Abstract
Object This study reviews the long-term clinical results of stereotactic radiosurgery in the treatment of pituitary adenoma patients. Methods We reviewed the outcomes of 298 patients who underwent Gamma Knife radiosurgery for recurrent or residual pituitary adenomas. These results are compared to other contemporary radiosurgical series. Results Pituitary tumors are well-suited for radiosurgery, since radiation can be focused on a well circumscribed region, while adjacent neural structures in the suprasellar and parasellar regions are spared. The overall rate of volume reduction following stereotactic radiosurgery is 85% for non-secretory adenomas that are followed for more than 1-year. The rates of hormonal normalization in patients with hypersecretory adenomas can vary considerably, and tends to be higher in patients with Cushing’s Disease and acromegaly (remission rate of approximately 53% and 54%, respectively) when compared with patients who have prolactinomas (24% remission) and Nelson’s syndrome (29%) remission. Advances in dose delivery and modulation of adenoma cells at the time of radiosurgery may further improve results. Conclusions Although the effectiveness of radiosurgery varies considerably depending on the adenoma histopathology, volume, and radiation dose, most studies indicate that radiosurgery when combined with microsurgery is effective in controlling pituitary adenoma growth and hormone hypersecretion. Long-term follow-up is essential to determine the rate of endocrinopathy, visual dysfunction, hormonal recurrence, and adenoma volume control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenomas represent 10–20% of all primary brain tumors [1, 2]. Tumors that secrete an excessive amount of a hormone are associated with various clinical syndromes, which include Cushing’s disease (Adrenocorticotrophic hormone, ACTH hypersecretion), acromegaly (growth hormone, GH hypersecretion), and hyperprolactinemia (Prolactin, PRL hypersecretion).
The most common secretory adenomas are prolactinomas, which result in amenorrhea-galactorrhea syndrome with infertility in women, and impotence and infertility in men. Growth-hormone-secreting pituitary adenomas cause acromegaly in adults and gigantism in children, and tumors secreting adrenocorticotropic hormone cause Cushing’s syndrome.
Non-secretory adenomas account for approximately 30% of all pituitary adenomas, and they are often macroadenomas on presentation. Adenomas in this group enlarge progressively in the pituitary fossa and often extend beyond the sella turcica [3]. Tumor enlargement may cause local mass effects and result in visual field dysfunction from direct compression of the optic apparatus. Patients with larger tumors might have headache from elevated intracranial pressure and hypopituitarism resulting from compression of the normal pituitary gland.
Transsphenoidal microsurgical resection of adenomas has been the mainstay of treatment in patients with symptomatic tumors and failed medical treatment. Radiation therapy was originally the adjuvant treatment of choice for recurrent or residual pituitary adenomas, but conventional radiotherapy had several limitations. The time to hormonal normalization was slow, and the rate of post-procedural hypopituitarism was high. Side-effects of conventional radiation treatment included impairment of neurocognitive function, visual dysfunction from optic neuropathy, stroke, and damage to other cranial nerves.
The advantage of stereotactic radiosurgery is that it delivers a high but highly focused dose of radiation to a tumor in a single session. With the aid of neuro-imaging guidance and blocking strategies, a steep radiation-dose fall-off is achieved which ideally spares surrounding tissues from the harmful effects of radiation [4]. This is particularly important in patients with pituitary adenomas since they are often located close to critical neural structures in the parasellar and suprasellar regions.
The radiobiology of radiosurgery and the rationale for its use in the treatment of pituitary adenomas
Cellular effects
Infliction of radiation damage at a cellular level occurs either through the direct action whereby the ionization breaks DNA strands or the indirect action where the DNA damage is produced by free radicals, which are products of radiation effect on other molecules, especially water molecules. Radiation results in a distribution of lethal and sublethal effects across the radiation field. Radiation damages the DNA of tumor cells as well as the DNA of normal cells in its path. Normal tissue, however, is generally more capable of DNA repair than tumors. This is partly due to aberrant cell cycle control mechanisms in tumors as well as differences in genetic features that permit damages to the abnormal tumor phenotype. Cells require time to repair DNA damage, and the normal cell response to irradiation is to delay the cell cycle. The length of G2 phase delay correlates with radiation resistance. Therefore, the radiobiology of the cell cycle and differences in cell repair are of paramount importance for radiotherapy. Repair plays a less critical role as the number of fractions decreases, especially in the setting of single, session radiosurgery. Normal and aberrant cells within the target volume receive lethal damage and the surrounding normal neuronal tissues are largely spared from radiation injury as a result of the steep dose fall-off.
