Abstract
Leptomeningeal metastasis is a devastating complication of the central nervous system in patients with late-stage solid or hematological cancers. Leptomeningeal metastasis results from the multifocal seeding of the leptomeninges by malignant cancer cells. Although central nervous system metastasis usually presents in patients with widely disseminated and progressive late-stage cancer, malignant cells may spread to the cerebrospinal fluid during earlier disease stages in particularly aggressive cancers. Treatment of leptomeningeal metastasis is largely palliative but will often provide stabilization and protection from further neurological deterioration and improve quality of life. There is a need to raise awareness of the impact of leptomeningeal metastases on cancer patients and its known and putative biological basis. Novel diagnostic approaches include identification of biomarkers that may stratify the risk for developing leptomeningeal metastasis. Current therapies can be used more effectively while waiting for advanced treatments to be developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leptomeningeal metastasis (LM) represents a devastating complication of malignant cancers that is characterized by the spread of the cancer to the central nervous system (CNS) and the formation of secondary tumors within the thin membranes (leptomeninges) surrounding the brain [1]. The incidence of LM in cancer patients varies with the type of primary cancer as well as the stage of the disease, but it is often broadly estimated to range between 5 and 15% [2–7]. In patients with solid tumors, LM occurs in an estimated 5–18% of patients [8]. A 5–15% incidence has been reported in patients with hematological cancer (including leukemia and lymphoma), while the lowest incidence appears to occur in patients with primary brain tumors (1–2%) [9]. It bears noting that the reported incidence of LM in epidemiological studies is likely underestimated, as some metastases may remain asymptomatic, and others may not be included in the discharge diagnosis [10]. This is supported by autopsy data, which estimate the rate of LM in cancer patients to be as high as 19% [11].
Central nervous system involvement is rarely diagnosed at the initial presentation. Rather, it is considered a late-stage manifestation that is most often identified at the time of relapse [12, 13]. LM is associated with severe morbidity and is nearly always fatal, with the median survival measured in months (or weeks in some studies) [10, 13]. Improved treatment strategies for systemic cancers that lead to longer survival and aggressive diagnostic evaluation have not only increased the rate of detection but also raised the inherent risk of developing CNS metastases [1]. CNS disease will progress in spite of otherwise excellent systemic control in the presence of the blood–brain barrier (BBB) and blood-cerebrospinal fluid (blood-CSF) barrier that allow the CNS to serve as a sanctuary site where malignant cells are shielded from systemic chemotherapy.
Current diagnostic methods are limited and may fail to identify LM early enough to prevent the escalation of neurological damage. Accordingly, an awareness and careful monitoring of early and subtle neurological signs and symptoms may help the clinician make an early diagnosis (Table 1) [1]. Clinical signs and symptoms may be focal but are more often varied, reflecting involvement of the neuroaxis at multiple sites [1, 2]. Traditional diagnostic approaches include cytological examination of the cerebrospinal fluid, flow cytometry, and neuroimaging [1, 12, 14]. Cytological examination of the CSF remains the leading diagnostic laboratory test for LM [14]; however, it suffers from several important deficiencies. A major limitation involves low assay sensitivity and specificity, leading to false-negative and, to a lesser extent, false-positive results [11, 14, 15]. In addition, CSF obtained from a site distant from pathologically involved meninges may not correlate with disease presence or therapeutic response [16]. CSF cytology may be supplemented by flow cytometry, which offers an objective and highly sensitive method to assist the clinician in an earlier diagnosis [17]. For specific tumor types, markers typically used to monitor systemic disease (serum) may also be detected in CSF (Table 2). Immunophenotyping using 2–4 different markers offers an alternative quantitative method to detect malignant cells [12]. Neuroimaging methods including magnetic resonance imaging (MRI) are also used to diagnose LM but may be difficult to interpret and are also subject to false-negative results [1, 14].
The goals of therapeutic intervention include palliation as well as stabilization, protection from further neurological deterioration, and improved quality of life. Based upon recent advances in understanding cancer and its treatment, this article reviews the pathophysiology of LM; discusses current and novel diagnostic approaches, including biomarkers that may identify patients at risk for developing LM; and introduces emerging options in the probable prophylaxis and treatment of LM.
Understanding and targeting the pathophysiology of leptomeningeal metastasis
Our current understanding of how brain metastases occur is limited. In the absence of such information, the rational design of new therapies to prevent or control LM is also limited. By considering the environment in which these metastatic cells thrive, we can begin to use available therapies more effectively and formulate improved therapies.
