Abstract
Hydroxyurea (HU), an orally administered chemotherapy, has become the de facto standard therapeutic agent in patients with surgically and radiation refractory meningiomas based on a limited literature. A retrospective case series of 60 patients with recurrent WHO grade 1 meningioma treated with HU following progression after surgery and radiotherapy was conducted with primary study objective progression free survival (PFS) at 6- and 12-months. Sixty patients (45 women; 15 men: median age 61.5 years, range 26–88) with recurrent meningioma were treated with HU (1000 mg/m2/day orally divided twice per day; one cycle operationally defined as 4-weeks of daily HU). All patients had progressed radiographically after prior therapy with surgery (60/60) and radiotherapy (external beam radiotherapy 60/60; stereotactic radiotherapy 53/60). No patient received prior chemotherapy or targeted therapy before instituting HU. Patients received 1-12 cycles (median 2.0) of HU with modest toxicity (10% grade 3 + anemia or fatigue). There were no radiographic responses, 35% of patients had stable disease and 65% manifested progressive disease. Duration of stable disease ranged from 3 to 12 months (median 4.0 months). The overall PFS was 10% (median PFS 2.0 months). The majority of patients (80%) following progression on HU were subsequently treated on an investigational trial. In this retrospective case series, HU though generally well tolerated and convenient, appeared to have very limited activity which raises questions of what constitutes effective salvage therapy and indicates an unmet need for alternative treatments for recurrent meningiomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrent meningiomas are managed by re-resection when clinically indicated and surgically accessible, otherwise radiotherapy is most often employed [1–8]. At present there does not appear to be survival difference in treatment with stereotactic or conventional fractionated external beam radiotherapy and consequently either modality is used for recurrent disease [9, 10]. Not infrequently and as illustrated in the present retrospective series, sequential radiotherapy is employed for multiply recurrent meningioma. Less clear from the literature is how best to manage patients with surgery and radiotherapy refractory recurrent meningioma [11–24]. The 2010 Central Nervous System National Comprehensive Cancer Network (CNS NCCN) guidelines suggest three possible treatments (hydroxyurea, alpha-interferon or somatostatin analogues such as Sandostatin LAR) recognizing that there is very meager literature (medical level evidence category 3) regarding the medical oncology management of recurrent meningioma [12–19, 21, 22].
This retrospective case series of 60 patients represents experience in treating surgery and radiation refractory World Health Organization (WHO) grade 1 recurrent meningiomas with hydroxyurea (HU).
Patients and methods
A retrospective study of patients treated with WHO grade 1 meningioma with hydroxyurea was performed and evaluated patients treated between 1/2000 and 12/2009. Approval for the retrospective analysis was obtained from the university human investigation review committee.
Objectives and end points
The two primary objectives of this retrospective study included determination of efficacy and toxicity of HU in the treatment of adults with surgery and radiation refractory recurrent WHO grade 1 meningiomas. The primary end point of the retrospective analysis was progression free survival (6- and 12-month). Secondary end points included, time to progression and response.
Patient selection
Patients had histologically proven WHO grade 1 meningioma that was recurrent neuroradiographically. All patients had progressed following definitive radiotherapy (RT) and surgery and were not considered eligible for further RT or surgery. All patients were chemotherapy naive. At least 3-months had elapsed since prior radiotherapy. Patients had radiographically measurable disease wherein recurrent tumor was bi-dimensionally measurable (at least 1.0 × 1.0 cm2) by cranial contrast-enhanced magnetic resonance imaging (MR). Histological confirmation of tumor recurrence was not required. Patients had a Karnofsky performance status greater than or equal to 60 and a life expectancy greater than 3 months. Adequate hematologic, renal and hepatic functions were required. No serious concurrent medical illnesses or active infection could be present that would jeopardize the ability of the patient to receive HU therapy.
Drug schedule
Hydroxyurea (HU; Hydrea; Bristol-Myers-Squibb, NY) was administered orally for 28-consecutive days (1000 mg/m2/day divided twice per day or rounded to the nearest dose level based on availability of HU capsule size) every 4-weeks (operationally defined as a cycle of therapy). HU was obtained commercially and billed to third party payers. No pharmaceutical sponsorship was provided in the conduct of this retrospective study. No premedication was required with oral HU. Concurrent medications included non-enzyme inducing anticonvulsants (20 patients), narcotics (15 patients), dexamethasone (15 patients), anti-constipation medication (14 patients) and anticoagulants (2 patients).
Treatment with HU was repeated every 28 days (4-weeks) provided that all toxicity from the previous cycle had resolved. If recovery had not occurred by day 28, the subsequent cycle of HU was delayed until recovery. All toxicities including hematologic due to HU therapy were rated retrospectively according to the NIH Common Toxicity Criteria (version 3.0).