Even though several of these effects are governed by volume considerations, there are differential responses that are based on the rate of proliferation of cells, resulting in increased sensitivity of endothelial, glial and subependymal plate cells. In addition to the cytotoxic effects, vascular obliteration seems to play a role in the death of tumor cells as well.
Tissue effects
The radiation doses prescribed for radiotherapy have been developed from decades of clinical experience. Early in the era of clinical radiotherapy, it was observed that multiple treatments (fractions) with reduced doses per fraction improved the therapeutic ratio when treating both benign and malignant tumors.
The radiobiological principles which govern the design of multifraction treatments do not necessarily apply to high-dose ionizing beams as used in radiosurgery. Radiotherapy uses a per-fraction prescription dose which is below the lethal dose threshold of normal tissue in the volume treated so as to purposely include a “margin” of normal tissue around the target lesion to account for daily setup error or subclinical disease. The time between fractions then allows this normal tissue to repair sublethal damage. In contrast, radiosurgery specifies a precise delivery of a high single-fraction dose of ionizing beams to a defined target volume. Normal tissue is excluded from the target volume as much as possible and the steep dose gradient at the margin of the target volume assures that this tissue receives minimal dosage. Thus, repair of normal tissue during the treatment is of little value in radiosurgery (Fig. 1).
The delivery of an inhomogeneous dose to the treatment field with a higher dose at the center of a tumor (the so-called hot spot) may be desirable for several reasons. First, it offsets the relative protection offered by the poor oxygenation of the tumor core; second, it increases the cell kill in the tumor cells adjacent to those in the hot spots due to the fact that the effect of a given dose to a population of cells is more damaging if the neighboring cells receive a high dose [5].
Radiosurgical planning and technique with the Gamma Knife
Pre-operative neuro-endocrine evaluation
All patients suspected of harboring a pituitary tumor should undergo a complete neurologic, ophthalmologic, endocrinologic, and radiologic work-up. This includes formal visual field and acuity testing and a dilated fundoscopic examination [2, 6]. Each facet of the hypothalamic-pituitary-end organ axis should be assessed. Mild elevations in serum prolactin commonly result from a stalk effect while levels greater than 200 ng/mL suggest a prolactin-secreting adenoma. Thyroid function is evaluated by measuring free thyroxine and thyroid-stimulating hormone. Adrenal function is assessed by a morning fasting serum cortisol and ACTH level. In cases of suspected Cushing’s syndrome, a 24 h urine free cortisol and a dexamethasone suppression test are performed. Serum GH and insulin-like growth factor-(IGF)-1 levels are measured to evaluate for acromegaly [7]. An oral glucose tolerance test with growth hormone measurements is the definitive test in cases of suspected growth-hormone secreting tumors [8]. In children, X-rays should be obtained to assess bone age in relation to chronologic age (Fig. 2).
Gamma plan software depiction of axial (left panel), coronal (middle panel) and sagittal (right panel) T1-weighted contrast enhanced magnetic resonance imaging in a patient with a residual non-functioning macroadenoma. A margin dose of 18 Gy was given to the 50% isodose line (yellow line). Using blocking and plugging strategies, radiosensitive structures such as the optic nerve (white arrow) can be shielded from the radiation dose
Imaging evaluation of the sellar region is achieved with thin slice pre- and post-contrast magnetic resonance (MR) imaging. Computed tomography (CT)-imaging may be useful in select cases where MR-imaging is not available or is otherwise contra-indicated [9, 10].
