Abstract
The role of repeat resection in the multimodal treatment of gliomas is unclear. Repeat surgery theoretically carries a higher risk of inducing neurological deficits, which might even out any advantage of cytoreduction. We sought to determine whether the occurrence of perioperative infarction is higher for repeat surgery than for first surgery, and sought to identify factors associated with the occurrence of postoperative infarction. Therefore, we searched our database to identify patients who were operated for primary or recurrent glial tumors between October 2007 and October 2010. We analyzed 177 procedures, of which 130 (73.4%) were first surgeries and 47 (26.5%) were repeat. Initial WHO grades, KPS scores, and age were evenly distributed between the groups. Forty-six (26.0%) patients had new DWI lesions on their postoperative MRI scan. Eighteen (10.2%) patients had new lesions greater than 4 cm3. Among these were 11 (6.2%) patients, for whom the new lesion caused neurologic deficit. There was no difference between first and repeat surgery with regard to the occurrence of new DWI lesions (27.7 vs. 21.3%, P = 0.77) or neurological deficits (10.0 vs. 10.6%, P = 1.0). Tumor location in the insula, operculum, and temporal lobe was found to be significantly associated with the occurrence of new DWI lesions. We conclude that repeat surgery should not be withheld as a treatment option for patients with recurrent gliomas for fear of a higher risk of postoperative infarction or new neurologic deficit than the first surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Therapeutic options for recurrent malignant gliomas are diverse. Solely chemotherapeutic [1], combined surgical and chemotherapeutic [2], and radiotherapeutic approaches [3] have been advocated [4, 5]. The role of repeat resection, when feasible, has also been discussed, but EBM-based guidelines are missing [6].
A surgical approach reduces tumor cell burden immediately when the tumor is resistant to standard radio-chemotherapy. Surgery can confirm recurrence if distinction from radiation-necrosis cannot be achieved radiologically. Nevertheless, repeat surgery is traditionally regarded as carrying a higher risk than conservative therapy, which might even out its advantages [7].
In addition to extracranial and extradural complications, for example wound-healing disorders, infections, and cerebrospinal fluid leaks, intraparenchymal surgery itself might be more dangerous. Surgery in a previously irradiated area, where a scar has formed, might lead to more perioperative complications. The glial scar alters the normal intraparenchymal architecture [8–10] and small vessels that can normally be saved may be injured and sacrificed. These vascular complications might lead to greater incidence of perioperative infarction, with potentially devastating effects.
We therefore sought to determine whether the incidence of perioperative infarction is higher in repeat surgery than in first surgery. We further determined factors associated with the occurrence of postoperative infarction.
Methods
Patient population
We retrospectively searched our glioma-patient database in which data are prospectively entered, and selected patients who were operated at our institution between October 2007 (introduction of a DWI procedure to standard postoperative imaging) and October 2010 for primary or relapsing glial tumors.
The medical records of 200 consecutive patients were reviewed and clinical and associated operative data were collected. After surgery, we routinely followed all glioma patients with clinical and MRI examinations every 3 months. We performed adjuvant treatment according to histopathology and prior treatment.
Patient selection for first or repeat surgery
At our institution, the decision to resect a primary or recurrent tumor is based on an interdisciplinary tumor board decision taking into account the patient’s clinical status, past therapeutic regimens, possible future therapeutic regimens, and resectability of the tumor. Patients with lesions suspicious of tumor recurrence usually undergo perfusion-weighted imaging or MR spectroscopy, in addition to conventional neuroimaging, to support diagnosis of tumor versus pseudoprogression.
Surgical technique
All patients underwent microsurgical tumor resection. Tumor resection was performed by use of regular microsurgical instruments, including an ultrasonic aspiration system (Stryker, Freiburg). Subpial dissection was the technique of choice for tumor dissection along the vasculature. Hemostasis was achieved by cauterization and placement of oxidized cellulose strips in the resection cavity. Patients received postoperative steroids for 5 days and mannitol for 3 days, to treat cerebral edema, and were monitored in our neurosurgical ICU for at least 12–24 h. When necessary, intraoperative neurophysiological monitoring techniques were used to enhance resection safety.
