Abstract
This retrospective audit was conducted to examine the changes in patient characteristics, referral, treatment and outcome over a 20-year period in a large regional neuro-oncology centre, focusing on the impact of the changes in pathological classification of gliomas. Using the Edinburgh Cancer Centre (ECC) database all cases of glioma were identified and patient, tumour and treatment characteristics noted. Survival was calculated from date of surgery or, if no operation was performed, the date of referral. Comparison was made between four periods 1988–1992 (c1), 1993–1997(c2), 1998–2002(c3) and 2003–2007 (c4). During the 20 years, 1175 patients with a glioma were referred to ECC. The median age increased from 53 years to 57 years (p < 0.001) but the proportion without pathology remained unchanged (10%). The distribution of pathological grades changed over time Grade I–II: 24, 6, 6, and 6%, Grade III: 42, 27, 17, and 13% and Grade IV: 24, 61, 68, and 68% in c1, c2, c3 and c4, respectively (p < 0.001). Immediate RT was given to 68% (c1), 70% (c2), 78% (c3) and 79% (c4). Median interval from resection to RT reduced from 43 days (c1) to 36 days (c4) (p < 0.001). 5-year overall survival for patients with Grade III lesions increased: 21% (c1), 35% (c2), 37% (c3), 33% (c4) as did 1-year overall survival for Grade IV lesions: 18% (c1), 26% (c2), 29% (c3), 27% (c4)). This improvement probably reflects the change in pathological classification rather than a change in management. Proportional hazards analysis of grade IV 1993–2007 only (to reduce pathological variation) showed that younger age, frontal lesions, excision, higher RT dose had reduced hazard of death. Interval from surgery to RT had no impact on survival in this series.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Though brain tumours constitute a small proportion of all cancers they have devastating effects on the patient and their family and very few are curable. In Edinburgh, the Cancer Centre and the Department of Neurosciences are co-located in the Western General Hospital and serve as the tertiary referral centre for a population of more than 1.25 million in South East Scotland. All newly diagnosed patients with primary brain tumours from this region are referred to this hospital for specialist opinion, surgery, radiotherapy and chemotherapy. The access to healthcare is universal and funded by the National Health Service so this represents all patients who have received a neuro-oncology opinion from the catchment area… Prognostic information is often quoted with reference to patients recruited into clinical trails [2, 28, 32]. These data reflect the restrictive entry criteria for the trial (e.g. good functional status, age exclusions, requirement for gross total surgical excision etc.) and may partly explain the better outcomes in more recent phase III randomized controlled trials [1] Therefore it is also important to examine the outcome in an unselected group of patients.
This retrospective audit was therefore conducted to
-
(1)
Examine changes in patients and their tumour characteristics referred to the neuro-oncology team over a 20-year period
-
(2)
Examine changes in patient management over this period
-
(3)
Investigate overall survival and whether or not this changed over time
-
(4)
Investigate the impact of the introduction of the WHO grading system for brain tumours.
Materials and methods
Using the Edinburgh Cancer Centre computerised database all patients with a primary intra-cerebral lesion referred for an oncology opinion between 1st January 1988 and 31st December 2007 were identified. This included four patients who had had a pathological diagnosis in December 1987 (though patients may have had a radiological diagnosis before this date). Patient and tumour characteristics were recorded, along with details of surgery, radiotherapy and chemotherapy. Radiotherapy and chemotherapy delivered within 4 months of each other was defined as combined modality treatment. Deferred treatment was defined as a gap between the dates of surgery and radiotherapy exceeding 4 months.
The cohort was sub-divided into four periods (1988–1992 (c1), 1993–1997 (c2), 1998–2002 (c3) and 2003–2007 (c4)) in order to detect changes in referral patterns, patient characteristics, pathological diagnosis, treatment and outcome over time. All patients are or were actively followed up in outpatient clinics, by telephone follow-up or by letters to general practitioner. Date and cause of death were usually already recorded on the database but if not were obtained by viewing the death certificate. Overall survival was calculated from date of surgery (resection if this occurred after initial biopsy) or, if no biopsy was performed, date of referral to oncology. Three patients were lost to follow up at 2, 5 and 15 months; otherwise the minimum follow up is two years.
