classified in the EIAEDs or VPA groups were kept in the corresponding group regardless any adding-on of non-EIAEDs. Lastly, patients who developed seizures after the end of TMZ therapy were kept in the non-AEDs group.
Statistical analysis
The primary end point of our study was to evaluate the impact of the different AED categories on thrombocytopenia. Thrombocytopenia was operationalized both as a continuous and as a dichotomic variable with a cut-off <100.000/mm3. This cut-off represents the threshold that mandates to delay or to discontinue TMZ. A linear and a probit pooled cross-sectional regression analysis, respectively, were used to study the impact of registered parameters on thrombocytopenia. Pooled analysis combines time series for several cross-sections. Provided that pooled data are characterized by having repeated observations on fixed units, pooled arrays of data combine cross-sectional data on N units and T time periods to produce a dataset of N × T observations. Here, thrombocytopenia data for 101 patients was structured in 11 time points of analysis, resulting in a potential dataset of 1,111 observations. Time points were defined as thrombocytopenia measures in 11 different instances during TMZ therapy: at baseline, at the end of radiotherapy treatment and before each adjuvant TMZ cycle. These 11 time points were designed to include the complete adjuvant treatment of the entire cohort, because 12 patients received more than six cycles of TMZ. In the same way, AEDs categories were taken into account in these time points. In order to prevent endogeneity, no further time observations were collected after patients had surpassed the thrombocytopenia threshold beyond which treatment decisions were altered. This was the case for 34 instances, for which 145 observations were discarded. Furthermore, mortality and occasional missing data led to a final dataset of 683 observations. Data evaluation was performed using STATA 10 statistical software package for Windows.
The secondary end points of the study were to evaluate the impact of AEDs on PFS and OS. The univariate analysis was made by constructing probability curves according the Kaplan–Meier method and comparing them by using a log-rank test. Subsequently, gender, RPA prognostic class, and the AEDs categorized group were introduced in a forward stepwise proportional-hazard analysis (Cox regression model) to identify independent predictors of PFS and OS. Data evaluation was performed using SPSS software package version for Windows 15.0 (SPSS, Chicago, Ill, USA).
Results
Patients
Demographics, clinical data, and tumor and treatment characteristics of the 101 patients included in the study are summarized in Table 2. Sixty patients (59%) were treated with AEDs during the course of TMZ treatment. Fifty-three of them (52%) had seizures, 35 (35%) at presentation and 18 (27%) during follow-up. Seven patients (7%) were treated with AEDs as prophylactic measure (6) or because of neuropathic pain (1). At baseline, 8 patients received EIAEDs therapy (6 phenytoin and 2 carbamazepine) and 33 non-EIAEDS therapy (25 VPA and 8 LEV). No patients were treated with single-agent non-EIAEDs other than VPA or LEV. No major differences were observed between patients who did or did not receive EIAEDs (online resource supplemental material, Table E-1). No patient received prophylaxis with trimethoprim-sulfamethoxazole.
Seizures were well controlled with AED monotherapy in the majority of patients (n = 46; 87%). Only 7 patients (13%) had refractory epilepsy: 5 (9.4%) needed two AEDs and 2 (3.8%) three AEDs to control seizures. Among patients with monotherapy, 13 were switched to a second AED. In 5 of these 13 patients, EIAEDs were replaced by LEV before the initiation of radiotherapy and the other 8 patients received VPA at first but were switched to LEV during the course of treatment. Hematological toxicity was the reason for VPA replacement in 3 of these 8 patients. In the other patients, the reason for AED replacement was not reported in the medical records. AED therapy categories update during TMZ therapy is summarized in online resource supplemental material, Table E-2.
Hematological toxicity
Twenty-eight (28%) patients presented hematological toxicity during TMZ treatment. Nine (9%) of them had grade 3–4. Thrombocytopenia was the most common (n = 25) with grade 3–4 toxicity in 8 (8%) patients. Only 3 patients presented neutropenia. Lymphocyte count was not included in the study. Hematological toxicity, mainly thrombocytopenia, was responsible for 95% of treatment delays but only for 5% of treatment discontinuation. The main reason for treatment discontinuation was disease progression.
Predictors of thrombocytopenia
Evaluation of thrombocytopenia as a continuous variable demonstrated that decrease in platelet count was related to accumulated TMZ dose (p < 0.001), age (p < 0.001), and VPA use (p = 0.004). On average, VPA treatment was associated with a decrease in 20.004 platelet units/mm3, holding for sex, age, and TMZ dose. On the other hand, on average, an additional year of age was significantly associated with a decrease in 1.328 platelet units/mm3, holding sex, TMZ dose, and AED category constant. In a second analysis, thrombocytopenia was analyzed as a dichotomic variable with a cut-off <100.000/mm3. This analysis showed that accumulated TMZ dose was the only variable with a significant impact on thrombocytopenia (p = 0.001). Age (p = 0.87) and VPA (p = 0.12) lost their influence (Table 3).