If a patient has a neurological deficit attributable to an adenoma, surgery is the initial treatment of choice for all tumors except prolactinomas. Transsphenoidal microsurgery (endoscopic or microscopic) affords the best chance of rapid relief of mass effect and reduction in excessive hormone levels in patients with Cushing’s disease and acromegaly [1, 2, 6, 11–13]. This approach is associated with a low rate of complications in the hands of experienced neurosurgeons [14]. For this reason, tumors located in areas such as the sellar floor or sphenoid that can be safely accessed surgically should undergo surgical removal, with radiosurgery being reserved for tumor residuals or recurrences.
Depending on the size and location of the tumor, one surgical goal may be to create a safe distance of 2 to 5 mm between the lesion and optic apparatus so that an adequate dose can be delivered to the adenoma with minimal risk of radiation injury to the optic pathways.
Temporary cessation of suppressive medications
In 2000, Landolt et al. reported a significantly lower hormone normalization rate in acromegalic patients who were receiving antisecretory medications at the time of radiosurgery [15]. Antisecretory medications likely alter cell cycling and halting them decreases tumor cell radiosensitivity [15, 16]. Since 2000, several other groups have documented a counterproductive effect of antisecretory medications on the rate of hormonal normalization following radiosurgery for patients with acromegaly, and prolactinomas [15, 17, 18]. Although the optimal time period to temporarily halt antisecretory medications is unclear, published studies have indicated that long-acting somatostatin analogues should probably be discontinued 8 weeks before treatment, and resumed 6–8 weeks after radiosurgery if the benefit of radiosurgery is to be optimized [15–19]. Class I evidence is still unavailable to support a temporary cessation of antisecretory medications, and it is possible that the centers that observed improved results in patients off anti-secretory medications were biased by the inclusion of patients with more severe disease who remained on anti-secretory treatment during radiosurgery).
Frame placement for pituitary radiosurgery
We find that patients are more comfortable when the frame is placed in the operating room using monitored anesthesia delivered by an anesthesiologist or nurse anesthetist. Prior to frame placement, the scalp is prepared with alcohol, and the areas of the pin placements are infiltrated with a long-acting local anesthetic. It is generally useful to use the angle of the optic chiasm as the axis of the frame. This angle approximates a line joining the lateral canthus and the top of the pinna, and makes the identification of the optic nerves, chiasm and tract easier by having an image that demonstrates the entire optic apparatus in a single MR-slice [20]. At times angling the plane of the frame base ring away from the axis of the optic pathway can be used to lower the dose to the optic apparatus (Fig. 3).
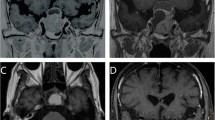
Pre- (left panel) radiosurgical coronal Gadolinium enhanced magnetic resonance imaging of a 52-year old patient with acromegaly. This patient had active acromegaly due to residual cavernous sinus tumor after successful surgical extirpation of a macroadenoma, and received a dose of 24 Gy to the tumor margin. (Right panel) Eighteen months following radiosurgery, the patient had significant reduction in the cavernous sinus residual and achieved endocrine remission
Imaging for pituitary radiosurgery
Regardless of the radiosurgical modality used, stereotactic radiosurgery requires clear and accurate imaging of that target. Advances in imaging over the past 20 years, have increased the efficacy and safety of radiosurgical treatment of pituitary lesions. Modern imaging consists of an MRI sequence consisting of post-contrast, thin-slice (e.g. 1 mm) volume acquisition to define the tumor within the sellar region. In patients with previous surgery, fat suppression techniques can prove useful for differentiating tumor from surgical fat grafts. In the pre-MRI era, CT was used routinely, but is now only used when a patient cannot tolerate an MRI. PET imaging may also be of value for detecting hypersecretory adenomas; such images can be co-registered and overlayed on stereotactic tomographic images (e.g. MR or CT) [21].
Treatment planning
After the acquisition of images with the stereotactic frame, multiple isocenter dose planning is performed to enclose the borders of the tumor within the prescription isodose line [22, 23]. The 50% isodose is the most common prescription isodose line for Gamma knife radiosurgery because this line is generally where the slope of the radiation fall off is the steepest [24]. However, one should analyze the dose gradient and determine the optimal fall off isodose, which generally varies between 40% and 60% [25].