Cerebrospinal fluid flow, volume, and pressure are regulated and maintained by transport processes at barrier interfaces [18]. Two principal interfaces serve this role: the BBB and the blood–CSF barrier. Together, these barrier systems maintain brain homeostasis by restricting the uncontrolled diffusion of blood-borne chemicals into the CNS parenchyma. The endothelial (BBB) and epithelial (blood–CSF barrier) cells that form these virtually impenetrable barriers are joined by tight junctions that restrict the free passage of water-soluble molecules and cells [18]. Penetration must occur either by a breach in the barrier or by some inherent characteristic(s) of the cells that allows passage through these barriers. Understanding the mechanism by which metastatic cells penetrate the BBB and blood–CSF barrier represents a major obstacle to overcome in the development of novel LM therapies. To this end, knowing the origin of the metastatic cells that cause LM and where/how they enter the CSF may help guide the design of novel therapeutic strategies. However, little is known regarding the origin of metastatic cells, i.e. whether they initially reside in a vascular or systemic niche. The blood–CSF barrier, which is composed of the choroid plexus and the arachnoid membrane, is widely distributed and may represent a route for tumor cells to reach the CNS [18]. The choroid plexus consists of many capillaries, loose connective tissue, and the choroid epithelium. While the epithelium is connected by largely impervious tight junctions, the capillaries possess a fenestrated endothelium through which solutes (and possibly cells) may pass [19]. This may serve as a portal of entry into the CSF for malignant cells. In principle, compounds or molecules could be developed to prevent tumor cells from entering the CSF by blocking choroid plexus cell surface markers to which tumor cells are attracted [20, 21].
Aside from understanding the site of entry, understanding the mechanism of tumor cell entry is also likely to reveal novel therapeutic targets. Malignant cells may reach the CSF and meninges via a variety of mechanisms, including hematogenous metastases to the choroid plexus, venous dissemination, or centripetal extension through neurovascular bundles [22]. Once tumor cells reach the leptomeninges, diffuse spread throughout the neuroaxis is achieved by the constant flow of CSF. Eventually, these distributed cells have the potential to settle and grow, forming leptomeningeal tumors [5]. These metastases result from complex interactions between the tumor cells and the tissue microenvironment mediated through cell–cell and cell–matrix contacts and through the release of cytokines and growth factors. A combination of different gene products expressed by certain cancer cells may potentially contribute to the enhancement of metastatic potential. Current research seeks to identify common pathways that regulate the expression of groups of metastasis-associated genes (e.g. those mediating tumor-stromal interactions). Indeed, emerging evidence suggests that there may be a distinct subpopulation of tumor cells with high metastatic potential, and that these cells, presumed to be cancer stem cells (CSCs), may possess a unique gene signature [23].
The cancer stem cell hypothesis proposes that CSCs not only drive tumor initiation and sustain growth but also may have a higher potential to invade and metastasize. Furthermore, in the context of this model, putative CSC subpopulations are believed to be highly resistant to chemotherapy and other conventional treatments. The potential for tumor regeneration therefore remains even after existing cancer therapies effectively remove a given tumor (Fig. 1) [24, 25]. This hypothesis has important clinical implications for LM therapy and prevention. The exact mechanism of LM is unknown in any particular patient. Because LM may represent the clonal expansion of stem cells in the CSF, targeted anti-CSC therapy may represent a novel therapeutic approach. The elucidation of pathways that regulate CSCs, such as Notch, Hedgehog, and Wnt, and the identification of molecular markers for this subpopulation of cells with high tumorigenic potential, may provide new targets for therapeutic development [24].
Cancer stem cell hypothesis: potential new target for the treatment of leptomeningeal metastases (LM). a Stem cells are defined by their ability to undergo self-renewal, as well as multi-lineage differentiation. Cancer stem cells may play a role in the growth and spread of cancer. b The cancer stem cell hypothesis proposes that primary tumors originate in tissue stem and/or progenitor cells through the dysregulation of the normally tightly regulated process of self-renewal. As a consequence, tumors represent a heterogeneous population of cells that contain a cellular component that retains key stem-cell properties including self-renewal, which initiates and drives carcinogenesis and differentiation. c Cancer stem cells may play an important role in mediating tumor metastasis, including metastasis to the leptomeninges. LM may represent the clonal expansion of cancer stem cells. d Cancer stem cells are believed to be relatively resistant to conventional anticancer therapy. If this is true, limitations of present therapies may relate to their inability to target the cancer stem cell component that is responsible for recurrence. Current therapies have been developed by virtue of their ability to induce tumor regression and may selectively target more differentiated cells in tumors, while leaving the tumor stem cell population intact, accounting for treatment resistance and relapse. e Aberrant stem cells may provide targets for the development of LM prevention strategies in the future. The elucidation of pathways that regulate cancer stem cells may provide new inroads for therapeutic development. Targeting tumorigenic cancer stem cells may decrease recurrence and improve long-term survival
Novel approaches to leptomeningeal metastasis diagnosis and treatment
Diagnosis
Apart from the more traditional diagnostic approaches discussed earlier (e.g. cytological examination), the use of biochemical markers may help to improve LM diagnosis, though for many years, these methods have not been without drawbacks. Poor sensitivity and specificity has limited the stand-alone use of biochemical markers for diagnosing LM. Nonspecific tumor markers including creatinine kinase BB isoenzyme, tissue polypeptide antigen, β2 microglobulin, β-glucoronidase, and lactate dehydrogenase isoenzyme-5, have served only as indirect indicators of LM and generally are useful only as adjunctive diagnostic assessment tools [14]. The identification of specific biochemical markers implicated in tumor invasion, angiogenesis, and metastasis may allow for earlier diagnosis and treatment of LM in the future. A recent study suggested that vascular endothelial growth factor (VEGF) represents a sensitive and specific biomarker for malignant cells in the CSF [3]. VEGF is known to induce endothelial cell proliferation and tumor angiogenesis [3, 26–28]. Groves et al. [3] demonstrated the positive predictive value of elevated CSF VEGF levels in patients with breast cancer, lung cancer, or melanoma and suggested that screening for VEGF levels in these high-risk patients is warranted to ensure early treatment. Evidence suggests that other correlative biomarkers also may help identify patients at risk of LM. Among these are molecules involved in chemotactic signaling, cell motility/adhesion, and stromal–cell interactions [29–31].