MRI modalities
All patients underwent postoperative high-field MRI within 72 h after tumor resection to determine the extent of resection and to rule out complications. MRI, including spin-echo DWI, was performed on a 1.5 T system (Intera; Philips Medical Systems, Best, The Netherlands) and, starting January 2009, on a 3.0 T system (Siemens Verio, Siemens, Erlangen, Germany). The protocol was identical for all patients, including transversal T2-weighted images (TR/TE 4,000/99 ms) and fluid attenuated inversion recovery (FLAIR, TR/TI/TE 10,000/2,000/120 ms). For detection of contrast enhancement, T1-weighted images (TR/TE 650/15 ms) were acquired before and after intravenous application of 0.1 mmol/kg gadolinium-DTPA. A diffusion-weighted single-shot EPI sequence with two b values (b = 0 and 1,000 mm2/s) was used. Both DWI and ADC maps were co-registered with T2-weighted and contrast-enhanced T1-weighted images and ADC maps were analyzed to exclude “shine through” effects.
Data analysis
We screened all postoperative MRI data to identify patients with lesions in the DWI scans not explained by hemostatic agents or fluid in the resection cavity.
We compared patients undergoing first or repeat resection with regard to occurrence and size of new post-operative lesions on DWI scans, new neurologic deficits, age, preoperative Karnofsky performance scale (KPS) score, and location of the tumor. Neurologic deficits with other causes, for example deliberate tissue removal, hemorrhage, or edema, were not considered.
We calculated DWI lesion-volumes and searched for clinical correlates in the patient files. Further, we classified patients with new DWI lesions into two groups:
-
1
those with small lesions, where micro-vessel injuries must be suspected; and
-
2
those with lesions >4 cm3, where bigger perforating vessels must have been injured (Fig. 1).
Exemplary cases of two different types of DWI lesion: a–c: Preoperative a and postoperative b, c scans of a patient with new DWI lesions classified as >4 cm3; d–f: Preoperative d and postoperative e, f scans of a patient with new DWI lesions classified as small new lesions not explainable by hemostatic agents
We defined tumor location according to preoperative MRI as (1) precentral, (2) postcentral, (3) opercular, (4) parafalcine (relation to anterior cerebral artery), (5) occipito-temporal, (6) occipito-parietal, and (7) insular (Fig. 2).
We also assessed survival after second surgery in the group of patients operated upon for recurrent tumors.
Statistics
Statistical analysis was performed by use of commercially available software (SPSS Statistics 19; IBM, Chicago, IL, USA). Binomial dichotomized data were compared by use of Fisher’s exact test, and categorical data were compared by use of the χ 2 test. Median or mean values were compared by use of Student’s t test when appropriate. Survival times were analyzed by the Kaplan–Meier method. P values <0.05 were considered statistically significant.
All patients gave written informed consent before the surgical procedure. The local ethics committee at the Johann Wolfgang Goethe-University approved this study (approval no. 4/09, project SNO_NCH_02_11).
Results
Two-hundred procedures were performed on 179 patients with gliomas of WHO grades II–IV. Mean age was 57 years (range: 23–84 years). Eight patients (4.5%) had initially WHO grade II tumors, nine patients (5.0%) were initially WHO grade III, and 162 patients (90.5%) had WHO grade IV tumors. Median KPS was 80. Postoperative DWI scans were not available after 23 procedures, which were excluded from the analysis. Thus, we analyzed 177 procedures, of which 47 (26.6%) were repeat surgeries for recurrent tumors. Initial WHO grades, KPS, and age were evenly distributed between groups (Table 1). All recurrent tumors were classified as glioblastomas, corresponding to WHO grade IV. Two of these were secondary glioblastomas and the others were recurrent glioblastomas.
Forty-six (26.0%) patients had new DWI lesions on their postoperative MRI scan. All of the lesions were directly adjacent or in close vicinity to the resection cavity and the lesions were not evident on preoperative T2-weighted images. For 25 (14.1%) patients, the new lesion was clinically silent. Eighteen (10.2%) patients had new lesions >4 cm3 (Fig. 3). Among these were 11 (6.2%) patients in which the new infarction led to a new neurologic deficit (Fig. 3). In 28 (15.8%) patients the postoperative DWI scan showed solely small new lesions. In 10 (5.6%) of these patients, these lesions led to an unspecific clinical status change, i.e. new delirium, confusion, or disorientation, which required a prolonged stay in the intensive care unit. Thus, 21 (11.9%) patients in total suffered a clinical status change.
