Abstract
Whole brain radiation therapy (WBRT) is one of the most effective modalities for treatment of brain metastases. With increasing cancer control there is growing concern regarding the long-term effects of treatment. These effects are seen as white matter change (WMC) on brain MRI. Severity of WMC is implicated in cognitive and functional decline in many patient groups. Our objective was to identify clinical factors associated with greater accumulation of WMC following WBRT. Through retrospective review of serial MRIs obtained from 30 patients surviving greater than 1 year after WBRT, treated at a single institution between 2002 and 2007, we calculated volumetric WMC over time using segmentation software. Changes related to tumor, secondary effects, surgery or radiosurgery were excluded. Factors that influenced the rate of WMC accumulation were identified through multivariate analysis. Following WBRT, patients accumulated WMC at an average rate of 0.07% of total brain volume per month. In multivariate analyses, greater rates of accumulation were independently associated with older age (β = 0.004, p < .0001), poor levels of glycemic control (β = 0.048, p < .0001) and hypertension diagnosis (β = 0.084, p < .0001). Long-term survivors of cancer allow assessment of late effects of treatment modalities. Radiation injury appears to be related to a steady rate of white matter damage over time, as indicated by progressive accumulation of WMC. Our results suggest that rate of WMC accumulation is enhanced by parameters such as hyperglycemia and hypertension. This has significant clinical impact by clearly identifying hyperglycemia, steroid-induced hyperglycemia, and other vascular risk factors as targets for intervention to decrease WMC in patients receiving WBRT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
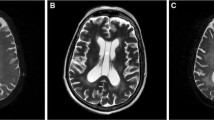
Whole brain radiation therapy (WBRT) is one of the most effective modalities for treatment of primary and metastatic brain lesions. As systemic therapy improves, a significant proportion of patients with brain metastases may be expected to be long-term survivors of their disease. With this improved disease control come growing concerns regarding the long-term toxicities of treatment, particularly long-term neurocognitive decline that may be associated with WBRT. White matter changes (WMCs), seen on MRI as hyperintense on the T2/FLAIR images, and mainly hypointense on the T1-weighted images, have been associated with neurocognitive decline in multiple settings [1, 2], including vascular dementia [3], and often develop in patients receiving radiation therapy to the brain. Quantifying the extent of WMCs has been challenging, due to differences in image acquisition over serial MRI’s and the qualitative nature of most previously established grading scales of WMC. Furthermore, few attempts have been made to establish clinical factors that predict the development of WMC’s following WBRT, or to correlate the severity of radiographically established WMC’s with the extent of clinically apparent neurocognitive changes.
In this study, our objective was to use volumetric analysis of serial MRI’s to identify clinically meaningful risk factors associated with more extensive/rapid WMC in patients living longer than 1 year after completing WBRT. Knowledge of these factors may alter the management approach, particularly among patients with known risk factors, allowing amelioration or reduction in the severity of late neurotoxicity among patients undergoing WBRT.
Methods
Patients
We conducted a retrospective review of the medical records of patients who underwent WBRT at the University of Maryland Marlene and Stewart Greenebaum Cancer Center, Department of Radiation Oncology between 2002 and 2007. All patients provided informed consent for radiation therapy, and the study was approved by the University of Maryland School of Medicine’s Institutional Review Board. Patients were included in the study if they (1) received either prophylactic or therapeutic WBRT (patients with primary brain tumors and/or CNS involvement of lymphoma and leukemia were excluded); (2) survived a year or more following the initiation of whole brain radiation; (3) had baseline and at least two follow-up scans available for review, the last of which was at least a year after completing WBRT; and (4) had data on pre-existing medical co-morbidities and follow-up after completing treatment. Of the 450 patients receiving WBRT during the data collection period, 30 patients met these inclusion criteria and formed the cohort of this study.
Data were collected on initial performance status, pre-existing medical co-morbidities, smoking history, WBRT treatment plan (total dose, fraction size, and treatment time), chemotherapy history, use of steroids and anti-epileptic medications, and use of other therapies such as stereotactic radiosurgery or surgical brain tumor resection. Serial neuro-cognitive testing was not routinely performed, unless clinically indicated. Glycemic control was calculated using all values in the electronic medical record over the treatment course after undergoing WBRT and a lack of control was defined as a mean glucose of more than 120 (the lower limit of abnormal for our laboratory. Information about the diagnosis and severity of clinical factors was obtained as part of a computerized chart review that included notes from the primary physicians and medical oncologists who were prescribing treatment. The granularity of the archival data source did not allow for prospective implementation of strict definitional criteria among the primary physicians. Due to the retrospective nature of our review we did not have enough information to be able to assess how well controlled the specific diagnoses were. Patients still alive at the time of data collection and analysis were censored at the time of their last follow-up.