Chemokines represent an important class of molecules that regulate the trafficking, proliferation, and adhesion of leukocytes [30]. Cell adhesion is a crucial step in tumor cell migration and is regulated by a cytoskeletal matrix of focal adhesion molecules. Cells with high CNS metastatic potential might express specific adhesion molecules that facilitate homing to the CNS. The chemokine receptor CXCR4 and its ligand, stromal cell-derived factor-1 (SDF-1), were found to play a key role in breast cancer cell invasion and migration [31]. This study demonstrated that CXCR4/SDF-1 signaling increased vascular permeability and resulted in increased migration and penetration of tumor cells through brain microvascular endothelial cells.
More recently, CXCR1 and CXCR2, both CXCL-8 receptors over-expressed in melanoma, were found to induce tumorigenicity and promote tumor growth and invasion in preclinical in vitro and in vivo studies [32]. Other recent data suggest that Menainv, an actin-binding protein involved in the regulation of cell motility, may be a promising new biomarker for metastatic breast cancer that may ultimately help identify patients at risk for LM. In vitro and in vivo data demonstrated that the expression of a specific protein isoform of Mena is restricted to the invasive subpopulation of breast tumor cells, whereas it is absent from those cells that do not metastasize [33]. Another key finding of this study involves tumor cell responsiveness to epidermal growth factor (EGF), which has important implications for treatment. EGF has been shown to increase a breast cancer cell’s metastatic potential [34]. Tumor cells that express Menainv are less likely to be responsive to newer breast cancer treatments that inhibit the EGF receptor (EGFR) [33]. The authors suggest that EGFR inhibitors may lack efficacy on Menainv-expressing tumor cells because of their enhanced sensitivity to EGF. Even a small signal that the drugs fail to block may be enough to stimulate the EGFR and promote tumor cell migration and metastasis. Perhaps, in the future, an antibody- or polymerase chain reaction (PCR)-based assay to identify Menainv-expressing tumor cells may be used to diagnose patients at high risk for progressive disease and possibly LM. These results may provide further insight into the molecular mechanisms underlying metastasis to the brain, and help develop appropriate treatment intervention early in the disease process, leading to improved outcomes and perhaps the prevention of LM.
In addition to the use of specific biomarkers, immunohistochemical analyses and nucleic acid-based assays may improve LM diagnosis and help identify reliable biological markers of the disease. In the case of leukemia and lymphoma, the use of monoclonal antibodies against surface markers may be used to distinguish between reactive and neoplastic lymphocytes in the CSF [14]. CSF flow cytometry can be helpful in distinguishing neoplasm from reactive lymphocytes. This is an important distinction, as morphologic evaluation of CSF cells by light microscopy may be confounded by the presence of reactive lymphocytes, which may generate false-positive results [12]. Cytogenetic studies have also been evaluated as a possible diagnostic aid in LM [35, 36]. The detection of numerical and structural genetic aberrations as a sign of malignancy using fluorescence in situ hybridization also may give additional diagnostic information. When used in conjunction with routine cytologic assessment, this method may yield a more accurate diagnosis [36]. Finally, PCR amplification to identify particular gene rearrangements has shown utility in CSF analysis [12, 37, 38]; however, this particular strategy requires precise knowledge of the genetic alterations which, in most cases, is simply not yet known [14].