Statistical analysis
Subgroup comparisons were made using the t test, chi-square test or analysis of variance (ANOVA), as appropriate. All survival rates are actuarial estimates calculated by the Kaplan–Meier method and subgroups were compared using the log-rank test. A Cox proportional hazards regression model was used to assess the independent prognostic significance of the variables.
Results
During the 20 years, 1175 patients with a primary intra-cerebral glioma were referred to the Edinburgh Cancer Centre (ECC) for a neuro-oncology opinion. The patient and tumour characteristics of the referred cases in the four time cohorts are shown in Table 1. Fifty-three percent more patients were referred to Oncology in the most recent cohort when compared with the first. This compares with a 28% increase in the number of registrations for primary malignant brain tumours recorded by Scottish Cancer Registry for South East Scotland (ICD-10 C71 malignant primary brain tumour) with 442 registrations 1988–1992, 493 between 1993–1997, 578 between 1998–2002, and 565 between 2003–2007, suggesting a higher proportion of patients are now referred for a neuro-oncology opinion.
There was no difference in the sex distribution in the four cohorts, but the age increased significantly over time (ANOVA p < 0.0001) (Fig. 1). There were also significant changes in the type of pathological diagnoses, most notably the higher proportion of patients diagnosed with a WHO grade IV glioma in the later three cohorts (χ² p < 0.001 for pathology and grade). The proportion of patients without pathological confirmation did not change over time (χ2 p = 0.3) despite the increased number of referrals.
Details of treatment are shown in Table 2. Resection was performed in 48% of the patients with WHO grade I or II lesions, 62% of those with WHO grade III tumours and 57% of patients with a WHO grade IV tumour. There was no change over time in the proportion of patients undergoing resection, for any of the grades of tumour.
For patients with confirmed low-grade tumours treatment was biopsy or resection followed by immediate radiotherapy and/or chemotherapy in 68%, deferred radiotherapy for 22%, and no further treatment for 11% of patients. For the patients with WHO grade III tumours treatment was surgery followed by immediate radiotherapy and/or chemotherapy for 85%, delayed treatment for 4% and no further treatment for 10%. For the 683 patients with WHO grade IV tumours biopsy or resection was followed by immediate radiotherapy and/or chemotherapy in 82% with 17% receiving no further treatment, and 1% delayed treatment (initially declined treatment).
There was no tissue diagnosis for 114 patients seen by the oncologists. In eight cases (10%) a biopsy had been performed, but was non-diagnostic. Thirty-nine patients without pathological confirmation received radiotherapy and one patient received chemotherapy.
There were no statistically significant changes between the cohorts in the proportion of patients receiving immediate, delayed or no treatment for any of the pathological groups. The fact that the proportion of patients not treated did not decrease despite an aging population and an increase the number of in referrals suggests under-referral in the first cohort.
The details of the radiotherapy are shown in Table 3. All patients were treated on a linear accelerator but the energy used changed from 4 MV photons to 6 MV in 1993. Excluding the patients managed with deferred treatment, the median interval between surgery and radiotherapy was 36 days. This improved from 38.5 days in the first cohort to 35.7 in the last but was only significant in patients undergoing a resection, where there was an improvement from 43 to 36 days (ANOVA p < 0.001).
Twenty-five of the immediate radiotherapy patients (3%) discontinued their treatment and received a dose of less than 30 Gy. Patients with WHO grade I or II glioma usually received 54–55 Gy (69%), but 14% received 60 Gy (usually because of enhancement on imaging). In 1991, a regime of 30 Gy in six fractions delivered on alternate days over 2 weeks was introduced for the treatment of poor prognosis patients with high-grade glioma [42, 45]. In this study 230 patients received such treatment, 219 with WHO grade III or IV lesion and 11 patients without pathology (see below for age and clinical differences). Prior to the introduction of this regimen a schedule of 45 Gy in 20 fractions was used for poor prognosis disease (26/31 of patients receiving this dose were in the first cohort).
