Abstract
We investigated pseudoprogression (psPD) in patients with malignant gliomas treated with radiotherapy (RT) and maintenance temozolomide (TMZ) in terms of incidence, outcomes, and predictive and prognostic factors. We evaluated p53 overexpression by immunohistochemical analysis of thirty-five tumor samples as a predictor for psPD. The time to progression and overall survival were compared between subgroups, psPD versus early progression (ePD) versus nonprogression (nonPD). Eight patients developed psPD among eighteen patients with lesion enlargement at the first MRI scan, and the others were classified as ePD. The remaining stable or improved patients were classified as nonPD. All patients with psPD were alive at last follow-up (median follow-up period was 12 months; range 5.8–58.5 months). Overall survival of psPD patients was significantly higher than ePD patients (P < 0.01). There was no significant survival difference between the psPD group and nonPD group (P = 0.25). Seven (87.5%) of eight tumors with psPD showed p53 overexpression, as compared to 3 (30%) of the ten tumors with ePD (P = 0.03). Our study indicates that psPD following chemoradiotherapy with TMZ is associated with significantly better overall survival compared to that of ePD, and is comparable to nonPD group. Overexpression of p53 was identified as a potential biomarker for predicting the development of psPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are the most common primary neoplasms of the human nervous system. Although the result of radiotherapy (RT) is greater with concurrent and adjuvant temozolomide (TMZ), median overall survival remains at approximately 12 months in patients with glioblastoma, the most aggressive form of glioma. Recently, studies of pseudoprogression (psPD), defined as spontaneous improvement of enhancing lesions without further treatment other than adjuvant TMZ, have been reported in patients with malignant gliomas treated with chemoradiotherapy along with TMZ [1–3]. The reported incidence ranged from 15 to 30%, and some studies demonstrated that the development of psPD is associated with favorable overall survival [3, 4]. Thus, if possible, differential diagnosis of early tumor responses, such as psPD and real progression, can assist physicians in identifying patients who may benefit from intensive salvage treatment. The methylation of O6-methylguanine DNA methyltransferase (MGMT) promoter is the only predictive molecular alteration for the development of psPD in patients with malignant gliomas treated with RT and maintenance TMZ to date. The further effort to identify the other useful predictive maker(s) is clearly required.
TP53 is a tumor suppressor gene commonly found in human cancers. p53 expression is induced by many types of stressors such as heat, cold, low-dose ionizing radiation, and low pH [5]. It is widely assumed that normal p53 functions play a crucial role in mediating cell death following DNA-damaging therapies. There is evidence to support the premise that wild-type p53 suppresses the suicide DNA repair enzyme MGMT, resulting in a higher efficacy of TMZ in p53 wild-type than p53 mutant tumors [6, 7]. Conversely, one study reported the increased sensitivity of mutant p53 glioma cells toward ultraviolet (UV)-C light by DNA damage-induced blockage of RNA polymerase II and transcription [8]. Therefore, p53 expression may be associated with chemotherapy or radiation sensitivity and subsequent development of psPD.
In the present study, we examined the incidence of psPD and correlated the development of psPD with clinical outcomes in patients with malignant gliomas undergoing treatment with concurrent chemoradiotherapy and TMZ. Further analysis was directed toward identifying clinical and molecular parameters associated with the development of psPD, including p53 overexpression.
Patients and methods
Study population
Between June 2004 and February 2009, a total of 40 malignant glioma patients treated with chemoradiotherapy and TMZ in the Seoul National University Bundang Hospital were reviewed. Five patients were excluded due to indeterminate biopsy results in four patients and loss of follow-up in one patient. Data for 35 patients were available and used for analysis. Clinical records were reviewed concerning performance status, the extent of surgery, histology, corticosteroid dose, neurologic signs and symptoms, and survival. The median duration of follow-up was 13.2 months (range 3.3–58.5 months) and the median follow-up duration of the survivors was 14.2 months (range 4.5–58.5 months).
Treatment details
Radiation therapy was delivered 5 days per week using a continuous course of treatment with photons produced with a megavoltage linear accelerator. The total radiation dose ranged from 59.4 to 61.2 Gy, using a conventional fractionation schedule of 1.8–2 Gy per fraction per day except in one patient whose tumor was located adjacent to the brainstem and who received a dose of 55.8 Gy. The radiation fields encompassed the residual tumor volume and tumor bed (as defined by a CT or MRI scan) with a 2-cm margin. Eight patients received fractionated stereotactic RT (FSRT) with intensity modulation with reduced margin after 40–50 Gy of irradiation. Concomitant TMZ (75 mg/m2/day) was given on all days during RT and was followed after 4 weeks by adjuvant TMZ (150–200 mg/m2/day) given on Days 1–5, every 28 days. The median number of cycles of adjuvant TMZ was 4 (range 1–15 cycles), and fourteen patients completed planned adjuvant treatment.