The occurrence of new DWI lesions on postoperative MRI scans did not differ significantly between first and repeat surgery (P = 0.44, Fisher’s exact test, odds ratio (OR) 0.7, 95% confidence interval (CI) 0.3–1. 6, Table 2). Furthermore, the proportion of patients suffering a neurologic worsening did not differ between first and repeat surgery (13 (10.0%) vs. 5 (10.6%), P = 1.0 Fisher′s exact test, (OR) 0.9, 95%-CI 0.3–2.28, Table 2). Lesion size (small vs. large) (P = 1.0, Fisher′s exact test, OR 0.9, 95% CI 0.3–2.9; Table 2) and “clinical status change” (6 (4.6%) vs. 4 (8.5%), P = 0.45, Fisher’s exact test, OR 1.9, 95% CI 0.5–7.2) also did not differ significantly between first and repeat surgery.
In multivariate logistic regression analysis (forward stepwise regression) with “occurrence of new DWI lesions” as the dependent variable, the factors “repeat operation”, “age > 65″, “KPS > 70”, and “initial WHO Grade” were not associated with new DWI lesions on a statistically significant level. However, we found “location” in the insular, opercular, or temporal region to be significantly associated with occurrence of new DWI lesions (Table 3; OR 7.9, 95% CI 3.8–16.5).
To rule out a selection bias in patients undergoing repeat craniotomy, we defined tumor location around the middle cerebral artery and its major branches as “risky” and all other locations as “non-risky”. According to this stratification, 59 of 130 procedures for primary tumors (45.4%) and 17 of 47 procedures for recurrent tumors (36.2%) were for tumors in “risky” location. This difference in proportion was not statistically significant (P = 0.30, Fisher’s exact test, OR 0.7, 95% CI 0.4–1.4).
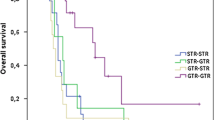
Of the 47 patients operated for tumor recurrence, one patient was lost to follow-up. At the time of analysis (Nov 15, 2011) 10 patients were alive; according to Kaplan–Meier analysis median survival since repeat surgery was 63.7 weeks.
Discussion
Repeat surgery for glioblastoma is still controversially debated [6, 11]. In general, it is thought to carry a higher risk of causing new neurological deficits [12], which in GBM patients has a proven effect on long-term survival [13]. Some authors advocate re-resection only for patients with severe symptoms [4], and perhaps only a subgroup of patients may profit from repeat surgery. Tumor volume reduction by means of surgery can facilitate re-irradiation [14–16], and repeat resection provides new tissue for molecular profiling, which may show changes in molecular characteristics, especially MGMT methylation status [17, 18]. Thus, repeat surgery might facilitate tailoring chemotherapeutic treatment.
There is little substantial evidence about the risks or benefits of repeat resection of a malignant glioma. While there are reports of benefits in terms of improved overall survival of patients undergoing repeat tumor resection [19], this has not been validated in larger studies [20]. Therefore, weighing risks and benefits of a second or even third surgical procedure requires facts for substantive discussion by tumor boards and with patients. Recently, Park et al. [21] introduced a clinical scale for prediction of survival of patients undergoing surgery for recurrent GBM, thus facilitating patient selection.
Most previous studies have focused on non-ischemic complications, e.g. wound-healing disturbances or cerebrospinal fluid leaks. They are known to occur more frequently after repeat surgery than after first surgery [12], but deficits explicitly attributable to perioperative ischemia are most often unreported. Our findings, together with the existing literature, can help clinicians to guide and advise patients in the event of tumor recurrence.
DWI sequences are widely used to detect acute ischemic brain injury [19]. We report here that the occurrence of new DWI lesions on postoperative MRI scans does not differ significantly between first and repeat surgery. Furthermore, the proportion of patients suffering neurologic worsening also did not differ between first and repeat surgery.
Estimating the general risk of developing postoperative infarction after neurosurgical procedures remains controversial. In our series, the incidence of perioperative infarction was 25%, which is rather low compared with previous studies: reportedly, as many as 60–70% [22–24] of patients show areas of reduced diffusion. New neurologic deficits, if they had been reported, were attributable to new DWI lesions in up to 20% [22]. More generally, encompassing any reason for neurologic deterioration, reported incidence of complications ranges between 42.5 and 4.2% [4, 12, 25–28].