Method of evaluation
All patients underwent cranial MR demonstrating progressive disease within 2 weeks of HU administration. Blood counts were obtained on day 1 of each HU cycle (or more often if clinically indicated), neurologic examination was performed every 4 weeks, and contrast-enhanced brain MR was performed after every two cycles of HU (i.e., every 8 weeks). Modified neuroradiographic response criteria as defined by Macdonald et al. were used [25]. All neuroradiography was reviewed by a neuroradiologist blinded to treatment and by the treating neuro-oncologist (MCC). Tumor measurements were determined by the neuro-oncologist (MCC). In patients with radiographic stable disease, partial response or complete response, 2 additional cycles of HU was administered and repeat MR was performed. Patients were continued on HU therapy until documentation of progressive disease at which time patients discontinued HU and were either monitored or offered alternative therapy. Alternative meningioma-directed therapy (or no therapy) was offered to patients that radiographically progressed.
Progression free survival (PFS) was defined as the time from the first day of treatment with HU until progression or death (PFS). Patients discontinued HU if there was progressive disease, development of unacceptable toxicity, patient refusal or noncompliance with treatment.
Results
Study population
Sixty patients (45 women; 15 men) ages 26–88 years (median 61.5), with recurrent WHO grade 1 meningioma (original and when applicable recurrent pathology reviewed and confirmed in all cases) were treated with HU (Table 1).
Patients presented at the time of tumor recurrence with the following signs and symptoms: worsening hemiparesis (n = 22), increased seizures (n = 20), headache (n = 16), gait disturbance (n = 6), and ophthalmoplegia (n = 5). Patient performance status using the Karnofsky scale ranged from 60 to 100 (median 70) at the time of documented tumor recurrence and initiation of HU therapy. Tumor locations were as follows: frontal (n = 32), parietal (n = 14), cavernous sinus (n = 10), sphenoid wing (n = 9), temporal (n = 8), tentorial (n = 4), cerebellopontine (n = 2) and multifocal (n = 7) (Table 1).
All patients had been treated previously with surgery in which a complete resection was accomplished in 20 at first resection, partial in 31 and biopsy only in 9 (Table 1). Twenty-nine patients (48%) underwent a second operation [in 4 (6.6%) a third resection] in which repeat tumor histology was consistent with WHO grade 1 meningioma.
All patients had previously been treated with limited-field radiotherapy (adjuvant in 34; at time of first recurrence in 26) (Table 1). In all, conventional fractionated radiotherapy was used in which 1.8–2.0 Gy was administered daily, with a median tumor dose of 54 Gy (range 50.4–55 Gy). Fifty-three patients were in addition treated with stereotactic radiotherapy (adjuvant in 5; at relapse in 48). Stereotactic radiotherapy dose ranged from 12 to 18 Gy (median 14). In all but one patient with multifocal disease (total of seven patients), all lesions were treated with both conventional fractionated radiotherapy and stereotactic radiotherapy.
HU was administered daily and initiated following documentation of tumor progression as demonstrated by neuroradiographic progression (in all patients) or clinical disease progression (in 65% of patients). Median time to initiation of HU following initial surgery was 35 months with a range of 15–132 months. Median time to initiation of HU following radiotherapy (including cyberknife) was 40 months with a range of 15–128 months. A total of 192 cycles of HU were administered. A minimum of one cycle of HU was administered to each patient with a median of 2 cycles (range 1–12). HU was administered at the proscribed dose in all patients (median dose 1500 mg/day; range 1000–2000 mg/day).
Toxicity
Toxicity was retrospectively recorded for all grades for all patients by type using the NCI common toxicity criteria (version 3.0). Table 2 lists all grade 2–5 toxicity observed with each figure representing the sum of the highest grade of toxicity attained, per toxicity, per cycle for all patients. A total of 192 treatment cycles were administered of which there were 6 (10% patients) grade 3 adverse events (AE) and no grade 4 or 5 AE. The most common grade 3 AE was anemia and fatigue (<1% of the total number of HU cycles each). No patient required transfusion nor were there any episodes of neutropenic fever. No treatment-related death occurred. Six patients required a dose reduction (to 1000 mg/day) otherwise all patients were treated at 1000 mg/m2/day.
Response
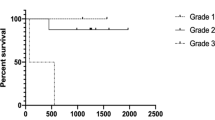
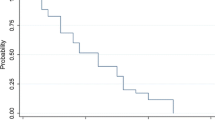
All patients were assessable for radiographic response and duration of response (Table 1; Fig. 1). Following 1–2 cycles of HU, 39 patients (65%) demonstrated progressive disease. Ten patients (17%) received six or greater cycles of HU. At the conclusion of TMZ, Karnofsky performance status ranged from 50 to 80 with a median of 70 in the entire study group.