Treatment
Standard treatment modalities for LM include radiotherapy to sites (if any) of bulky disease and the CSF as well as systemic and/or intra-CSF chemotherapy (Table 3) [39–41]. Many of the current approaches to treatment are subject to restrictions related to toxicities, ease of administration, and efficacy. For example, because of poor CNS penetration at conventional doses, systemic therapy with methotrexate must be given at high doses (≥3 g/m2) to ensure that sufficient quantities reach the CSF, placing the patient at risk of considerable systemic toxicity. Intra-CSF administration of chemotherapeutics has many advantages, including circumventing the BBB, ensuring direct drug delivery to the leptomeninges, and minimizing the potential for systemic toxicity through low drug dose requirements [41]. The disadvantages and risks associated with intra-CSF therapy center upon the route of administration, which occurs via lumbar puncture or directly to the ventricles via Ommaya reservoir. Surgical complications or inflammatory reactions may develop shortly after drug delivery [42] while late complications such as leukoencephalopathy may be progressive and even fatal [43]. Additionally, CSF flow abnormalities can impede adequate distribution of intra-CSF drugs to all diseased areas and may therefore constitute one reason for treatment failure [44]. Increased toxicity may result from intra-CSF therapy when CSF flow abnormalities are present due to decreased volume of distribution, and thus CSF flow study should be done prior to treatment. Focal irradiation may also aid in the normalization of CSF flow if block is present [45].
Ventriculoperitoneal shunting is often used to alleviate hydrocephalus, a complication of LM and an important prognostic factor [46, 47]. Persistent hydrocephalus, and associated intracranial hypertension, are associated with neurological deterioration and may lead to debilitating symptoms including headache, nausea, vomiting, ataxia, and cognitive impairments [48, 49]. Ventriculoperitoneal shunts may improve the prognosis of LM and may be an effective palliative tool and viable option in treating LM alongside chemotherapy [49, 50]. However, the risks associated with ventriculoperitoneal shunting which requires an invasive surgical procedure must be taken into consideration. These involve hemorrhage, shunt malfunction or infection, peritonitis, and in rare instances, peritoneal carcinomatosis [49, 51]. Another consideration with ventriculoperitoneal shunting involves selection of the appropriate type of valve, optimal drainage rate/volume, and the precise type of shunting, which remains controversial [52]. A programmable pressure valve is designed to accommodate different pressure settings as needed and allows noninvasive adjustments to be made externally through the use of micromagnets [52]; however, unintentional reprogramming may occur with exposure to magnetic fields (e.g. during MRI, metal detector exposure) [53]. Additionally, programmable shunts produce significant MRI artifacts, hindering the use of the latter modality for intracranial disease surveillance.
Newly emerging alternatives to standard LM treatment options include sustained-release formulations of conventional chemotherapies, pathway-specific inhibitors, and monoclonal antibody therapy. Six alternative treatment options will be discussed: liposomal cytarabine, lapatinib, gefitinib, capecitabine, bevacizumab, and rituximab.
Liposomal cytarabine
A cell-cycle-specific synthetic nucleoside, this sustained-release formulation of cytarabine is indicated for the treatment of LM and is designed for direct administration into the CSF. Its extended half-life allows for a less-frequent dosing schedule compared with standard intrathecal chemotherapy, such as methotrexate or unencapsulated cytarabine, and promotes a consistent distribution of cytarabine throughout the neuraxis [54]. The only randomized trial that compared liposomal and unencapsulated cytarabine in patients with lymphomatous meningitis (N = 28) reported complete cytological responses in 71% of patients receiving liposomal cytarabine, versus only 15% of patients receiving unencapsulated cytarabine (P = 0.006) [54]. Patients treated with liposomal cytarabine also experienced a longer time to neurological progression (78 days) compared with patients receiving unencapsulated cytarabine (42 days). A more recent, and larger, retrospective chart review reported similar safety and efficacy results of intrathecal liposomal cytarabine in patients with lymphomatous meningitis (N = 55) [55]. Complete and partial neurologic responses, defined as disappearance of all or at least 50% of neurological symptoms, respectively, were achieved in 27 and 12 patients, respectively, with an overall neurological response rate of 72%. Median time to neurological progression was 105.5 days. The authors concluded that liposomal cytarabine should be the treatment of choice in patients with LM in the setting of lymphoma.
Lapatinib
Specific pathway inhibitors may provide a therapeutic strategy for eliminating subpopulations of chemotherapy-resistant cells (e.g. those with a high potential for CNS metastasis). Lapatinib is a small tyrosine kinase inhibitor that acts as a dual inhibitor of two members of the EGFR family, EGFR and human epidermal growth factor receptor (HER)-2/neu. EGFR is overexpressed in many solid tumors, including non-small cell lung, breast, colorectal, head/neck, and prostate cancers [56]. Overexpression of HER-2/neu is seen in approximately 25% of breast cancers and also in a variety of other cancers, including ovarian, prostate, lung, and gastrointestinal cancer [56]. Lapatinib has been reported to cross the BBB and might therefore have a role in treating or preventing CNS progression [57]. The addition of systemic lapatinib (750 mg twice daily) was evaluated in 242 patients with HER-2+ breast cancer and progressive brain metastases following treatment with trastuzumab and cranial radiotherapy [58]. Although patients with LM only were excluded, CNS objective responses were seen in 6% of patients. The results demonstrate the feasibility of a targeted systemic therapy in the treatment of LM and a recent case report has documented response [59].