In one of the largest series published on complications in glioma surgery, Chang et al. [12] reported significantly different neurological worsening between first and repeat surgery (18 vs. 8%). Their results, based on 499 patients undergoing first or repeat surgery, and data from Barker et al. [25] with a 25% incidence of neurological worsening after repeat surgery, seem to contradict our findings. However, we do not report the incidence of neurologic worsening attributable to any cause except new DWI lesions. We left out cases in which worsening was attributable to the surgery itself, e.g. in a patient with a temporal or parietal tumor experiencing a new visual field deficit explained by deliberate surgical disruption of the optic radiation after tumor removal in the temporal or parietal lobe.
Our results are comparable with those of Khan et al. [29] who, in a heterogeneous series including metastases and gliomas, saw only 19% new DWI-positive lesions with 11% of patients having new neurologic deficits. Our observed frequency of DWI lesions is not explained by lower sensitivity for detecting DWI-signal alterations, because MRI scanning modalities of the former studies were comparable with ours, and lesions as small as 0.3 cm3 were recorded [24].
In our study the site of the tumor, but not repeat resection, was the only risk factor in the development of new DWI lesions according to multivariate analysis. This strengthens the idea that location should be a major factor in risk assessment for postoperative ischemic injury. Park et al. [21] have already included and validated brain areas directly adjacent to the M1 and/or M2 segments of the MCA in their scale for prediction of survival after recurrent GBM operations, but they did not address postoperative patient status. On this basis, we stratified tumor location into “risky” and “non-risky” areas in our series; there was no statistically significant difference between patients undergoing first and repeat surgery, which makes a selection bias unlikely and strengthens our conclusion that repeat surgery alone does not impose greater risks in terms of ischemic deficits.
Kumabe et al. [30] investigated ischemic complications associated with resection of opercular gliomas and found infarction in 100% of the patients studied, possibly related to disruption of arteries from the insula to the corona radiata. Incidence of new DWI lesions in insular gliomas has, unfortunately, not been reported in the largest series published so far [31, 32] but this location per se carries a higher risk of ischemic injury [33]. Our findings seemingly support the aforementioned studies, but the small number of insular and opercular tumors in our series is a limitation.
Different mechanisms can lead to ischemic injury and DWI lesions [32]. Because all lesions in this study occurred around the resection cavity and because T2-shine-through effects were excluded, vessel injury—arterial or venous—is the most likely cause of the DWI lesions. We did not differentiate between arterial infarction, venous stasis, and other possible mechanisms, which eventually does not matter for the individual patient suffering a new deficit. Avoiding vessel injury during resection is the best way of preventing ischemia and applies to both arterial and venous vessels.
Most of our patients are referred to our service from many different institutions; all patients had undergone pre and postcontrast T1 and T2-weighted imaging outside our institution. Although DWI scans were not routinely available preoperatively, it is unlikely they were present preoperatively, because the lesions were all located around the resection cavity and these lesions were not evident on preoperative T2-weighted images, indicating that the lesions occurred in the perioperative phase [34].
Retraction of brain tissue during surgery is sometimes made responsible for the smaller areas of restricted diffusion around the resection cavity [22]. This might explain some of the small DWI lesions around the resection cavity. Because less brain retraction is required in surgery for recurrent gliomas, and because we did not see a difference in occurrence between newly diagnosed and recurrent gliomas, this mechanism becomes less likely.
Rather, our small number of patients with large DWI lesions and symptomatic ischemic areas supports our surgical strategy to avoid vascular injury. With subpial dissection techniques, we avoid vessels coming from the subarachnoid space to nourish the deep white matter, which may cross the resection area and lead to infarction of more deeply seated structures [35]. IOM techniques can help to stop resection in critical subcortical areas [36, 37], but there is no reliable warning sign when vasculature is disrupted.