No patient (0%) demonstrated a complete or partial response and 21 patients (35%) demonstrated stable disease. Overall median time to tumor progression was 4.0 months (range 3–12 months). Progression free survival at 6- and 12-months was 10% (PFS-6) and 0% (PFS-12).
Forty-eight (80%) patients received an investigational therapy (temozolomide, CPT-11, alpha-interferon or Sandostatin LAR) following progression on HU [21–24].
Discussion
The utility of chemotherapy in the treatment of recurrent meningioma remains ill-defined notwithstanding limited evidence for the use of HU, α-interferon and somatostatin analogues in this setting [12–19, 21, 22]. HU has constituted the primary medical therapy based on several studies and upon the initial report of both in vitro and in vivo activity by Schrell (Table 3) [12, 13]. Schrell demonstrated in vitro that HU, an oral chemotherapy with a variety of anti-tumoral effects, was a potent inhibitor of cultured meningioma cells by inducing apoptosis [12, 13]. Several subsequent clinical trials suggest in vivo efficacy with modest and acceptable toxicity (Table 3). Problematic with the various HU trials however is that many patients had not failed radiotherapy or that radiotherapy was administered concurrently. In the present retrospective study and what arguably is now usual and customary practice in the management of meningiomas, all patients treated with HU had prior radiotherapy as well as histological confirmation of a WHO grade 1 meningioma. In this subset of patients, all previously treated with surgery and radiotherapy and in whom no further surgery or radiotherapy was applicable, there were no radiographic responses, 35% of patients had stable disease and 65% manifested progressive disease following 2 cycles of HU. The duration of stable disease ranged from 3 to 12 months (median 5.0 months). The overall PFS was 10% at 6 months (median PFS 2.0 months). These results in this retrospective case series, demonstrate that HU though generally well tolerated and convenient appeared to have very limited activity in recurrent WHO grade 1 meningioma which raises questions of what constitutes effective salvage therapy and indicates an unmet need for alternative treatments for recurrent meningiomas.
At present, there is very limited data regarding outcome measures that best define response to an investigational agent for recurrent meningiomas. As has become customary in brain tumor trials, PFS-6 serves as the endpoint for the majority of trials for recurrent gliomas. A similar strategy has been adopted for recurrent meningiomas, however what constitutes a clinically relevant PFS-6 varies [11, 14–24]. In the imatinib trial, a PFS-6 of 45% was seen in patients with recurrent grade 1 meningiomas and was felt to indicate lack of efficacy [20]. By contrast, in the α-interferon and Sandostatin trials for recurrent meningioma, a PFS-6 of 40% was felt to reflect an active agent [21, 22]. In part these differences reflect prior treatment administered (surgery and radiotherapy) as well as differing interpretations of the meager literature regarding disease progression in treated recurrent meningiomas. In the only randomized placebo controlled trial of patients with recurrent grade 1 meningioma previously treated with radiotherapy (SWOG-9005) were treated with the investigational agent, mifepristone (RU-486), a progesterone antagonist, or placebo. There were 45 subjects for analysis (22 from the treatment arm and 23 on placebo) [11]. Of the 45 patients, 30 died at time of reporting, with a median survival of 31 months (95% confidence interval of 15–50 months). Forty-two of the 45 subjects have progressed with at median time to tumor progression of 6 months (95% confidence interval of 5–7 months). Time to tumor progression was similar in both patients groups (placebo and mifepristone) suggesting mifepristone was an inactive therapy. This study suggests a 50% PFS-6 as a baseline outcome measure from which to compare other medical therapies in similarly treated patients. What has changed in the contemporary management of recurrent meningiomas is the frequent utilization of both fractionated external beam radiotherapy as well as stereotactic radiotherapy [9, 10]. Whether the PFS-6 in the present study (treated with two radiation modalities and surgery) is comparable to that seen in the SWOG-9005 trial (treated with a single radiotherapy modality) is unknown. Regardless, the present may serve as the statistical benchmark for new clinical trials for recurrent meningiomas with targeted agents such as bevacizumab, sunitinib, vatalanib and the somatostatin analogue, pasireotide. A recent phase IIa study (reported only in abstract form) from the Southwest Oncology Group (SWOG S9811) administered HU for recurrent WHO grade 1 meningiomas [26]. All study patients (n = 29) were felt not to be candidates for surgery however the number of patients treated with either RT or surgery was not stated. HU at a dose of 20 mg/kg/day resulted in a response rate of 0% and median PFS of 27 months (PFS-3 years 43%). The authors conclude the natural history of meningioma at present is unknown and consequently were uncertain if HU represented an effective cytostatic chemotherapy for recurrent meningioma.