Good prognosis patients with high-grade glioma patients received 60 Gy in 30 fractions; 70% of WHO grade III and 54% of WHO grade IV patients received this dose. The proportion of patients with WHO grade III tumours receiving this dose increased over time (53% c1 vs. 78% c2 vs. 78% c3 vs. 79% c4 χ2 p = 0.01) but not WHO grade IV tumours (43% vs. 58% vs. 52% vs. 55% χ2 p = 0.42). No patient received a radio-surgical boost. When the characteristics of the patients with WHO grade IV lesions who received 60 Gy (n = 299) were compared with those receiving 30 Gy (n = 204); they were younger with a median of 52 years (range 18–72) compared with 65 years (range 41–77) (p < 0.001) and more likely to have undergone a resection (73% compared with 53%) (p < 0.001).
Chemotherapy was the primary treatment for 33 patients, although 11 of these also received radiotherapy within 90 days. A further 18 patients received sequential radiotherapy and chemotherapy. Concurrent chemo-radiation using temozolomide was introduced in August 2005 with 16 patients treated with the schedule in the final cohort [40]. Five patients received delayed chemotherapy. At progression 270 patients received chemotherapy. This included 35% of patients with a WHO grade III and 21% with a WHO grade IV lesion.
Survival
The median survival for all patients with a WHO grade I or II lesion was 72 months (95% CI 52.0–135.3) and the 3 year survival rate was 74.3%. The 3 year survival was 75.9% in c1, 72.71% in c2, 73.7% in c3 and 71.3% in c4. The estimated actuarial survival rates were 56.6% at 5 years and 43% at 10 years. In the first cohort the 5 year survival rate was 57.4% compared to 43.6% in c2, 57.9% in c3 and 71.3% in c4 (log rank p = 0.845) (Fig. 2a).
The estimated median survival for all patients with WHO grade III tumours was 27.1 months (95% CI 20.7–36.4) and 1 year survival rate 70.1%. The median survival improved with each successive cohort (c1 17.1 (12.8–23.4) c2 32.8 (17.5–46.2), c3 47.1 (25.3–60.0) and c4 25.3 months (15.5–60.0), as did the 1 year survival (c1 62.5%, c2 71.6%, c3 78.0%, c4 73.3%) and 5 year survival (c1 20.8%, c2 35.3%, c3 37.30% and c4 32.8%) (log rank p = 0.047) (Fig. 2b). Though the proportion of patients receiving high dose radiotherapy increased, it is more likely that majority of this improvement in survival is a consequence of the re-classification of some WHO grade III lesions as WHO grade IV lesions in the revised WHO classification.
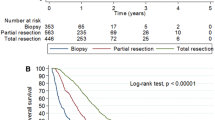
The median survival for all patients with WHO grade IV tumours was 7.2(95% CI 6.7–7.8) months with a 1 year survival rate 26.6% and 2 year rate of 6.8%. The 1 year survival was 17.9% in c1, 26.3% in c2, 28.6% in c3 and 26.9% in c4 with 2 year survival 1.8, 7.9, 6.4 and 7.6%, respectively, (log rank p = 0.121) and median survival of 5.4, 6.7, 7.5 and 7.6 months, respectively (Fig. 2c). Three patients with WHO grade IV tumours remain alive after 5 years, two from c3 and one from c4.
Patients receiving immediate post-operative radiotherapy
The median survival rate for patients with WHO grade III tumours who received immediate radiotherapy was 31.0 months (95% CI 23.0–43.6) with 75.9% alive at 1 year and 30.7% at 5 years and for the patients with WHO grade IV the median survival was 8.8 months (95% CI 7.9–9.5) and the 1 year survival rate was 31.8%.
For the 204 patients with a WHO grade IV tumour treated with 30 Gy the median survival was 6.6 months (95% CI 6.1–7.1) and the 1 year survival rate 8.3%. For the 299 patients with a glioblastoma receiving 60 Gy the median survival was 12.4 months (95% CI 11.3–14.0) with a 1 year survival rate of 51.5% and 13.9 at 2 years. The 1 year survival rate was 58.1% for patients who had had an excision and 33.8% for those who had had biopsy only. The difference in the outcome following the two treatment regimes primarily reflects the selection of the 30 Gy regime for poor prognosis patients (Fig. 3).