Predictors of PFS and OS
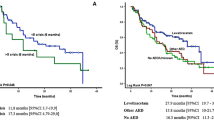
Median PFS of the overall cohort was 6.95 months (range: 2.26–63.18 months). According to the RPA prognostic class, median PFS was 10.5, 8.2, and 4.4 months in RPA class III, IV and V, respectively. On the other hand, median PFS was 6.1, 8.1, 6.9, and 3.2 months in non-AEDS, VPA, LEV, and EIAEDs groups, respectively (Fig. 1a). Although patients treated with VPA therapy had a marked tendency to indicate a better prognosis, only RPA, class III (p = 0.02) and class IV (p = 0.002), was independently associated with longer PFS in Cox regression analysis. No significant effect of AED therapy on PFS was found (Table 4).
In the same way, median OS of the entire cohort was 17.25 months (range: 2.89–72.46 months) and survival ratios at 12 and 24 months were 66 and 39.5%, respectively. The median outcomes by AED category were 14.03 months (range: 2.89–71.48) for non-AEDs; 14.69 months (range: 3.9–31.93) for EIAEDs; 26.39 months (range: 3.02–72.46) for VPA; and 23.44 months (range: 3.9–40.23) for LEV (Fig. 1b). However, despite these better survival tendencies in the VPA and LEV categories, the univariate analysis did not show significant differences in OS between AEDs categories. Only the RPA prognostic class was independently associated with longer survival in Cox regression analysis (Table 4).
Discussion
The present study suggests that age, VPA therapy, and accumulated TMZ dose had a negative effect on total platelet count in GBM patients treated with TMZ. However, only accumulated TMZ dose was an independent predictor of critical thrombocytopenia that indicated a change in TMZ treatment.
Interactions between AEDs and chemotherapy, especially the risk of hematological toxicity and their impact on survival in brain tumor patients, are a growing matter of interest [11–18, 32–36]. However, since the introduction of TMZ, the potential impact of AEDs on hematological toxicity has been poorly analyzed [17]. Nowadays, there is a strong trend among neuro-oncologists to switch from classical to newer AEDs in brain tumor patients. In the setting of TMZ therapy, this change in the seizure treatment is based on theoretical reasoning without support of clear evidence.
Previous studies on hematological toxicity of brain tumor patients treated with AEDs present several problems to support the trend to use only second generation AEDs. Some of these studies were done in patients under chemotherapy protocols without TMZ [16, 18] and they included both grade III and IV gliomas [18]. Other studies reported a higher incidence of side effects attributed to classical AEDs compared to new generation AEDs. However, these studies were retrospective in nature and included heterogeneous samples with low- and high-grade gliomas, treated with different chemotherapy schedules that makes it difficult to elucidate the role of AEDs on toxicity [34, 36]. Another important limitation of all these studies was that AEDs therapy was not updated during chemotherapy treatment. Recently, a retrospective study of the EORTC-NCI TMZ trial demonstrated that patients treated with VPA had more grade 3–4 hematological toxicity than patients treated with EIAEDS or non-AEDs [17]. However, this increased hematological toxicity had no influence on the total number of TMZ cycles with dose reduction received by VPA-treated patients. Nevertheless, this study presents some weaknesses. The dynamic change of AEDs during TMZ treatment and the total dose of TMZ administered were not taken in consideration. Both variables are more relevant than the number of TMZ cycles in the study of drug interactions.
Hematological toxicity (grade 3–4) in our cohort was similar to that previously reported (12–19%) [2, 3]. Up to 25% of patients developed thrombocytopenia, but it was severe (grade 3 or 4) in only 8%. However, thrombocytopenia was responsible for only 5% of TMZ withdrawal in our cohort, a lower rate than previously reported (11–17%) [2, 3]. It is unclear why a lower percentage of patients discontinued TMZ due to thrombocytopenia in our series, because demographic and tumor-related characteristics of our cohort were similar to previous reported studies with the exception of AEDs use, which was not reported in these TMZ trials [2, 3].
In this study, VPA use resulted in a negative effect on total platelet count, but it did not influence on clinically relevant thrombocytopenia. Nevertheless, these findings should be interpreted with caution. The low incidence of TMZ withdrawal due to thrombocytopenia, in addition to the sample size of our series, are two factors that may limit the ability to detect a significant association between VPA and critical thrombocytopenia. Age was the only biological risk factor with significant impact on total platelet count, but like VPA, it had no effect on critical thrombocytopenia. This fact could be explained because elderly patients have a less efficient metabolism, carrying a higher risk of treatment interactions and adverse events [37]. In addition, contrary to previously reported data, gender did not have a significant influence on total platelet count [38].