Shielding strategies
Beam blocking plug patterns are often used to shift the prescription isodose curves away from the optic apparatus because of the radiosensitivity of this structure. These “plugging patterns” may be generated manually or by using planning software and are then implemented by replacing collimators in the helmet with solid “plugs.” With the Gamma Knife Perfexion, shielding can be accomplished by closing off any one or combination of eight sectors that comprise the 192 source collimator. Sectors are closed in an automated fashion, and dynamic shielding is applied in a four tiered grading scheme.
The need to use plug patterns can be reduced by adjusting the gamma angle so that the anteroposterior axis of the peripheral isodose curves is parallel to the optic apparatus in the sagittal plane. If the frame is placed parallel to the course of the optic apparatus, then a gamma angle of 70° or 90° may be used. This maneuver takes advantage of the extremely steep fall off of radiation dorsal to the isocenter. Selection of the prescription dose is based partially on the integrated logistic formula as well as specific strategies for protecting the optic apparatus, controlling tumor growth, and establishing and maintaining normal endocrine function [26, 27].
Neuro-anatomical considerations: radiation effects on the optic apparatus, cavernous sinus and pituitary
The mechanisms of radiation injury to the cranial nerves are among the critical determinants of the feasibility of pituitary radiosurgery. Most of this damage is thought to be a result of secondary to damage of small vessels and protective Schwann cells or oligodendroglia. There is a difference in the tolerance of different cranial nerves; with sensory nerves (optic and acoustic) tolerating the least radiation and the nerves in the parasellar region, the facial nerves and the lower cranial nerves tolerating higher doses. This may be due to the fact that both the optic and acoustic nerves are actually fiber tracts of the central nervous system and carriers of much more complex data. Clinical experience suggests that these specialized sensory nerves do not show a great capacity to recover from injury.
The radiosensitivity of the cranial nerves often necessitates limitation in the doses given for tumors and vascular lesions in close relation to these structures. Although the precise dose tolerance of the cranial nerves is unclear, the anterior visual pathways seem to be least radio-resistant, and single doses above 8 Gy should be avoided [28–30]. The distance between optic nerves and chiasm and the lesion being treated should be carefully assessed. A distance of 5 mm between the tumor and the optic apparatus is ideal, but a distance of as little as 2 mm may be acceptable, due to the shielding capabilities of the Gamma Knife. It appears that the risk may be related to the volume of the optic apparatus receiving the dose [31–34]. The tolerable distance is a function of the degree to which a dose plan can be designed to deliver a suitable radiation dose to the tumor yet spare the optic apparatus.
In treating patients with secretory pituitary adenomas, it is not uncommon that cranial nerves in the parasellar region receive doses between 20 Gy to 25 Gy. Tishler et al. [35] noted that the maximum dose delivered to cranial nerves were related to neurologic deficits in 29 patients after LINAC radiosurgery and 33 patients after Gamma Knife surgery (GKRS). Twelve new neuropathies were observed that were related to the nerves in the parasellar region, but they were all unrelated to a maximum dose in the range of 10–40 Gy. The conclusion of this study was that doses up to 40 Gy are relatively safe for nerves in the parasellar region.
In our recently published paper on radiosurgery for Cushing’s disease, four of 10 patients who had repeat GKRS developed visual acuity reduction (two of whom also developed oculomotor nerve palsies). Two additional patients who had previous fractionated radiation also developed oculomotor neuropathies [28]. Three of these six patients ultimately had improvements in their visual deficits.
The majority of cranial nerves in the cavernous sinus appear to be more resistant to radiation effects than the optic nerve, but reports of cranial neuropathy, particularly after repeat radiosurgery are well-documented [28]. Although the tolerable limit to the cavernous sinus nerves is unknown, authors have described effective radiosurgical doses of between 19 Gy and 23 Gy to this region without major side effects [36–41]. Injury to the cavernous segment of the carotid artery is rare after radiosurgery, with a few isolated case reports [42]. In cases where the tumor appears to extend into the cavernous sinus, shielding can be used to reduce radiation to the medial temporal lobe.