Cox’s proportionate hazards model
A significant shift in the pathological classification occurred during the period of this audit. The major shift in classification appeared to have occurred between 1992 and 1993 (all patients 1988–1992 24% WHO Grades I–II, 42% Grade III, 25% Grade IV, 1993 6% Grades I–II, 28% Grade III, 64% Grade IV). Therefore the Cox’s proportional hazards analysis was performed only on the three later cohorts where the pathological grading was more consistent.
To examine the factors associated with an increased hazard of death the following variables were entered into the model: year, gender, age (continuous variable), tumour grade, tumour location (frontal lobe or not), resection or not, delay to radiotherapy (continuous variable), radiotherapy dose, and radiotherapy field arrangement. When grade III and IV were combined the significant variables were identical, with the HR death for Grade IV 3.55 (95% CI 2.77–4.55). No variables of prognostic significance were found for patients with low grade tumours. The results for patients with high grade glioma are shown in Table 4. The expected factors of grade IV pathology and older age were associated with an increased hazard of death as were biopsy only and non-frontal lobe location. For patients undergoing resection those with a non-frontal lobe tumour had an improved outcome as did those with a frontal lobe tumour having a resection suggesting there is indeed a survival advantage of more extensive surgery regardless of tumour location. The interval from resection or biopsy to starting radiotherapy had no effect on overall survival whether this interval was calculated as a continuous or categorical interval with weekly intervals
Discussion
This series reports the characteristics, treatment and survival or more than 1100 patients referred to a regional neuro-oncology centre over a 20 year period. For comparison, Table 5 sets out recently (2000–2009) published papers quoting population-based data and Table 6 those reporting patients referred for an expert opinion. The search strategy used to identify the studies was: PUBMED, limited to papers related to adults published in English 2000–2009 using search terms glioma and population based (potential 87), outcome (potential 1251), patterns of care (potential 12) and glioblastoma with the same second terms (potential 29, 478 and 6). The titles were searched and 22 articles were felt to likely to be relevant and the papers obtained. From review of these papers and their citations a total of 19 relevant papers were identified.
Ideally to reflect the outcome of all patients, population-based studies should include patients with both pathologically confirmed and clinical diagnosis [18], but many of the population-based studies [4–7, 10, 12, 15, 21, 22, 31] include only cases where there is pathological confirmation, and hence would have been referred to a neurosurgical centre. This case selection is likely to omit very frail patients unsuitable for invasive procedures. The studies from Australia [11, 14, 36] and Switzerland [23] include patients with and without pathological confirmation so are true ‘population-based’ studies.
This is a referral based study using the Edinburgh Cancer Centre computerised database including patients with and without pathological confirmation. It includes all patients from a region (no funding or referral biases) but only those referred to Oncology and omits patients who had radiological only diagnosis or whose clinical condition declined after surgery. It was not possible to find out further details of those who underwent surgery but not referred for an oncology opinion as many of the case-files had been destroyed. We have also not performed a data-linkage with the cancer registry to identify those within surgery or an oncology opinion as the initial raison d’etre of this study was to produce a set of patients with pathology specimens with known treatment and outcome data for studies examining prognostic markers.
Pathology
In order to improve the consistency of reporting and to ensure the best match with outcome the pathological classification of brain tumours has evolved over the last 30 years. In 1979 the World Health Organisation produced the first classification of brain tumours [47] which was revised in 1993 to take account of advances in immuno-histochemistry [25] and again in 2000 [24]. The Edinburgh series confirms observations of other authors [13, 35, 46] that the later classifications appear to be more discriminatory and predict outcome more accurately. Also, that the main shift was to ‘up-grade’ tumours, particularly from grade III to grade IV. Recognising the impact of the newer classification on survival is important for the readers of papers, particularly those published prior to 1993, as the clinical entities described may be different from current practice.