In an attempt to provide a rationale to support repeat surgery, Xu et al. [13] reported the survival of 21 patients undergoing re-craniotomy to be better than that of a control group, although groups may not have been truly balanced for prognostic factors such as patient age, WHO grade, or KPS score in their study. The median survival of our patients was ~15 months after repeat surgery and thus longer than for their series and for another recent report [13, 19]. However, all patients in our series received adjuvant therapy after repeat resection, which is known to be more effective than repeat surgery alone [4, 7]. It is a limitation of our study that we did not compare survival and quality of life after repeat surgery with a control group of patients not undergoing repeat surgery in the event of tumor regrowth. Thus, we cannot prove that repeat resection is beneficial to the individual patient. Our data may rather help to guide clinicians on multidisciplinary tumor boards.
Repeat surgery, especially where complete resection of the recurrence can be achieved [38], should not be withheld as a treatment option for patients with recurrent gliomas for fear of a higher risk of postoperative infarction or new neurologic deficit than the primary surgery. Adjuvant treatment is nonetheless warranted.
Conclusion
Repeat surgery for gliomas does not per se carry a higher risk of development of new postoperative DWI lesions than first resection, if meticulous microsurgical techniques are applied. Thus, weighing risk and benefits, repeat surgery should not be withheld as a treatment option for patients with recurrent gliomas for fear of a greater risk of postoperative infarction or new neurologic deficit than the primary surgery. The main risk factor for new postoperative DWI lesions seems to be tumor location in the insular, opercular, or temporal region.
References
Schafer N, Tichy J, Thanendrarajan S, Kim Y, Stuplich M, Mack F, Rieger J, Simon M, Scheffler B, Bostrom J, Steinbach JP, Herrlinger U, Glas M (2011) Ifosfamide, carboplatin and etoposide in recurrent malignant glioma. Oncology 80(5–6):330–332
Gilbert MR, Kuhn J, Lamborn KR, Lieberman F, Wen PY, Mehta M, Cloughesy T, Lassman AB, Deangelis LM, Chang S, Prados M (2011) Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neurooncol (Epub ahead of print)
Nieder C, Astner ST, Mehta MP, Grosu AL, Molls M (2008) Improvement, clinical course, and quality of life after palliative radiotherapy for recurrent glioblastoma. Am J Clin Oncol 31(3):300–305
Mandl ES, Dirven CM, Buis, Postma TJ, Vandertop WP (2008) Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surg Neurol 69(5):506–509. doi:10.1016/j.surneu.2007.03.043 discussion 509
Easaw JC, Mason WP, Perry J, Laperriere N, Eisenstat DD, Del Maestro R, Belanger K, Fulton D, Macdonald D (2010) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol (Toronto, Ont) 18(3):e126–e136
Barbagallo GM, Jenkinson MD, Brodbelt AR (2008) ‘Recurrent’ glioblastoma multiforme, when should we reoperate? Br J Neurosurg 22(3):452–455. doi:10.1080/02688690802182256
Terasaki M, Ogo E, Fukushima S, Sakata K, Miyagi N, Abe T, Shigemori M (2007) Impact of combination therapy with repeat surgery and temozolomide for recurrent or progressive glioblastoma multiforme: a prospective trial. Surg Neurol 68(3):250–254
Tofilon PJ, Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radiat Res 153(4):357–370
Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO (2006) Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis 21(3):457–467
Bernstein M, Gutin PH (1981) Interstitial irradiation of brain tumors: a review. Neurosurgery 9(6):741–750
Quant EC, Drappatz J, Wen PY, Norden AD (2010) Recurrent high-grade glioma. Curr Treat Options Neurol 12(4):321–333
Chang SM, Parney IF, McDermott M, Barker FG 2nd, Schmidt MH, Huang W, Laws ER Jr, Lillehei KO, Bernstein M, Brem H, Sloan AE, Berger M (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 98(6):1175–1181. doi:10.3171/jns.2003.98.6.1175
Xu JF, Fang J, Shen Y, Zhang JM, Liu WG, Shen H (2011) Should we reoperate for recurrent high-grade astrocytoma? J Neurooncol 105(2):291–299
Combs SE, Thilmann C, Edler L, Debus J, Schulz-Ertner D (2005) Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol 23(34):8863–8869
Bauman GS, Sneed PK, Wara WM, Stalpers LJ, Chang SM, McDermott MW, Gutin PH, Larson DA (1996) Reirradiation of primary CNS tumors. Int J Radiat Oncol Biol Phys 36(2):433–441