Diagnosis of pseudoprogression
Early evaluation of treatment response was initiated 4 weeks after the end of concomitant chemoradiotherapy (CCRT) with MRI. The size of enhancing lesion in the gadolinium-enhanced T1 weighted image was measured, and tumor progression was defined according to MacDonald’s Criteria [9] as a 25% increase in tumor size with two-dimensional measurement, newly appeared lesions, an increased need for corticosteroids, or neurological deterioration. Early progression (ePD) was defined as progression on the first and second MRI scan after CCRT and adjuvant TMZ was suspended at second MRI scan. If the progressive lesion decreased in size or stabilized on subsequent follow-up MRI without additional treatment, it was defined as psPD. Since majority of lesions were not spherical, we performed serial volumetric analysis of enhancing lesions to calculate a change of enhancing volume using RT planning software (BrainLAB, Feldkirchen, Germany). In this case progression was defined as a 40% increase in volume, which is in accordance with previous work [10]. If patients presented stable disease or had no lesion at the first evaluation after CCRT, additional cycles of TMZ were continued, and these were defined as the nonPD subgroup.
Immunohistochemistry of p53
The expression of p53 in tumor samples was determined by immunohistochemistry using the p53 monoclonal mouse antihuman antibody DO-7 (DAKO, Glostrup, Denmark). Four-micrometer-thick tissue sections were taken from formalin-fixed, paraffin-embedded, archival tumor blocks. For antigen retrieval, tissue sections on silanized slides (DAKO) were boiled in 0.01 M citrate buffer (pH 6.0, SIGMA, St. Louis, MO) for 15 min using a microwave. A commercially available kit (ChemMate DAKO EnVision Detection Kit, Peroxidase/DAB, Rabbit/Mouse, DAKO) was used, and the slides were developed with DAB (diaminobenzidine tetrahydrochloride) and counterstained with Harris-hematoxylin (DAKO). Positively-stained tumor cells were counted under a microscope. Expression of p53 in over 10% of the tumor cells was considered as overexpression, which is highly suggestive of p53 mutation, because the wild-type protein is not generally detectable due to its short half-life [11–13].
Statistical analysis
Statistical analysis was performed using SPSS version 17.0 (SPSS, Chicago, IL). The Kaplan–Meier method was employed to calculate the time to progression (TTP) and overall survival (OS). We designated the TTP as the time period from the date of surgery to the point when radiologic or clinical evidence of PD was noted and the OS as the interval between the date of surgery and the date of death. The difference in survival between different subgroups (nonPD vs. ePD vs. psPD) was assessed using the log-rank test. To define the association between clinical or biological factors and the development of psPD, the Fisher’s exact test was used, comparing psPD versus ePD. Differences were considered statistically significant at P < 0.05 (two-sided test).
Results
Patient characteristics
The patient population was comprised of seventeen men and eighteen women aged 23–82 years (median: 57 years). The histologic diagnosis was anaplastic oligodendroglioma in two patients, anaplastic astrocytoma in eight patients, and glioblastoma in twenty-five patients. Cytoreductive surgery was performed in twenty-two patients. Radical resection was accomplished in thirteen patients (59%), nine patients (41%) underwent subtotal resection, and the remaining eight patients were diagnosed by biopsy (Table 1).
Incidence of psPD
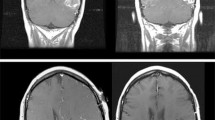
An early increase in the size of enhancing lesions was observed in eighteen patients at the first MRI scan after CCRT. Eight patients were classified as having psPD (23%), and the remaining ten patients were considered to have ePD (29%) after a second MRI scan. Among the patients with ePD, half had increased enhancing lesions and the others had a clinically or neurologically progressed condition. For volumetric measurement, only two patients had changes in their MRI volumetric response compared with diametric MacDonald’s Criteria: one patient went from ePD to psPD and the other from ePD to nonPD. The volumetric changes in enhancing lesions were demonstrated in Fig. 1a (psPD) and Fig. 1b (ePD).
Factors predictive for the development of psPD
Clinical and biological factors were analyzed to detect predictors for the development of psPD (Table 2). The p53 overexpression was found to be the most relevant predictive factor for the development of psPD (P = 0.03 by Fisher’s exact test). Seven of the eight tumors (87.5%) from patients with psPD showed overexpression of p53, as compared to 3 (30%) of the ten tumors in patients with ePD. In contrast, other factors, such as age, sex, extent of surgery, histology, surgery to RT interval, and use of FSRT boost were not significantly correlated with the early MRI response.