Patient characteristics
The principal change observed over the 20 year period was the increasing age of the patients. Similar trends have been observed elsewhere [4] and are likely to reflect improved healthcare for other competing conditions, such as, ischaemic heart disease (i.e. patients survive these conditions to then get a glioma) and increased access to cross-sectional imaging resulting in scanning of a wider group of patients, for example elderly patients with ‘stroke-like’ symptoms. Also the improvements in the surgical and non-surgical treatments of brain tumours are likely to encourage referral of patients with a suspected brain tumour for further investigation. It is likely that this trend for an increased age of patents with brain tumours, particularly glioblastoma, is likely continue and it is predicted that Scotland will have a 15% increase in the number of cases from 2005–2015 [17], primarily due to an aging population.
Treatment
Surgery
Of the patients referred for an oncology opinion 52% underwent at least partial resection of their tumour. This is lower than many of the American [3, 10, 12, 21, 26, 31] series (range 61–78%) even when only quoting data from glioblastoma patients or the elderly. The rates of resection are also lower than the series from European specialist centres [20, 27, 38] (69–94% of pathologically confirmed glioblastoma vs. 58% same group). However, the resection rates are similar to the population-based series from Australia (55% resection) [36] and Switzerland (52%) [23]. The optimal resection rate for any particular population requires prospective studies of the underlying clinical decision making and it maybe that a resection rate of 52% is appropriate for our population. However, it is now clear from many studies [37, 39], particularly in the era of concurrent chemo-radiation [28], that optimal resection is associated with an improved outcome. For this reason the UK national guidance recommends that patients with brain tumours are operated on by specialist neuro-surgeons (neuro-oncology constitutes at least 50% workload) who are members of the multi-disciplinary team [30].
Radiotherapy
Of the patients referred for an oncology opinion (in UK neuro-oncologists are dual trained in radiotherapy and chemotherapy) 75% (81% glioblastoma) received radiotherapy. This is not dissimilar to the North American series though a little lower than the Italian series of referred cases (94%). For the patients with low grade tumours 64% received immediate radiotherapy which is higher than the US (62%) [12] and Norwegian series (45%) [22]. However, with the publication of the EORTC trial [44] the proportion of patients who are now receiving delayed radiotherapy has increased (11% c1 vs. 45% c4).
Of the patients with high grade glioma 72% of patients with grade III and 52% with grade IV lesions received a ‘radical dose’ of radiotherapy. This is lower than the other European institutional series [20, 27, 38] but it should be noted that the ‘palliative’ hypo-fractionated schedule of 6 × 5 Gy is radio-biologically similar to 60 Gy [42] and has been widely adopted in the UK and elsewhere for patients with a shorter prognosis [34].
‘Delays’ in treatment are a highly emotive subject and much British healthcare policy over recent years has been focused on reducing delays in treatment. Two previous publications have suggested a significant detrimental effect of delays in commencing radiotherapy for patients with high-WHO grade glioma [9, 16]. However, in our study we could not identify such an effect and the majority of our patients were treated in a relatively short time; 73% within 6 weeks of surgery and 91% within 8 weeks. A similar result has been identified in a recent analysis of patients recruited to RTOG trials [8]. The interval up to the 6 weeks allowed for entry into the trials had no impact on outcome, and in fact an inverse relationship was noted where those who had a delay of 4–6 weeks had the lowest hazard of death. Closer inspection of our data also revealed a similar ‘n-shaped’ curved (though non-significant) with those with the shortest and longest intervals having inferior survival. It is likely that those patients ‘rushed for treatment’ were young patients in a poor clinical condition.
Chemotherapy
Only 30% of our patients (51% glioblastoma) received chemotherapy at some point during their illness. This is lower than the other institutional series [20, 26, 27] but is similar to that observed in the Australian series [11]. In August 2005 we started to deliver concurrent and adjuvant temozolomide for selected patients (young, good performance status and resection) but did not extend the use to the trial indications until full funding approval from the Scottish Medicines Consortium was obtained in December 2006. However, in such a large ‘all-comers’ series the small improvement (2.5 months) in median survival of this therapeutic intervention will be diluted out, though we hope to see more patients alive 5 years in the final cohort as their follow-up continues [40]. Prior to this the very marginal survival benefit of adjuvant nitrosoureas [29] was not felt to be clinically significant and so was not routinely used in our unit, as it was in many US and European centres during this period.