In terms of outcome, median PFS was similar to data previously reported [2], although OS was a little longer, probably due to the implementation of bevacizumab regimens as salvage treatment, and the slight increase of patients with better RPA class and gross total resections [39–41]. Nevertheless, RPA class was the only variable with independent prognostic impact on PFS and OS. The response evaluation in our series was performed before the publication of RANO criteria [42], and pseudoprogression could have been misdiagnosed, implying a potentially bias in PFS. However, a retrospective response evaluation was performed using these new RANO criteria without any change in the results of the multivariate analysis.
The impact of AEDs therapy on survival is controversial. Experimental studies have demonstrated an antitumor effect of VPA through the inhibition of histone deacetylase [43–45] and a possible synergistic effect of the combination VPA/TMZ through a redox regulation mechanism [46]. LEV has also demonstrated an antitumor effect through the inhibition of methyl-guanine-DNA-methyltransferase [47]. However, the impact of VPA use on survival in clinical studies revealed contradictory results [15–17]. A retrospective study with 160 patients with GBM treated with lomustine demonstrated a better outcome of patients treated with VPA compared to those who received EIAEDS (13.9 vs. 10.8 months) [16]. Conversely, a recent retrospective analysis of three trials found a better survival in patients treated with EIAEDs when compared with non-EIAEDs (12.3 vs. 10.7 months) [15]. The principal limitation of these studies is the absence of follow-up in AEDs schedule, making it difficult to know how many of these patients initially treated with EIAEDs discontinued therapy or were switched to non-EIAEDs therapy or vice versa. Interestingly, another recent retrospective study based on the EORTC-NCI TMZ trial suggested that patients receiving VPA had longer survival than those on EIAED [17]. However, this study had not performed adjustments for multiple comparisons in the analysis and underestimated the potential impact of treatments given after GBM progression. Despite the fact that VPA-treated patients had a marked tendency to indicate better prognosis, our series did not identify differences among AEDs in terms of outcome, including PFS and OS. On the assumption that there was a potentially undetected effect due to the retrospective nature of our study, or a potential lack of power provided by our sample size, we presume that the impact of VPA use on survival may be minor in comparison with classical survival prognostic factors as RPA.
In conclusion, accumulated TMZ dose was the main determinant factor of critical thrombocytopenia in treated GBM patients with epilepsy. VPA use and age had an independent and negative effect on total platelet count without influencing critical thrombocytopenia. Therefore, the systematic withholding of VPA in the management of GBM patients treated with TMZ might not be justified. Nevertheless, this negative effect may be taken into account, especially in elderly patients, when choosing AED therapy in clinical practice.
References
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 4:278–299
Stupp R, Mason WP, Van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L (2007) The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol 9:47–52
Hau P, Koch D, Hundsberger T et al (2007) Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology 68:688–690
Vecht CJ, Wilms EB (2010) Seizures in low- and high-grade gliomas: current management and future outlook. Expert Rev Anticancer Ther 10:663–669
Van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430
Vecht CJ, Van Breemen M (2006) Optimizing therapy of seizures in patients with brain tumors. Neurology 67(12 suppl 4):S10–S13
Badyal DK, Dadhich AP (2001) Cytochrome P450 and drug interactions. Indian J Pharmacol 33:248–259
Patsalos PN, Fröscher W, Pisani F, Van Rijn CM (2002) The importance of drug interactions in epilepsy therapy. Epilepsia 43:365–385
Anderson GD (2004) Pharmacogenetics and enzyme induction/inhibition properties of antiepileptic drugs. Neurology 63(suppl 4):S3–S8
Vecht CJ, Wagner GL, Wilms EB (2003) Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol 2:404–409
Vecht CJ, Wagner GL, Wilms EB (2003) Treating seizures in patients with brain tumors: drug interactions between antiepileptic and chemotherapeutic agents. Semin Oncol 30(6 suppl 19):49–52
Gajjar A, Chintagumpala MM, Bowers DC, Jones-Wallace D, Stewart CF, Crews KR (2003) Effect of intrapatient dosage escalation of irinotecan on its pharmacokinetics in pediatric patients who have high-grade gliomas and receive enzyme-inducing anticonvulsant therapy. Cancer 97(suppl 9):2374–2380