Gefitinib
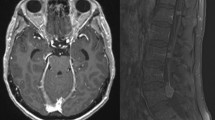
Other EGFR-targeted therapies, such as gefitinib, have been subject to comprehensive clinical development. Two pivotal in vitro tumor tissue studies have shown that a favorable clinical response to gefitinib is closely associated with the presence of somatic mutations in the EGFR gene [60, 61]. A recent prospective phase-2 study demonstrated that first-line gefitinib in patients with non-small cell lung cancer (NSCLC) expressing EGFR mutations and very poor performance status resulted in a 66% response rate and a 1-year survival rate of 63% [62]. However, a high incidence of disease recurrence in the brain and leptomeninges of patients who are initially responsive to gefitinib has been reported [63]. A higher-than-standard dose of gefitinib might be an effective LM treatment option if adequate drug concentrations could be achieved in the CSF. In support of this, beneficial effects of high-dose gefitinib in the treatment of LM in select NSCLC patients have recently been reported [64, 65]. Yi et al. [65] analyzed, retrospectively, the response of patients with NSCLC and LM, and known or highly probable EGFR mutations (N = 11), to high-dose gefitinib (500 and 750 mg/day) followed by erlotinib (n = 2). The two patients treated with high-dose gefitinib survived beyond 18.6 and 8.6 months since diagnosis of LM, exceeding the known survival rate after 4–6 months of treatment. In another study, brain MRI showed marked improvement in a patient with NSCLC, progressive intracranial metastases (Fig. 2a) and de novo meningeal involvement following high-dose gefitinib therapy (Fig. 2b) [66]. Erlotinib, which similarly targets EGFR, achieves therapeutic CSF levels, particularly at higher-doses [67] and clinical responses have been reported [68].
a Brain magnetic resonance imaging (MRI) demonstrating evidence of progressive intracranial metastases, new leptomeningeal involvement, and enhancement of the seventh and eighth nerve complex in the left auditory canal. b MRI demonstrating marked improvement with only minimal leptomeningeal enhancement. Reprinted with permission [64] ©2006 American Society of Clinical Oncology
Capecitabine
Capecitabine, an oral analog of 5-fluorouracil, acts as an inhibitor of DNA synthesis [69]. Several case reports have suggested that capecitabine treatment yields good responses and durable disease control for LM from breast cancer [69–71] and lung cancer [72]. Tham and colleagues [71] reported a long-term (up to 3.7 years) complete clinical response in patients with LM secondary to breast cancer following capecitabine monotherapy. Shigekawa et al. [69] recently reported the successful treatment of LM from breast cancer with a combination of trastuzumab and capecitabine. However, the mechanism underlying this potential synergy is unknown. An earlier study of capecitabine combined with temozolomide in the treatment of brain metastases from breast cancer resulted in an 18% objective tumor response rate [73]. Furthermore, neurocognitive function improved or stabilized in patients who exhibited a partial or complete response. To our knowledge, no further studies have been conducted on this combination.
Bevacizumab
As discussed earlier, VEGF and its receptors have been implicated in tumor growth and propagation through an enhancement of angiogenesis. Bevacizumab is an anti-VEGF monoclonal antibody that blocks VEGF receptor–ligand binding interactions [56]. New vessels formed through VEGF-induced neo-vascularization may cause increased leakage at the BBB [74]. In support of this concept, preclinical studies have demonstrated that while the BBB is intact in patients with small metastases, it is disrupted in those with larger tumors [74]. A case study of a patient with two visible parenchymal brain metastases from colorectal cancer also demonstrated that the addition of bevacizumab to systemic therapy resulted in a complete response in one lesion, and a partial response in the other [75]. Anti-VEGF treatment also could prevent cells from metastasizing to the CNS through a “normalization” of the vasculature [76]. This is relevant, because the most common cause of symptomatic CNS metastases is believed to be the hematogenous spread of malignant cells to the brain [77]. An encouraging clinical response was reported in a patient with LM from colorectal cancer [78]. This patient received a combination of bevacizumab, temozolomide, and irinotecan.
Rituximab
Rituximab is a genetically engineered chimeric mouse/human monoclonal antibody that binds to the CD20 transmembrane antigen on the majority of circulating B-cells [79]. It has a general regulatory effect on the cell cycle and induces a pronounced, rapid, and prolonged near-depletion (lysis) of circulating B-cells and sensitizes them to cytotoxic chemotherapy. The Fc domain of rituximab mediates B-cell lysis through the recruitment of immune effector functions [80]. In a small-scale (N = 10), phase-1 study of intraventricular rituximab in patients with recurrent CNS non-Hodgkin’s lymphoma and LM, meningeal responses were detected in six patients: intraocular responses in two patients and the resolution of brain parenchymal lymphoma in one patient [81]. Similarly, a recent clinical series of 14 patients with recurrent LM tested the feasibility of combining liposomal cytarabine and rituximab [82]. All patients received intraventricular therapy via Ommaya reservoir. Ten patients exhibited a partial response, defined as a conversion from positive to negative CSF with stable, or improved, neurological deficits, and the overall survival was 5 months (range 1.5–7). These results suggest that there is no additive toxicity of combining these two agents with modest palliative activity. Four patients remain alive and continue to be followed. These preliminary results further suggest that rituximab may be feasible and effective in this patient population.