Survival
Table 3 reports the effect of the first MRI finding after RT. For the entire study population, the median TTP was 8.4 months with 1- and 2-year TTP rates of 41 and 32%, respectively. The estimated median OS was 25.2 months with 1- and 2-year OS rates of 81 and 54%, respectively. The median TTP for patients with psPD, ePD, and nonPD was 7, 3.1, and 22.1 months, respectively (Fig. 2a). The TTP after ePD was inferior to that after psPD (P < 0.01) or nonPD (P < 0.01). All patients with psPD were living at last follow-up, and median OS for patients with ePD and nonPD was 10.8 and 25.6 months, respectively (Fig. 2b). The OS after ePD was worse than that for the other subgroups (P < 0.01 for each comparison). The difference in TTP and OS between patients with psPD and patients with nonPD was not statistically significant (P = 0.15 and 0.25, respectively).
Discussion
The phenomenon of “pseudoprogression” of malignant glioma was mentioned by Hoffman et al. [14] in 1979 and described by de Wit et al. [15] in 2004, and since has become well understood. It has been suggested that psPD may represent a result of treatment-related cellular hypoxia that leads to expression of hypoxia-regulated molecules from tumor and surrounding cells with a subsequent increase in vascular permeability and increased tumor enhancement [16]. In addition, vascular changes and leakiness caused by radiation, which can lead to edema and enhancement of tumor, is considered crucial for the development of psPD [17]. However, much work is needed to elucidate the underlying mechanisms of psPD. In current study, we tried to identify the potential biologic marker(s) to predict this unique phenomenon.
We observed psPD in 23% of malignant glioma patients treated with chemo-radiothearpy with TMZ. In addition, our results indicate that mutant p53 was expressed in one-third of malignant gliomas, and more importantly, significantly associated with the development of psPD. To the best of our knowledge, the present study is the first to report the predictive potential of p53 overexpression for the development of psPD. One can assume that discerning the early MRI response by such a predictor is of value in practice, because favorable survival is expected for patients with psPD, as confirmed in our study.
Contradicting result has been published about the relationship between p53 status and radiosensitivity of malignant glioma cells [18]. The clinical impact of p53 mutations in malignant glioma, in the same context, remains debatable [19–22]. With respect to a relationship between p53 and TMZ, Hermisson et al. [23] demonstrated that abrogation of p53 wild-type function strongly attenuated TMZ cytotoxicity. In contrast, an alternative pathway to induce apoptosis in p53 mutant gliomas after TMZ treatment was shown by Roos et al. [24].
Recently, studies have shown that tumor samples with methylated MGMT promoters or low active MGMT have a tendency to harbor mutant p53 [11, 25, 26], although conflicting data were reported in another study [27]. As shown by Brandes et al. [4], MGMT promoter methylation is known to positively correlate with the development of psPD; thus, our findings seem to support the relationship between MGMT promoter methylation and psPD. Taken together, these findings suggest that malignant gliomas with MGMT promoter methylation are highly sensitive to chemoradiotherapy with TMZ. Furthermore, these cells have impaired double-strand DNA break repair capability, and p53 mutation may be a surrogate marker for MGMT promoter status. However, various mutations have been found in TP53 [28], and G:C–A:T transition mutation in p53 could be related to a defect in MGMT activity, which leads to changes in G:C–A:T nucleotides during DNA replication [26].
Although patients with psPD were not followed-up long enough to calculate a median overall survival, no patient died after exhibiting psPD. The survival benefit from the addition of TMZ to radiotherapy in glioblastoma patients with methylated MGMT promoters, compared with those with unmethylated MGMT promoters, has been shown in a study by the European Organization for Research and Treatment of Cancer–National Cancer Institute of Canada [29]. Good response after chemoradiotherapy with TMZ may affect prolonged OS, and the development of psPD seems to reflect a better response of the tumor to treatment. Another possible explanation for the high OS rates in patients with psPD is the good salvage response after progression. Although the difference was insignificant, the duration of TTP in patients with psPD was shorter than in those with nonPD. Thus, it can be assumed that tumors responsive to first chemoradiotherapy will have good response to salvage treatment.
In conclusion, our findings suggest that p53 overexpression may be a potentially useful marker to predict the development of psPD in patients with malignant gliomas after chemoradiotherapy with TMZ.