Radiation therapy
All patients underwent WBRT delivered via opposed lateral fields. Inferior field borders were set at the C1-2 intervertebral space with a 1.5–2 cm flash beyond the cranium superiorly, anteriorly, and posteriorly. Custom blocks were designed to shield the cornea and oral cavity/oral pharynx in all patients. A variety of fractionation schemes were used; range 26.25 Gy (2.8 Gy, 9 fractions) to 45.0 Gy (1.8 Gy, 25 fractions), with modal regimen of 37.5 Gy (2.5 Gy, 15 fractions). Forty-six percent of patients received a stereotactic radiosurgery boost, and 26% had craniotomy with resection of a brain metastasis in addition to WBRT. Seventy percent of patients received steroids during and after WBRT, with a mean duration of steroid usage of 11 weeks (range 1–83 weeks).
Radiographic imaging
Standard diagnostic sequences were obtained on hospital scanners (1.5 T Siemens or 1.5 T/3T GE), including T1 with and without contrast, T2, and T2-weighted turbo spin echo FLAIR images with typical TR (repetition time) of 8,000 ms, TE (echo time) of 102 ms, and TI (inversion time) of 2,500 ms. A total of 166 brain MRIs (mean 5.5 per person, median 5, range 2–16) were identified in patients’ medical records and were available for review in a format that allowed for volumetric analysis.
Volumetric analyses
WMC were measured volumetrically using the medical image processing, analysis, and visualization (MIPAV) segmentation application, available through the NIH [4]. All analyses were performed on FLAIR sequence images. Tissues outside of cortical grey matter were removed from the images using a process of volume of interest (VOI) level-setting, painting, and masking. Next, the “Brainstrip” plug-in was used to set first and last slice bounds, exclude ventricular areas from the image, and adjust upper and lower image intensity thresholds to better visualize WMC. The resulting scan was used for subsequent volumetric analyses.
VOI level setting and freehand contouring were used to enclose the whole brain, ventricle, and tumor volumes. Next, a pixel–voxel intensity value was determined which adequately characterized areas of WMC while minimizing non-specific signal in non-WMC areas of the brain (commonly in the base of the temporal lobe).The voxel intensity threshold was ~390 with a range from 230 to 550. The statistics generator tool in MIPAV was used to quantify the total ventricular and whole brain volumes before quantifying WMC. WMC volume was assessed in the statistics generator by excluding areas of whole brain and tumor volumes with pixel–voxel intensities below the predetermined threshold value before again calculating volume. Subsequent calculations were used to obtain WMC volume, as a percent of whole brain volume, excluding WMC attributable to tumor, stereotactic radio-surgical, or surgical effects.
The images were analyzed by one of the authors (CR). The person doing the analysis was trained in the use of the software and was completely blinded to any information having to do with the identity or the timing of the scan.
Statistical analysis
All data analyses were performed using SAS 9.2 (SAS Institute, Cary, NC), and judged with respect to statistical significance at p < .05. Prior to the analyses we evaluated patient characteristics, risk factors determined by diagnosis codes in the medical records, and WMC volumes using descriptive statistics. Survival was estimated using Kaplan–Meier procedures. We then employed a multilevel model of change with time since WBRT as a predictor to determine the average trajectory of WMC accumulation. This model was then expanded to identify factors (i.e., age, WBRT dose, hypertension, hyperglycemia, smoking, and diabetes) influencing the rate of WMC accumulation following WBRT. Non-significant factors were trimmed. Final models indicate the moderating effects of hyperglycemia and hypertension on rates of WMC accumulation, controlling for age.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. Seventeen of the 30 patients (57%) were women, and the median age was 49.3 years (range 35–73 years). The most common tumor histology was non-small cell lung cancer (63%), followed by breast cancer (10%). Twenty-three percent of the patients received PCI. A large majority of patients (83%) were smokers. Noted co-morbid conditions included diabetes (13%), hyperglycemia (33%), and hypertension (36%) Of the nine hyperglycemia patients four had history of diabetes and five did not; seven of them (only 2 of whom had history of diabetes) received steroids and two did not. Seventy-six percent of patients had a Karnofsky score of 80 or above at the start of WBRT.