Prophylaxis
Despite the lack of a clear consensus, several new chemotherapies show efficacy in treating LM and hold promise for future LM prophylaxis. Combining the most appropriate systemic chemotherapy for the primary tumor with effective intra-CSF therapy for leptomeningeal involvement may be a feasible approach for the future. However, the decision to treat prophylactically must be based on the relative risk of CNS metastases of the primary tumor and the toxicities associated with additive prophylactic therapy. Twenty-year follow-up data from a randomized trial of patients with intermediate- or high-grade non-Hodgkins lymphoma with bone marrow involvement compared patients who received prophylactic intrathecal methotrexate and unencapsulated cytarabine [83]. While relapse was uncommon (2.8%), CNS relapse occurred on average 5.4 months after diagnosis, when most patients are responding to primary systemic treatment. This suggests that patients with apparent relapse in the CNS may actually have had occult CNS involvement at the time of cancer diagnosis. Moreover, there was no difference in the CNS relapse rate between patients who did or did not receive prophylaxis. This suggests that prophylaxis may not be warranted, and that the paradigm be shifted from prophylaxis to the detection of sub-clinical CNS metastasis at an earlier stage to maximize treatment efficacy. These results should be interpreted keeping in mind the limited statistical power of the analyses and the choice of prophylactic agents and regimens used. The GEL-TAMO group conducted a retrospective study investigating the efficacy and safety of liposomal cytarabine in patients with diffuse large B-cell lymphoma and cytologically negative for lymphomatous meningitis [84]. Patients included those at risk of CNS metastasis, defined as having either testicular or bone marrow involvement, bulky abdominal disease, and/or involvement of paranasal or sinus tissues. After a 12-month follow-up, the authors reported no evidence of CSF involvement as assessed by flow cytometry, suggesting that liposomal cytarabine may be an option for CNS prophylaxis in patients with aggressive non-Hodgkin’s lymphoma. Despite these promising results, the efficacy and utility of LM prophylaxis remains unresolved because of the lack of prospective trial data and consensus on CNS relapse risk factors [85].
Conclusions
It is clear that LM occurs more frequently in cancer patients than is understood or appreciated. Several new diagnostic tools are available to help physicians diagnose the disease earlier, which may improve treatment outcomes and enhance preventative measures. Newly emerging treatment strategies, including sustained-release formulations, antibody-based and pathway-targeted approaches, and multimodal therapeutic combinations may dramatically improve the treatment of LM and offer improved prophylactic interventions. Advances in the treatment and diagnosis of primary cancer, as well as further advances in our understanding of the pathophysiology of LM, may ultimately lead to a new and more effective treatment paradigm.
References
Kesari S, Batchelor TT (2003) Leptomeningeal metastases. Neurol Clin N Am 21:25–66
Gleissner B, Chamberlain MC (2006) Neoplastic meningitis. Lancet Neurol 5:443–452
Groves MD, Hess KR, Puduvalli VK et al (2009) Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol 4:229–234
Mitchell MS (1989) Relapse in the central nervous system in melanoma patients successfully treated with biomodulators. J Clin Oncol 7:1701–1709
O’Meara WP, Borkar SA, Stambuk HE et al (2007) Leptomeningeal metastasis. Curr Probl Cancer 31:372–424
Theodore WH, Gendelman S (1981) Meningeal carcinomatosis. Arch Neurol 38:696–699
Yap HY, Yap BS, Rasmussen S et al (1982) Treatment for meningeal carcinomatosis in breast cancer. Cancer 50:219–222
Jorda M, Ganjei-Azar P, Nadji M (1998) Cytologic characteristics of meningeal carcinomatosis. Increased diagnostic accuracy using carcinoembryonic antigen and epithelial membrane antigen immunocytochemistry. Arch Neurol 55:181–184
Chamberlain MC (2006) CSF Disseminated primary brain tumors. In: Newton HB (ed) Handbook of brain tumor chemotherapy. Elsevier Science, San Diego, pp 316–326
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14
Glass JP, Melamed M, Chernik NL et al (1979) Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 29:1369–1375
Gleissner B, Chamberlain MC (2007) Treatment of CNS dissemination in systemic lymphoma. J Neurooncol 84:107–117
Hatton C (2005) Lymphomatous meningitis. Haematologica Rep 1:108–109
Chamberlain MC (2008) Neoplastic meningitis. Curr Neurol Neurosci Rep 8:249–258
Yoshida S, Morii K, Watanabe M et al (2000) Characteristic features of malignant lymphoma with central nervous system involvement. Surg Neurol 53:163–167
Glantz MJ, Cole BF, Glantz LK et al (1998) Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 82:733–739