References
Chamberlain MC, Glantz MJ, Chalmers L, van Horn A, Sloan AE (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82:81–83
Jefferies S, Burton K, Jones P, Burnet N (2007) Interpretation of early imaging after concurrent radiotherapy and temozolomide for glioblastoma. Clin Oncol (R Coll Radiol) 19:S33
Taal W, Brandsma D, de Bruin HG, Bromberg JE, Swaak-Kragten AT, Smitt PA, van Es CA, van den Bent MJ (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Takahashi A, Ohnishi K, Wang X, Kobayashi M, Matsumoto H, Tamamoto T, Aoki H, Furusawa Y, Yukawa O, Ohnishi T (2000) The dependence of p53 on the radiation enhancement of thermosensitivity at different let. Int J Radiat Oncol Biol Phys 47:489–494
Kock H, Harris MP, Anderson SC, Machemer T, Hancock W, Sutjipto S, Wills KN, Gregory RJ, Shepard HM, Westphal M, Maneval DC (1996) Adenovirus-mediated p53 gene transfer suppresses growth of human glioblastoma cells in vitro and in vivo. Int J Cancer 67:808–815
Srivenugopal KS, Shou J, Mullapudi SR, Lang FF Jr, Rao JS, Ali-Osman F (2001) Enforced expression of wild-type p53 curtails the transcription of the O(6)-methylguanine-DNA methyltransferase gene in human tumor cells and enhances their sensitivity to alkylating agents. Clin Cancer Res 7:1398–1409
Batista LF, Roos WP, Kaina B, Menck CF (2009) p53 mutant human glioma cells are sensitive to UV-C-induced apoptosis due to impaired cyclobutane pyrimidine dimer removal. Mol Cancer Res 7:237–246
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Henson JW, Ulmer S, Harris GJ (2008) Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol 29:419–424
Wiewrodt D, Nagel G, Dreimuller N, Hundsberger T, Perneczky A, Kaina B (2008) MGMT in primary and recurrent human glioblastomas after radiation and chemotherapy and comparison with p53 status and clinical outcome. Int J Cancer 122:1391–1399
Curtin K, Slattery ML, Holubkov R, Edwards S, Holden JA, Samowitz WS (2004) p53 alterations in colon tumors: a comparison of SSCP/sequencing and immunohistochemistry. Appl Immunohistochem Mol Morphol 12:380–386
Sarkar C, Karak AK, Nath N, Sharma MC, Mahapatra AK, Chattopadhyay P, Sinha S (2005) Apoptosis and proliferation: correlation with p53 in astrocytic tumors. J Neurooncol 73:93–100
Hoffman WF, Levin VA, Wilson CB (1979) Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg 50:624–628
de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ (2004) Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63:535–537
Jensen RL (2009) Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol 92:317–335
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Khodarev NN, Labay E, Darga T, Yu J, Mauceri H, Gupta N, Kataoka Y, Weichselbaum RR (2004) Endothelial cells co-cultured with wild-type and dominant/negative p53-transfected glioblastoma cells exhibit differential sensitivity to radiation-induced apoptosis. Int J Cancer 109:214–219
Schmidt MC, Antweiler S, Urban N, Mueller W, Kuklik A, Meyer-Puttlitz B, Wiestler OD, Louis DN, Fimmers R, von Deimling A (2002) Impact of genotype and morphology on the prognosis of glioblastoma. J Neuropathol Exp Neurol 61:321–328
Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG 2nd, Aldape K (2001) Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res 61:1122–1128
Tada M, Matsumoto R, Iggo RD, Onimaru R, Shirato H, Sawamura Y, Shinohe Y (1998) Selective sensitivity to radiation of cerebral glioblastomas harboring p53 mutations. Cancer Res 58:1793–1797
Shiraishi S, Tada K, Nakamura H, Makino K, Kochi M, Saya H, Kuratsu J, Ushio Y (2002) Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 95:249–257
Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M (2006) O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem 96:766–776
Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF, Kaina B (2007) Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene 26:186–197
Watanabe T, Katayama Y, Komine C, Yoshino A, Ogino A, Ohta T, Fukushima T (2005) O6-methylguanine-DNA methyltransferase methylation and TP53 mutation in malignant astrocytomas and their relationships with clinical course. Int J Cancer 113:581–587
Shamsara J, Sharif S, Afsharnezhad S, Lotfi M, Raziee HR, Ghaffarzadegan K, Moradi A, Rahighi S, Behravan J (2009) Association between MGMT promoter hypermethylation and p53 mutation in glioblastoma. Cancer Invest 27(8):825–829
Jesien-Lewandowicz E, Jesionek-Kupnicka D, Zawlik I, Szybka M, Kulczycka-Wojdala D, Rieske P, Sieruta M, Jaskolski D, Och W, Skowronski W, Sikorska B, Potemski P, Papierz W, Liberski PP, Kordek R (2009) High incidence of MGMT promoter methylation in primary glioblastomas without correlation with TP53 gene mutations. Cancer Genet Cytogenet 188:77–82
Bouchet BP, de Fromentel CC, Puisieux A, Galmarini CM (2006) p53 as a target for anti-cancer drug development. Crit Rev Oncol Hematol 58:190–207
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Acknowledgments
This work was supported by the grants from Korean Ministry of Education, Science & Technology (BAERI # 2007-2001198) to IAK.
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, HC., Kim, CY., Han, J.H. et al. Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: potential role of p53. J Neurooncol 102, 157–162 (2011). https://doi.org/10.1007/s11060-010-0305-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0305-7