Survival
The 16 patients with known death dates lived between 13.2 and 44.1 months past WBRT. The Kaplan–Meier overall survival curve for the entire cohort of patients is shown in Fig. 1. Estimated median survival from WBRT was 24.1 months. Two- and three-year survival probabilities were 45 and 11%, respectively, underscoring the selective nature of this “long-term survivor” cohort.
Accumulation of WMC
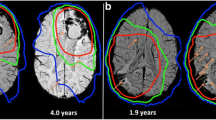
WMCs were identified in all MR scans (169 scans obtained from 30 patients), accounting for between 0.036 and 5.63% of total brain volume. In scans obtained prior to WBRT, WMC accounted for, on average, 0.47% (SD 0.40, range 0.04–1.54%) of total brain volume. Long-term changes in WMC were modeled with respect to time since WBRT using multilevel regression, with explicit accommodation of individual differences in initial levels of WMC and rates of change. Average and individual trajectories are shown in Fig. 2. Patients accumulated, on average, WMC at a rate of 0.07% per month. Therefore, over the course of 1 year, WMCs would be expected to increase on average from 0.36% of total brain volume to [0.36 + (0.07 × 12)] = 1.20% of total brain volume. By 2 years, WMC would be expected to account for 2.04% of brain volume. However, there were significant between-person differences in rate of accumulation (σ 2 = 0.003, p < .01), with some individuals accumulating WMC relatively slowly and others relatively quickly. Representative longitudinal MRI scans from two patients are shown in Fig. 3.
Graph showing the percent of white matter change (measured as a volume of white matter change as a function of whole brain tissue volume) as a function of time after whole brain radiation treatment. Each line represents a patient. Although there is some fluctuation the solid line represents the best fit line for all 30 patients showing a general trend of increasing white matter change as more time elapses since treatment (0.07% per month)
We attempted to examine if the differences in rates of WMC accumulation were present early on (e.g., 6 months of data, 12 months of data, 18, etc.). Distinguishable differences may have been present already 8 months in, but given the relatively small overall size of the sample, and that power to detect statistically significant differences in this study is derived in large part from the longitudinal aspect of the data, we cannot confidently say that there were significant differences within a couple of weeks or months after WBRT. Larger samples, or more frequent measurement of WMCs, will be necessary to obtain a more precise picture of how and when the process of WMC accumulation diverges with the presence or absence of specific risk factors. At the descriptive level, there was a slight rise in WMC shortly after radiation in some patients and for some patients it is quite dramatic. These changes may be related to a mixture of inflammatory cytokines and a slight transient (days to weeks) breakdown in blood brain barrier. However, further study will be necessary to tease out such processes.
Patient characteristics associated with an increased rate of WMC accumulation included age (β = 0.004, p < .0001), total dose of radiation (β = 0.006, p < .0001), hyperglycemia (β = 0.048, p < .0001) and hypertension (β = 0.084, p < .0001). Smoking and diabetes did not appear to affect the rate of WMC accumulation. In multivariate analyses controlling for age (β = 0.001, p > .05), and total dose (β = 0.003, p < .05), hyperglycemia and hypertension remained significant factors remained independent moderators of change. In particular, hyperglycemia (β = 0.045, p < .05) and hypertension (β = 0.056, p < .05) each contributed to greater rates of accumulation, while not being related to initial levels of WMC. Differences in average change are shown in Fig. 4.