Ward MS (1999) The use of flow cytometry in the diagnosis and monitoring of malignant hematological disorders. Pathology 31:382–392
Johanson CE, Duncan JA, Stopa EG et al (2005) Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res 22:1011–1037
Kobayashi Z, Tsuchiya K, Machida A et al (2009) Metastatic CNS lymphoma presenting with periventricular dissemination–MRI and neuropathological finding in an autopsy case. J Neurological Sci 277:109–113
Levine S (1987) Choroid plexus: target for systemic disease and pathway to the brain. Lab Invest 56:231–233
Oldstone MB, Southern PJ (1993) Trafficking of activated cytotoxic T lymphocytes into the central nervous system: use of a transgenic model. J Neuroimmunol 46:25–31
Groves MD (2003) The pathogenesis of neoplastic meningitis. Curr Oncol Rep 5:15–23
Alix-Panabieres C, Riethdorf S, Pantel K (2008) Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 14:5013–5021
Kakarala M, Wicha MS (2008) Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 26:2813–2820
Li X, Lewis MT, Huang J et al (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Fidler IJ, Langley RR, Kerbel RS et al (2005) Angiogenesis. In: De Vita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice in oncology. Lippincott Williams & Wilkins, New York, pp 129–135
Kraft A, Weindel K, Ochs A et al (1999) Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 85:178–187
Stockhammer G, Poewe W, Burgstaller S et al (2000) Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology 54:1670–1676
Goswami S, Phillippar U, Sun D et al (2009) Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis 26:153–159
Kadoch C, Treseler P, Rubenstein JL (2006) Molecular pathogenesis of primary central nervous system lymphoma. Neurosurg Focus 21:E1
Lee B-C, Lee T-H, Avraham S et al (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res 2:327–338
Singh S, Nannuru KC, Sandanandam A et al (2009) CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer 100:1638–1646
Philippar U, Roussos ET, Oser M et al (2008) A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15:813–828
Wyckoff J, Wang W, Lin EY et al (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64:7022–7029
Van Oostenbrugge RJ, Hopman AHN, Ramaekers FCS et al (1998) In situ hybridization: a possible diagnostic aid in leptomeningeal metastasis. J Neurooncol 38:127–133
Van Oostenbrugge RJ, Hopman AHN, Arends JW et al (2000) Treatment of leptomeningeal metastasis evaluated by interphase cytogenetics. J Clin Oncol 18:2053–2058
Baehring JM, Hochberg FH, Betensky RA et al (2006) Immunoglobulin gene rearrangement analysis in cerebrospinal fluid of patients with lymphoproliferative processes. J Neurol Sci 247:208–216
Gleissner B, Siehl J, Korfel A et al (2002) CSF evaluation in primary CNS lymphoma patients by PCR of the CDR III IgH genes. Neurology 58:390–396
Berg SL, Chamberlain MC (2003) Systemic chemotherapy, intrathecal chemotherapy, and symptom management in the treatment of leptomeningeal metastasis. Curr Oncol Rep 5:29–40
Berg SL, Chamberlain MC (2005) Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management. Cancer Treat Res 125:121–146
Chamberlain MC, Nolan C, Abrey LE (2005) Leukemic and lymphomatous meningitis: incidence, prognosis and treatment. J Neurooncol 75:71–83
Chamberlain MC, Kormanik PA, Barba D (1997) Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg 87:694–699
Siegal T, Lossos A, Pfeffer MR (1994) Leptomeningeal metastases: analysis of 31 patients with sustained off-therapy response following combined-modality therapy. Neurology 44:1463–1469
Glantz MJ, Hall WA, Cole BF et al (1995) Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer 75:2919–2931
Glantz MJ, Hall WA, Cole BF et al (1995) Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer 75(12):2919–2931
Chamberlain MC, Glantz M (2006) Ventriculoperitoneal shunt in patients with leptomeningeal metastases. Neurology 66:783
Omuro AM, Lallana EC, Bilsky MH et al (2005) Ventriculoperitoneal shunt in patients with leptomeningeal metastases. Neurology 10:1625–1627
Mocco J, Tomey MI, Komotar RJ et al (2006) Ventriculoperitoneal shunting of idiopathic normal pressure hydrocephalus increases midbrain size: a potential mechanism for gait improvement. Neurosurgery 59:847–851
Schiff D, Kline C, Meltzer H et al (2009) Palliative venticuloperitoneal shunt in a pediatric patient with recurrent metastatic medulloblastoma. J Palliat Med 12:391–393
Lokich J, Levine H, Nasser I (1998) Malignancy-related hydrocephalus: clinical features and results of ventricular peritoneal shunt procedure in three patients. Am J Clin Oncol 21:366–368
Eralp Y, Saip P, Aydin Z et al (2008) Leptomeningeal dissemination of ovarian carcinoma through a ventriculoperitoneal shunt. Gynecol Oncol 108:248–250
Matsumae M, Sato O, Itoh K et al (1989) Quantification of cerebrospinal fluid shunt flow rates. Assessment of the programmable pressure valve. Childs Nerv Syst 5:356–360