Since the systemic effects of secretory adenomas can be so devastating, a margin dose of 25 Gy or 30 Gy may utilized. However, it is not known to what degree a higher margin dose (e.g. 20 Gy vs. 30 Gy) will result in delayed hypopituitarism. In cases of functioning adenomas with imaging defined targets in the cavernous sinus, radiosurgical plans can be devised with higher range treatment doses while shielding much of the normal stalk, gland, and optic apparatus. Nonfunctioning pituitary adenomas appear to require a lower radiosurgery treatment dose than functioning adenomas [32, 43–45]. An effective dose for a nonfunctioning tumor is 13–15 Gy to the margin.
Results of using stereotactic radiosurgery for pituitary adenomas
The goals of stereotactic radiosurgery in the treatment of pituitary tumors are to stop tumor growth, and, in those with secretory adenomas, to reverse hormonal hypersecretion. Moreover, radiosurgery should be carried out in such a way so as to avoid both delayed radiation injury to neural structures in the parasellar regions and secondary tumor formation.
Radiosurgery for pituitary adenomas
Non-secretory tumors
In our experience of 90 patients treated for nonfunctioning tumors (with mean follow-up of 44.9 months), tumor volume decreased in 59 patients (65.6%), remained unchanged in 24 (26.7%), and increased in seven (7.8%) [46]. Eight patients were treated with Gamma Knife as primary therapy, for medical reasons or patient preference, and in these patients, with 34 months mean folow-up, a decrease in tumor volume occurred in three (42%); 5 patients had stabilization of tumor volume (58%). In the 82 patients who were treated for recurrent or residual tumor after surgery, reduction in tumor volume occurred in 56 (68%), no change was detected in 19 (23%), and an increase occurred in seven (8%). The median time to tumor shrinkage on MR-imaging was 9 months (range, 6–48 months) following radiosurgery. This is consistent with a recently published series that demonstrated pituitary adenomas were 90%, 80%, and 70% of their initial volume at 1, 2, and 3 years post-GK radiosurgery [47].
Tumors involving the parasellar space require special consideration, as they would be otherwise untreatable. Of the 61 tumors treated at the University of Virginia that involved the parasellar space, 39 decreased in volume (63%) and 17 remained unchanged (27%).
Most other contemporary series involving stereotactic radiosurgery for non-functioning tumors (Table 1) have demonstrated excellent control of tumor growth, with a mean tumor control rate of 93% (range, 68%–100%) [1, 32, 48–55]. In patients with four or more years of follow-up, the reported mean control rate is 95% (range, 83–100%) [45, 51, 56–61]. Some series have even demonstrated improvement in visual function following radiosurgery after shrinkage of the tumor [31, 61–63]. Nevertheless, prevention of tumor growth, without volume reduction, is still considered a radiosurgical success.
The CyberKnife (Accuray, Calif., USA), is a newer radiosurgical device which is mounted on a maneuverable robotic manipulator and tracks the target with the aid of real-time guidance [64, 65]. Early experience with the Cyberknife has been promising for nonfunctioning adenomas, with a growth control rate of 95%, and lower prescription doses (14–16 Gy) than described for the Gamma Knife, although long-term clinical follow-up is lacking [66].
Radiosurgery for secretory adenomas
Most published results on radiosurgery for secretory adenomas have differed based on methodology, endocrine criteria for remission, the study population and length of follow-up. Most series typically report a higher prescription (margin) dose to patients with functioning adenomas, with a range between 20 Gy and 25 Gy in most reports [17, 28, 55, 67]. Because hormone normalization has been followed by relapse, we prefer the term “remission” to “cure.”
Acromegaly
The most widely accepted guidelines for endocrine remission in acromegaly consist of a GH level less than 1 ng/ml in response to an oral glucose challenge and a normal serum IGF-1 when matched for age [8, 68].