Villikka K, Kivistö KT, Mäenpää H, Joensuu H, Neuvonen PJ (1999) Cytochrome P450-inducing antiepileptics increase the clearance of vincristine in patients with brain tumors. Clin Pharmacol Ther 66:589–593
Jaeckle KA, Ballman K, Furth A, Buckner JC (2009) Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 73:1207–1213
Oberdornfer S, Piribauer M, Marosi C, Lahrmann H, Hitzenberger P, Grisold W (2005) P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol 72:255–260
Weller M, Gorlia T, Cairncross JC et al (2011) Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 77:1156–1164
Bourg V, Lebrun C, Chichmanian RM, Thomas P, Frenay M (2001) Nitroso-urea-cisplatin-based chemotherapy associated with valproate: increase of haematologic toxicity. Ann Oncol 12:217–219
Friedman HS, Kerby T, Calvert H (2000) Temozolomide and treatment of malignant glioma. Clin Cancer Res 6:2585–2597
Blackburn SC, Oliart AD, García Rodríguez LA, Pérez Gutthann S (1998) Antiepileptics and blood dyscrasias: a cohort study. Pharmacotherapy 18:1277–1283
Nasreddine W, Beydoun A (2008) Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia 49:438–445
Kimland E, Höjeberg B, Von Euler M (2004) Levetiracetam-induced thrombocytopenia. Epilepsia 45:877–878
Mesche A, Runge U, Sabolek M (2008) Thrombocytopenia during levetiracetam therapy. Epilepsy Res 80:91–92
Gallerani M, Mari E, Boaria B, Carletti R, Marra A, Cavallo M (2009) Pancytopenia associated with levetiracetam treatment. Clin Drug Investig 29:747–751
Elouni B, Ben Salem C, Biour M (2009) Levetiracetam-induced pancytopenia. Ann Pharmacother 43:985
Oghlakian R, Nock C, Koubeissi M (2010) A case of levetiracetam-induced thrombocytopenia. Epileptic Disord 12:335–337
Sahaya K, Goyal MK, Sarwal A, Singh NN (2010) Levetiracetam-induced thrombocytopenia among inpatients: a retrospective study. Epilepsia 51:2492–2495
Scott CB, Scarantino C, Urtasun R et al (1998) Validation and predictive power of radiation therapy oncology group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90–06. Int J Radiat Oncol Biol Phys 40:51–55
Mirimanoff RO, Gorlia T, Mason W et al (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24:2563–2569
Chang SM, Parney IF, Huang W et al (2005) Patterns of care for adults with newly diagnosed malignant glioma. JAMA 293:557–564
Macdonald D, Cascino T, Schold SJ, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Maschio M, Albani F, Jandolo B et al (2008) Temozolomide treatment does not affect topiramate and oxcarbazepine plasma concentrations in chronically treated patients with brain tumor-related epilepsy. J Neurooncol 90:217–221
Van Breemen M, Rijsman RM, Taphoorn MJB, Walchenbach R, Zwinkels H, Vecht CJ (2009) Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol 256:1519–1526
Maschio M, Dinapoli L, Vidiri A et al (2009) The role side effects play in the choice of antiepileptic therapy in brain tumor-related epilepsy: a comparative study on traditional antiepileptic drugs versus oxcarbazepine. J Exp Clin Cancer Res 28:60
Maschio M, Dinapoli L, Saveriano F et al (2009) Efficacy and tolerability of zonisamide as add-on in brain tumor-related epilepsy: preliminary report. Acta Neurol Scand 120:210–212
Merrell RT, Anderson SK, Meyer FB, Lachance DH (2010) Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg 113:1176–1181
Klotz U (2009) Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41:67–76
Armstrong TS, Cao Y, Scheurer ME et al (2009) Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neuro Oncol 11:825–832
Koshy M, Villano JL, Dolecek TA et al (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 107:207–212
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, Bialer M (2004) The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 45:737–744
Li XN, Shu Q, Su JM, Perlaky L, Blaney SM, Lau CC (2005) Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther 4:1912–1922
Fu J, Shao CJ, Chen FR, Ng HK, Chen ZP (2010) Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol 12:328–340
Chen CH, Chang YJ, Ku MS, Chung KT, Yang JT (2011) Enhancement of temozolomide-induced apoptosis by valproic acid in human glioma cell lines through redox regulation. J Mol Med 89:303–315
Bobustuc GC, Baker CH, Limaye A et al (2010) Levetiracetam enhances p53-mediates MGMT inhibition and sensitizes glioblastoma cell to temozolomide. Neuro Oncol 12:917–927
Acknowledgment
The authors thank Rosó Sala and Ana Baños for their excellent nursing and secretarial support, respectively.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simó, M., Velasco, R., Graus, F. et al. Impact of antiepileptic drugs on thrombocytopenia in glioblastoma patients treated with standard chemoradiotherapy. J Neurooncol 108, 451–458 (2012). https://doi.org/10.1007/s11060-012-0836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0836-1