Chiafery M (2006) Care and management of the child with shunted hydrocephalus. Pediatr Nurs 32:222–225
Glantz MJ, LaFollette S, Jaeckle KA et al (1999) Randomized trial of a slow-release versus standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol 17:3110–3116
Garcia-Marco JA, Panizo C, Garcia ES et al (2009) Efficacy and safely of liposomal cytarabine in lymphoma patients with central nervous system involvement from lymphoma. Cancer 115:1892–1898
Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23:1147–1157
Cameron DA, Stein S (2008) Drug insight: intracellular inhibitors of HER2—clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol 5:512–520
Lin NU, Diéras V, Paul D et al (2009) Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 15:1452–1459
Onishi H, Morisaki T, Nakafusa Y et al. (2011) Objective response with lapatinib in patients with meningitis carcinomatosa derived from HER2/HER1-negative breast cancer. Int J Clin Oncol. doi:10.1007/s10147-011-0195-5
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Inoue A, Kobayashi K, Usui K et al (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27:1394–1400
Omuro AM, Kris MG, Miller VA et al (2005) High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 103:2344–2348
Jackman DM, Kesari S, Cioffredi L et al. (2009) Phase I study of high dose gefitinib in patients with leptomeningeal metastases from EGFR-mutant non-small cell lung cancer. Presented at the 13th World Congress on Lung Cancer San Francisco, 31st July–4th August 2009; Poster P1.189
Yi HG, Kim HJ, Kim YJ et al (2009) Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer 65:80–84
Jackman DM, Holmes A, Lindeman N et al (2006) Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 24:4517–4520
Clarke JL, Pao W, Wu N et al (2010) High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 99(2):283–286
Tetsumoto S, Osa A, Kijima T et al (2011) Two cases of leptomeningeal metastases from lung adenocarcinoma which progressed during gefitinib therapy but responded to erlotinib. Int J Clin Oncol. doi:10.1007/s10147-011-0256-9
Shigekawa T, Takeuchi H, Misumi M et al (2009) Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast Cancer 16:88–92
Ekenel M, Hormigo AM, Peak S et al (2007) Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol 85:223–227
Tham YL, Hinckley L, Teh BS et al (2006) Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer 7:164–166
Paydas S, Bicakci K, Yavuz S (2009) Dramatic response with capecitabine after cranial radiation to the brain parenchymal and leptomeningeal metastases from lung cancer. Eur J Intern Med 20:96–99
Rivera E, Meyers C, Groves M et al (2006) Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107:1348–1354
Fidler IJ, Yano S, Zhang RD et al (2002) The seed and soil hypothesis: vascularization and brain metastases. Lancet Oncol 3:53–57
Bhaskara A, Eng C (2008) Bevacizumab in the treatment of a patient with metastatic colorectal carcinoma with brain metastases. Clin Colorectal Cancer 7:65–68
Kim LS, Huang S, Lu W et al (2004) Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis 21:107–118
Posner JB (1996) Brain metastases: 1995 a brief review. J Neurooncol 27:287–293
Ku GY, Krol G, Ilson DH (2007) Successful treatment of leptomeningeal disease in colorectal cancer with a regimen of bevacizumab, temozolomide, and irinotecan. J Clin Oncol 25:e14–e16
Cvetkovic RS, Perry CM (2006) Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Drugs 66:791–820
Marcus R, Hagenbeek A (2007) The therapeutic use of rituximab in non-Hodgkin’s lymphoma. Eur J Haematol Suppl 67:5–14
Rubenstein JL, Fridlyand J, Abrey L et al (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25:1350–1355
Chamberlain MC, Johnston SK, Van Horn A et al (2009) Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol 91:271–277
Bernstein SH, Unger JM, Leblanc M et al (2009) Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516—the southwest oncology group. J Clin Oncol 27:114–119
Canales M, Peñarrubia MJ, Salar A et al. (2008) Efficacy and safety of intrathecal liposomal cytarabine injection given prophylactically in patients with high-risk diffuse large B-cell lymphoma: a report of 24 patients in Spain. Abstract Presented at: the 13th Congress of the European Hematology Association, Copenhagen, Denmark, 12–15 June
Lim HY, Thiel E, Glantz MJ (2008) To protect and defend: central nervous system prophylaxis in patients with non-Hodkin’s lymphoma. Curr Opin Oncol 20:495–501
Acknowledgment
This work was supported in part by grants from NIH (NIH 3P30CA023100-25S8) to S. Kesari.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grewal, J., Saria, M.G. & Kesari, S. Novel approaches to treating leptomeningeal metastases. J Neurooncol 106, 225–234 (2012). https://doi.org/10.1007/s11060-011-0686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0686-2