This graph depicts percent white matter change as a function of amount of time in months since treatment. Top panel shows the difference in white matter change accumulation for the patients that were hyperglycemic (solid line average glucose above 120; dashed line average glucose below 120; β = 0.048, p < .0001). Bottom panel shows the different rate of change for patients with a diagnosis of hypertension (β = 0.084, p < .0001). Solid line represents patients with a diagnosis of hypertension. Dashed line represents patients who do not have a hypertension diagnosis
Discussion
WMCs on MRI are a non-specific indicator of disturbance of brain vasculature and metabolism [3]. WMCs are associated with several neuropsychiatric disorders, including vascular dementia, and have been associated with cognitive decline in multiple settings [5–8]. Radiation injury to the brain is frequently manifested on post-therapy MRIs as WMCs [1, 2]. The most important target cells for radiation-induced central nervous system damage are currently thought to be glial and endothelial cells. Radiation effects on the cerebral arterial wall are mediated by a number of cellular processes taking place in the endothelium and vascular smooth muscle. Some of these changes are thought to be dose dependent, and can be seen as early as 2 weeks after radiation. Perfusion scans have shown a transient increase in the rate of breakdown of the blood brain barrier from 2 to 6 weeks after WBRT, followed by a period of several weeks when the amount of WMC is stable, followed again by steadily progressing WMC for several months. This reflects early and late radiation changes. Capillaries are the most radiosensitive component of cerebral vessels, making their integrity key to overall tissue radiosensitivity [9–11].
Long-term cancer survivors who have undergone WBRT are at an increased risk for developing WMCs [12]. In the past, qualitative scales such as the Fezekas scale have been used to assess WMC, with good correlation between all such scales [13]. Previous studies have been limited by small numbers of subjects, short follow-up (particularly in the setting of a condition like WMC that develops long after the completion of WBRT), and a lack of quantitative grading scales for WMCs. The association between WMCs induced by radiochemotherapy and the cognitive function of the patients has been evaluated by several groups. In a 2004 study by Correa et al. [14] 28 PCNSL patients in disease remission received a post-treatment baseline neuropsychological evaluation. Assessment of extent of WMCs on MRI was also performed. They found that PCNSL survivors treated with WBRT +/− chemotherapy displayed more pronounced cognitive dysfunction than patients treated with MTX-based chemotherapy alone. In another study by Neuwelt et al. 16 PCNSL patients were followed after complete response. Neuropsychological cognitive testing and MR imaging or CT were performed before treatment and at completion of the 12-month treatment (blood–brain barrier disruption + chemotherapy) for each patient. Interestingly, a significant association between neurocognitive data and WMCs was found at baseline after tissue diagnosis but before chemotherapy, but no correlation existed at the end of treatment. Moreover, patients who attained a complete response showed improved or maintained cognitive functioning [15]. Determining the differential effects of theses variables on cognition has been a challenge and whether more extensive WMCs are associated with worse neurologic toxicity is not yet clear.
There have been only a few reports of MRI findings of long-term sequential changes following WBRT [1, 16–18]. MRI can identify WMC with great sensitivity, secondary to the alterations in water content corresponding to these lesions, though several problems exist with evaluation of longitudinal MR imaging examination. Differences in image acquisition technique can lead to disparities in image quality and windowing, making comparisons difficult, even within the same patient. Other problems in longitudinal studies exist regarding the assessment of white matter over time [16, 17]. Historically, this was assessed qualitatively with visual rating scales. These scales rated the amount of WMC. However, although well validated for qualitative assessment, these techniques do not quantitatively assess the amount of tissue damage [13, 17]. Each of the visual rating scale grades may encompass a wide variety of imaging characteristics. Subtle WMC may be missed if they remain within the parameters of the prior scaling unit.
Volumetric evaluation provides a continuous scale along which to measure WMCs, allowing significantly more sensitive assessment of WMC, and overcoming the floor and ceiling effects associated with visual rating scales. Quantitative volumetric analysis of WMC has successfully been used to show differences between patient groups, and has increased sensitivity of assessment of WMC relative to visual rating scales [19]. There are many methods of volumetric analysis of brain tissue. One such method is a thresholding technique, which uses individual values of voxel intensity, keeping voxels greater than or equal to this value in the scan to be quantified. The same thresholding number is subsequently used to analyze all scans belonging to a given patient, and to compare between patients. This method can be problematic when scanning techniques vary within and between patients over time as is often the case in hospital settings. Another method sets the threshold for each scan individually, basing the threshold value on differences in MRI signal between WMC and normal parenchyma. However, this method can incorrectly include areas outside of the area of interest that meet the voxel intensity threshold criteria. Therefore, it is important to use area masks (i.e. a defined area of interest outlined on the scan) to ensure specificity of WMC calculations [19]. In this report, we used multiple masks to exclude areas of the brain that would give us false positives and set the thresholding value based on each individual scan to accommodate different scanning techniques thereby overcoming some of the above outlined difficulties.