Veninga T, Langendijk HA, Slotman BJ, Rutten EH, van der Kogel AJ, Prick MJ, Keyser A, van der Maazen RW (2001) Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol 59(2):127–137
Brandes AA, Franceschi E, Tosoni A, Bartolini S, Bacci A, Agati R, Ghimenton C, Turazzi S, Talacchi A, Skrap M, Marucci G, Volpin L, Morandi L, Pizzolitto S, Gardiman M, Andreoli A, Calbucci F, Ermani M (2010) O(6)-methylguanine DNAmethyltransferase methylation status can change between first surgery for newly diagnosed glioblastoma and second surgery for recurrence: clinical implications. Neurooncology 12(3):283–288
Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, Schackert G, Kreth FW, Pietsch T, Loffler M, Weller M, Reifenberger G, Tonn JC (2011) Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer 129(3):659–670
Helseth R, Helseth E, Johannesen TB, Langberg CW, Lote K, Ronning P, Scheie D, Vik A, Meling TR (2010) Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand 122(3):159–167
Clarke Dagger JL, Ennis Dagger MM, Yung WK, Chang SM, Wen PY, Cloughesy TF, Deangelis LM, Robins HI, Lieberman FS, Fine HA, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Lamborn KR, Prados MD (2011) Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neurooncology 13(10):1118–1124
Park JK, Hodges T, Arko L, Shen M, Dello Iacono D, McNabb A, Olsen Bailey N, Kreisl TN, Iwamoto FM, Sul J, Auh S, Park GE, Fine HA, Black PM (2010) Scale to predict survival after surgery for recurrent glioblastoma multiforme. J Clin Oncol 28(24):3838–3843
Ulmer S, Braga TA, Barker FG 2nd, Lev MH, Gonzalez RG, Henson JW (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67(9):1668–1670
Smith JS, Lin H, Mayo MC, Bannerjee A, Gupta N, Perry V, Cha S (2006) Diffusion-weighted MR imaging abnormalities in pediatric patients with surgically treated intracranial mass lesions. J Neurooncol 79(2):203–209
Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, Dillon WP, Berger MS (2005) Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 103(3):428–438
Barker FG 2nd, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, Wilson CB (1998) Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42(4):709–720 discussion 720–703
Nimsky C, Ganslandt O, Tomandl B, Buchfelder M, Fahlbusch R (2002) Low-field magnetic resonance imaging for intraoperative use in neurosurgery: a 5-year experience. Eur Radiol 12(11):2690–2703
Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM (1998) Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42(5):1044–1055 discussion 1055–1046
Vives KP, Piepmeier JM (1999) Complications and expected outcome of glioma surgery. J Neurooncol 42(3):289–302
Khan RB, Gutin PH, Rai SN, Zhang L, Krol G, DeAngelis LM (2006) Use of diffusion weighted magnetic resonance imaging in predicting early postoperative outcome of new neurological deficits after brain tumor resection. Neurosurgery 59(1):60–66 discussion 60–66
Kumabe T, Higano S, Takahashi S, Tominaga T (2007) Ischemic complications associated with resection of opercular glioma. J Neurosurg 106(2):263–269
Sanai N, Polley MY, Berger MS (2010) Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg 112(1):1–9
Simon M, Neuloh G, von Lehe M, Meyer B, Schramm J (2009) Insular gliomas: the case for surgical management. J Neurosurg 110(4):685–695
Smith GC, Pell JP (2003) Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ (Clinical research ed) 327(7429):1459–1461
Lutsep HL, Albers GW, DeCrespigny A, Kamat GN, Marks MP, Moseley ME (1997) Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 41(5):574–580
Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F (2003) The microvasculature of the cerebral white matter: arteries of the subcortical white matter. J Neuropathol Exp Neurol 62(2):154–161
Sanai N, Berger MS (2010) Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus 28(2):E1
Wiedemayer H, Fauser B, Sandalcioglu IE, Schafer H, Stolke D (2002) The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg 96(2):255–262
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003
Acknowledgment
The authors would like to thank H. Ackermann, PhD for statistical advice.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dützmann, S., Geßler, F., Bink, A. et al. Risk of ischemia in glioma surgery: comparison of first and repeat procedures. J Neurooncol 107, 599–607 (2012). https://doi.org/10.1007/s11060-011-0784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0784-1