Survival
Our reported survival for the patients with low grade glioma is similar to that reported in the US [12] and Norwegian [22] series however, unlike the latter series we did not identify an improvement in survival over time; they demonstrated increased median survival from 4.1 to 9.2 years over a 12 year period, possibly reflecting earlier imaging diagnosis (lead time bias). The median survival of 7.2 months and one-year overall survival of 27% for the patients with glioblastoma is similar to some reported series [10, 23, 31, 41, 43, 46], but shorter than that observed in Australia [36] and the European [20, 27, 38] and US neurology institutes [26], probably reflecting referral bias towards fitter patients and also an increased tendency for second tumour resections in these latter series.
The outcome of the patients with WHO grade III tumours improved over the period of this study, however rather than being an effect of treatment it is most likely due to effects of the later WHO classification moving poorer prognosis grade III patients into the grade IV category (the so called Will Roger’s effect [19]).
Conclusions
We report a large series of patients diagnosed with intra-cerebral glioma over a 20 year period. The principal findings are that the revised pathological classifications of glioma appears to discriminate more effectively, particularly with respect to grade III tumours Also, that the population of patients with glioma is aging and a higher proportion of patients are being referred for a specialist opinion. The proportion of patients receiving radiotherapy was similar to other series but fewer patients underwent resection of their tumour and received chemotherapy. The outcomes observed were similar to several series of patients from the same era, but slightly inferior to reported figures from some specialist centres, probably reflecting the lack of referral bias in our series. In this series we could not identify an impact of delay from surgery to commencing radiotherapy.
References
Anderson E, Grant R, Lewis SC et al (2008) Randomized phase III controlled trials of therapy in malignant glioma: where are we after 40 years? Br J Neurosurg 22(3):339–349
Anonymous (1990) Prognostic factors for high-grade malignant glioma: development of a prognostic index. A Report of the Medical Research Council Brain Tumour Working Party. J Neurooncol 9(1):47–55
Barnholtz-Sloan JS, Sloan AE, Schwartz AG (2003) Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer 98(3):603–609
Barnholtz-Sloan JS, Sloan AE, Schwartz AG (2003) Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973–1997. J Neurosurg 99(3):458–466
Barnholtz-Sloan JS, Sloan AE, Davis FG et al (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 22(14):2865–2872
Barnholtz-Sloan JS, Maldonado JL, Williams VL et al (2007) Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol 85(2):171–180
Barnholtz-Sloan JS, Williams VL, Maldonado JL et al (2008) Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg 108(4):642–648
Blumenthal DT, Won M, Mehta MP et al (2009) Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol 27(5):733–739
Burnet N, Jena R, Jefferies S et al (2006) Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin Oncol 18(2):93–103
Chang SM, Barker FG 2nd (2005) Marital status, treatment, and survival in patients with glioblastoma multiforme: a population based study. Cancer 104(9):1975–1984
Cher L, Rosenthal MA, Drummond KJ et al (2008) The use of chemotherapy in patients with gliomas: patterns of care in Victoria from 1998–2000. J Clin Neurosci 15(4):398–401
Claus EB, Black PM (2006) Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer 106(6):1358–1363
Coons SW, Johnson PC, Scheithauer BW et al (1997) Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 79(7):1381–1393
Dally M, Rosenthal M, Drummond K et al (2009) Radiotherapy management of patients diagnosed with glioma in Victoria (1998–2000): a retrospective cohort study. J Med Imaging Radiat Oncol 53(3):318–324
Deorah S, Lynch CF, Sibenaller ZA et al (2006) Trends in brain cancer incidence, survival in the United States: surveillance, epidemiology, end results program, 1973 to 2001. Neurosurg Focus 20(4):E1
Do V, Gebski V, Barton M (2000) The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol 57(2):131–136