Whether associated with radiation therapy, CNS disease, or normal aging, WMCs are thought to be associated with cognitive decline [8, 20–22]. The amount of WMC is thought to correspond to more severe cognitive dysfunction. This hypothesis is predicated on a cognitive model of neural networks, that to work efficiently complex cognitive functions require intact connections of these networks. O’Sullivan et al. [23] showed (using DTI) that more extensive anisotropy and disconnection in the white matter of elderly patients is associated with more severe cognitive decline. Risk factors predisposing individuals to cerebrovascular disease have been associated with more extensive white matter disease [24, 25]. However, cerebrovascular disease, itself, has not been linked to greater post-WBRT WMCs in previous series, likely because the scales used were not sufficiently sensitive to distinguish differences among relatively small patient populations [2].
Acknowledging the small sample size and its limits in terms of generalizability, this report is unique in that it represents a quantitative analysis of WMCs among a large sample of long-term adult survivors of brain metastases who received whole brain radiation [17]. In this cohort of patients, WMCs were observed on all MR scans, beginning as soon as 1 month after the completion of after WBRT. The rate of accumulation of WMCs in our sample was modeled as linear over time. However, this may not be entirely accurate given the relative number of patients and the changes in the rate of WMC accumulation that may manifest with late radiation injury. In a few patients, a transient decrease in WMC was observed in initial MRI scans, followed by a resumption of WMC accumulation. Whether this represents the difference between early and late radiation changes is uncertain.
It is intuitive to think that vascular co-morbidities like hypertension would put patients at risk for a higher rate of WMC accumulation [25]. This effect on white matter was seen by Welzel et al. [26] in a smaller short term study using white matter anisotropy. Hypertensive patients already have vascular damage at the time of treatment, so the addition of WBRT-associated vascular damage would be predicted to have a combined or even synergistic effect on the rate of WMC accumulation. This relationship warrants further study. The effect of proper control of hypertension on WMC could not be assessed in our data set, but understanding of this relationship is key to possible remediation of one potentially significant risk factor for adverse cognitive effects of radiation therapy.
One clinical factor we investigated was the effect of diabetes on WMC, specifically the effect of glucose control on WMC accumulation. Unfortunately, an insufficient number of diabetic patients were available for inclusion in our patient population. However, we were able to look at the effect of hyperglycemia (regardless of diabetes diagnosis status). Hyperglycemia induces a variety of biochemical changes within endothelial cells. Endothelial cells transport glucose via the GLUT-1 transporter. Because this transporter continuously transports glucose based on concentration gradient, extracellular hyperglycemia induces intracellular hyperglycemia in endothelial cells. Ultimately, intracellular hyperglycemia increases free radical production and damage to the endothelial lining, which is compounded by radiation-mediated free radical damage. These short term chemical effects are only related to glucose. So presumably controlling glucose levels in the cerebral vasculature could affect or ameliorate this cascade [27].
Our glycemic measurements were rather crude, with information obtained from hospital labs, and thus susceptible to possible isolated, transient hyperglycemic spikes at the time of measurement only, related to illness or steroid treatment. Most cancer patients lack routine follow-up of glucose management, even though they are at significant risk for hyperglycemia. In the future, measurement of Hemoglobin A1c could be used to better gauge long-term glucose control in cancer patients. Our data may in fact underestimate the effect of prolonged hyperglycemia on WMC, simply because the duration of hyperglycemia was not measured in a refined way in the present patient population. This patient population (with hyperglycemia related to steroid treatment and illness vs. diabetes) presents a unique opportunity to determine the contribution of hyperglycemia itself to the pathophysiology of radiation damage to the brain parenchyma and vasculature, excluding the effects of molecular deregulation that occurs in a diabetic patient [28]. One recently published New England Journal of Medicine article suggested that glucose control did not have an effect on vascular outcomes in diabetics [24]. Their result implies that diabetic vascular damage is not the result of hyperglycemia itself, but rather that it is the result of some dysfunction related to diabetes molecular pathophysiology. Here we see an effect of hyperglycemia itself regardless of diabetic status.
We were unable to observe an effect of chemotherapeutic regimen on WMC accumulation. The wide variety and differential timing of patients’ medication routines required that we treat medications as a random factor and thus precluded investigation of specific associations with accumulation of WMCs. We hope in a larger prospective study to be able to follow this line of inquiry. Other papers have found a relationship between some drugs, mostly radiosensitizers, and increased WMC. Given the many combinations of chemotherapy drug classes used in this patient cohort, and a wide array of chemotherapeutic agents during the treatment course, individual chemotherapy effects were likely obscured.