Challenges in treating recurrent meningiomas with targeted and chemotherapeutic agents are several including a lack of interest by the pharmaceutical industry (the most common funding source for cancer clinical trials), minimal interest by neuro-oncology cooperative groups that are predominantly glioma focused, a perception that patients eligible for study are uncommon notwithstanding that meningioma constitutes the most frequent primary brain tumor and a bias by the neuro-oncology community that treatment following failure of surgery and radiotherapy is futile. As a consequence, there are very few open trials for patients with surgery and radiotherapy refractory recurrent meningioma (all comparatively small, single arm Phase 2 studies) attesting to an unmet need in neuro-oncology.
In conclusion though HU is relatively non-toxic and convenient as an orally administered medication with no acute side effects, in patients with recurrent and refractory grade 1 meningiomas, HU appears to have very limited activity in this large retrospective case series.
References
Claus EB, Bondy ML, Schildkraut JM et al (2005) Epidemiology of intracranial meningioma. Neurosurgery 57:1088–1095
Rockhill J, Mrugala M, Chamberlain MC (2007) Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus 23:E1
Bondy M, Ligon BL (1996) Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 29:197–205
Goldsmith B, McDermott MW (2006) Meningioma. Neurosurg Clin N Am 17:111–120, vi
McMullen KP, Stieber VW (2004) Meningioma: current treatment options and future directions. Curr Treat Options Oncol 5:499–509
Ragel B, Jensen RL (2003) New approaches for the treatment of refractory meningiomas. Cancer Control 10:148–158
Wen PY, Drappatz J (2006) Novel therapies for meningiomas. Expert Rev Neurother 6:1447–1464
Whittle IR, Smith C, Navoo P, Collie D (2004) Meningiomas. Lancet 363:1535–1543
Rogers L, Mehta M (2007) Role of radiation therapy in treating intracranial meningiomas. Neurosurg Focus 23(4):E4 (Review)
Elia AE, Shih HA, Loeffler JS (2007) Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus 23(4):E5 (Review)
Grunberg SM, Rankin C, Townsend J et al (2001) Phase II double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unresectable meningioma. Proc Am Soc Clin Oncol 20:56a (#222)
Schrell UM, Ritting MG, Anders M et al (1997) Hydroxyurea for treatment of unresectable and recurrent meningiomas. I. Inhibition of primary human meningioma cells in culture and in meningioma transplants by induction of the apoptotic pathway. J Neurosurg 86(5):845–852
Schrell UM, Ritting MG, Anders M et al (1997) Hydroxyurea for treatment of unresectable and recurrent meningiomas II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg 86:840–844
Newton HB, Scott SR, Volpi C (2004) Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg 18:495–499
Newton HB, Slivka MA, Stevens C (2000) Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neuro-Oncol 49(2):165–170
Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE (2002) Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg 97:341–346
Rosenthal MA, Ashley DL, Cher L (2002) Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci 9(2):156–158
Hahn BM, Schrell UMH, Sauer R et al (2005) Prolonged oral hydroxyurea and concurrent 3f-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neuro-Oncol 74:157–165
Loven D, Hardoff R, Sever ZB et al (2004) Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol 67:221–226
Wen PY, Yung WK, Lamborn KR et al (2006) Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res 12:4899–4907
Chamberlain MC, Glantz MJ (2008) α-interferon for recurrent WHO grade I intracranial meningiomas. Cancer 113:2146–2151
Chamberlain MC, Glantz MJ, Fadul CE (2007) Recurrent meningioma: salvage therapy with sandostatin. Neurology 9:969–973
Chamberlain MC, Tsao-Wei D, Groshen S (2006) Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neuro-Oncol 78(3):271–276
Chamberlain MC, Tsao-Wei D, Groshen S (2004) Temozolomide for treatment resistant recurrent meningioma. Neurology 62(7):1210–1212
Macdonald DR, Cascino TL, Schold SC, Cairncross JG (1990) Response criteria for phase 2 studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Swinnen LJ, Renkin C, Rushing EJ et al (2009) In: Proceedings SNO, annual meeting of the society of neuro-oncology, 18–21 Nov 2010, Montreal, QC. Abstract OT-08:iv70
Conflicts of interest
MCC reports participation in advisory board and speaker’s bureau for Genentech, MundiPharma, Schering Plough, Sigma Tau. SK Johnston reports no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamberlain, M.C., Johnston, S.K. Hydroxyurea for recurrent surgery and radiation refractory meningioma: a retrospective case series. J Neurooncol 104, 765–771 (2011). https://doi.org/10.1007/s11060-011-0541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0541-5