Erridge S, Featherstone C, Chalmers R et al (2007) What will be the radiotherapy machine capacity required for optimal delivery of radiotherapy in Scotland in 2015? Eur J Cancer 43(12):1802–1809
Erridge S, Moller H, Price A et al (2007) International comparisons of survival from lung cancer: pitfalls and warnings. Nat Clin Pract Oncol 4(10):570–577
Feinstein A, Sosin D, Wells C (1985) The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med 312(25):1604–1608
Filippini G, Falcone C, Boiardi A et al (2008) Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol 10(1):79–87
Iwamoto FM, Reiner AS, Panageas KS et al (2008) Patterns of care in elderly glioblastoma patients. Ann Neurol 64(6):628–634
Johannesen TB, Langmark F, Lote K (2003) Progress in long-term survival in adult patients with supratentorial low-grade gliomas: a population-based study of 993 patients in whom tumors were diagnosed between 1970 and 1993. J Neurosurg 99(5):854–862
Kita D, Ciernik IF, Vaccarella S et al (2009) Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology 33(1):17–22
Kleihues P, Cavenee W (2000) World Health Organization Classification of Tumours. Pathology and genetics of tumours of the nervous system. IARC Press, Lyon
Kleihues P, Burger P, Scheithauer B (1993) Histological typing of tumours of the central nervous system. World Health Organization international histological classification of tumours. World Health Organization, Heidelberg
Laws ER, Parney IF, Huang W et al (2003) Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 99(3):467–473
Mineo JF, Bordron A, Baroncini M et al (2007) Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir 149(3):245–252 discussion 252–253
Mirimanoff R, Gorlia T, Mason W et al (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24(16):2563–2569
MRC (2001) Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol 19(2):509–518
NICE (2006) Improving Outcomes for People with Brain and Other CNS Tumours. National Institute for Health and Clinical Excellence, UK
Paszat L, Laperriere N, Groome P et al (2001) A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys 51(1):100–107
Pignatti F, van den Bent M, Curran D et al (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20(8):2076–2084
Rachet B, Mitry E, Quinn M et al (2008) Survival from brain tumours in England and Wales up to 2001. Br J Cancer 99(Suppl 1):S98–S101
RCR (2006) Radiotherapy dose-fractionation. Royal College of Radiologists, London
Revesz T, Scaravilli F, Coutinho L et al (1993) Reliability of histological diagnosis including grading in gliomas biopsied by image-guided stereotactic technique. Brain 116(Pt 4):781–793
Rosenthal MA, Drummond KJ, Dally M et al (2006) Management of glioma in Victoria (1998–2000): retrospective cohort study. Med J Aust 184(6):270–273
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62(4):753–764 discussion 264-6
Stark AM, Nabavi A, Mehdorn HM et al (2005) Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol 63(2):162–169 discussion 169
Stummer W, Reulen H, Meinel T et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–576
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Tait MJ, Petrik V, Loosemore A et al (2007) Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg 21(5):496–500
Thomas R, James N, Guerrero D et al (1994) Hypofractionated radiotherapy as palliative treatment in poor prognosis patients with high grade glioma. Radiother Oncol 33(2):113–116
Tseng JH, Merchant E, Tseng MY (2006) Effects of socioeconomic and geographic variations on survival for adult glioma in England and Wales. Surg Neurol 66(3):258–263 discussion 263
van den Bent MJ, Afra D, de Witte O et al (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366(9490):985–990
Whittle IR, Basu N, Grant R et al (2002) Management of patients aged >60 years with malignant glioma: good clinical status and radiotherapy determine outcome. Br J Neurosurg 16(4):343–347
Wrensch M, Rice T, Miike R et al (2006) Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro Oncol 8(1):12–26
Zülch K (1978) Histological typing of tumours of the central nervous system. World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erridge, S.C., Hart, M.G., Kerr, G.R. et al. Trends in classification, referral and treatment and the effect on outcome of patients with glioma: a 20 year cohort. J Neurooncol 104, 789–800 (2011). https://doi.org/10.1007/s11060-011-0546-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0546-0