Conclusions
In this study, we analyzed post-WBRT WMCs in a quantitative manner in an attempt to identify clinical factors that were associated with more rapidly developing and more severe WMCs in long-term survivors of brain metastases. Because brain vasculature is relatively radiosensitive, it is not surprising that a relationship between pre-existing hypertensive vascular disease and a greater rate of accumulation of WMC was observed. Our findings also suggest that age, impaired glycemic control, and uncontrolled hypertension are important risk factors for worsened radiation effects on the brain. These findings require confirmation through future longitudinal follow-up studies that utilize more refined glycemic measurements, such as hemoglobin A1c. Prospective study of glycemic control in WBRT patients, employing close monitoring and tight regulation of blood glucose levels, would be invaluable.
References
Fujii O et al (2006) White matter changes on magnetic resonance imaging following whole-brain radiotherapy for brain metastases. Radiat Med 24(5):345–350
Johannesen TB et al (2003) Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol 69(2):169–176
Armstrong CL et al (2004) A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev 14(1):65–86
Bazin PL et al (2007) Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods 165(1):111–121
Breteler MM et al (1994) Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam study. Stroke 25(6):1109–1115
Deary IJ et al (2006) White matter integrity and cognition in childhood and old age. Neurology 66(4):505–512
Deary IJ et al (2003) Cerebral white matter abnormalities and lifetime cognitive change: a 67-year follow-up of the Scottish mental survey of 1932. Psychol Aging 18(1):140–148
Leaper SA et al (2001) Neuropsychologic correlates of brain white matter lesions depicted on MR images: 1921 Aberdeen Birth Cohort. Radiology 221(1):51–55
Emami B et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122
O’Connor MM, Mayberg MR (2000) Effects of radiation on cerebral vasculature: a review. Neurosurgery 46(1):138–149 (discussion 150–1)
Schultheiss TE et al (1995) Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 31(5):1093–1112
Armstrong CL et al (2002) Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology 59(1):40–48
Pantoni L et al (2002) Visual rating scales for age-related white matter changes (leukoaraiosis): can the heterogeneity be reduced? Stroke 33(12):2827–2833
Correa DD et al (2004) Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 62(4):548–555
Neuwelt EA et al (2005) Imaging changes and cognitive outcome in primary CNS lymphoma after enhanced chemotherapy delivery. AJNR Am J Neuroradiol 26(2):258–265
Shan ZY et al (2006) Quantitative morphologic evaluation of white matter in survivors of childhood medulloblastoma. Magn Reson Imaging 24(8):1015–1022
van den Heuvel DM et al (2006) Measuring longitudinal white matter changes: comparison of a visual rating scale with a volumetric measurement. AJNR Am J Neuroradiol 27(4):875–878
Bastin ME et al (2009) Diffusion tensor and magnetization transfer MRI measurements of periventricular white matter hyperintensities in old age. Neurobiol Aging 30(1):125–136
Wu M et al (2006) A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 148(2–3):133–142
Starr JM et al (2003) Brain white matter lesions detected by magnetic resonance [correction of resonance] imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry 74(1):94–98
Shenkin SD et al (2005) Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebrovasc Dis 20(5):310–318
Reddick WE et al (2006) Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer 106(4):941–949
O’Sullivan M et al (2001) Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 57(4):632–638
Duckworth W et al (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360(2):129–139
Murray AD et al (2005) Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 237(1):251–257
Welzel T et al (2008) Diffusion tensor imaging screening of radiation-induced changes in the white matter after prophylactic cranial irradiation of patients with small cell lung cancer: first results of a prospective study. AJNR Am J Neuroradiol 29(2):379–383
Martini SR, Kent TA (2007) Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 27(3):435–451
Gallagher KA et al (2006) Hyperbaric oxygen and bone marrow-derived endothelial progenitor cells in diabetic wound healing. Vascular 14(6):328–337
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Szerlip and C. Rutter are equal contributors.
Rights and permissions
About this article
Cite this article
Szerlip, N., Rutter, C., Ram, N. et al. Factors impacting volumetric white matter changes following whole brain radiation therapy. J Neurooncol 103, 111–119 (2011). https://doi.org/10.1007/s11060-010-0358-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0358-7