Our recently published experience with radiosurgery involves 135 patients with mean follow-up of 57 months [69]. At least 18 months of follow-up was available on 95 patients, all of whom had failed previous transsphenoidal surgery. The overall remission rate for the entire series was 53% with a mean time to remission of 30 months. Of note was the fact that the remission rate was 59% in patients off suppressive medications compared with 37% in patients receiving a suppressive medication (most commonly octreotide) at the time of GKRS treatment. No substantial difference in the margin dose (22 Gy for both groups) was present between the two groups. As a result of this finding, we currently recommend a cessation of somatostatin analog medication 8 weeks before and for 8 weeks after GKRS.
Published remission rates following radiosurgery for acromegaly vary widely from 0% to 100%, with the majority of patients achieving tumor growth control (Table 2) [15, 18, 19, 34, 70–74]. Jezkova et al. reported a remission rate of 50% at 42 months follow-up in 96patients with acromegaly who received radiosurgery [75]. Nearly one-third of these patients, however, had radiosurgery as primary treatment, without surgical extirpation of the adenoma. Pollock et al. [18] demonstrated a remission rate of 50% in 46 patients with a higher remission rate in patients who were off suppressive medications at the time of radiosurgery.
Cushing’s disease
Cushing’s disease is one of the most devastating pituitary disorders, and is associated with significant morbidity and premature death. Even after transsphenoidal surgery, up to 30% of patients may have persistent o recurrent disease [2, 14, 43]. Most centers define an endocrine remission as a UFC in the normal range associated with the resolution of clinical stigmata or a series of normal post-operative serum cortisol levels obtained throughout the day [7, 33].
We have treated 107 patients with Cushing’s disease, with 53% of patients achieving normalization of 24 h UFC-levels with a mean margin dose of 23 Gy. The rate of remission statistically correlated with tumor volume, but not with tumor invasion into the cavernous sinus or the suprasellar region [28].
In our experience, the rate of hormone normalization after radiosurgery for Cushing’s disease is difficult to predict, with remission occurring as early as 2 months and as late as 8 years after GKRS [56, 63]. Most patients who have remission however, will do so within the first 2 years following radiosurgery. Patients with persistent disease should thus consider alternative treatments such as adrenalectomy, or repeat radiosurgery (although this may be associated with a higher rate of cranial nerve damage) [28]. (Fig. 4).
Published endocrine remission rates following radiosurgery (Table 3) vary considerably, from 10% to 100%, with higher remission rates when radiosurgery follows surgical debulking [28, 34, 49, 50, 58, 76–79]. In series with at least ten patients and a median follow-up of 2 years, endocrine remission rates range from 17% to 83% [58, 78–80]. Rähn and associates (10,27) reported their experience at the Karolinska Institute involving 59 patients with Cushing’s disease who were treated using the Gamma Knife and followed for 2–15 years. The efficacy rate of the initial treatment was 50%, with retreatment eventually providing normalization of cortisol production in 76% of patients [81].
Prolactin-secreting adenomas
We use GKRS as a treatment for prolactinomas after failure of medical and/or surgical treatment. Of the 23 patients treated at our institution, complete normalization of prolactin levels occurred in 26%, at an average time of 24.5 months, with a mean prescription dose of 19 Gy [17]. Consistent with the work of Landolt and Lomax, we also found that the remission rate was lower in patients receiving an anti-secretory medication at the time of GKRS [16, 17].
In published studies of radiosurgery for prolactinomas, the mean prescription dose has varied from 13.3 Gy to 33 Gy, and remission rates varied from 0% to 84% (Table 4) [1, 16, 17, 55, 67, 82, 83]. Variations in success rate are likely related to the dose delivered to the tumor as well as other factors. Witt et al. noted no remissions with a prescription dose of 19 Gy [34, 84]. Pan et al. [85] reported a 52% endocrine “cure” rate in a retrospective study of 128 patients in whom GKRS was used as first-line treatment for prolactinomas with a prescription dose of 30 Gy. This study is on a large sample size, and is interesting in that GKRS was used as a first-line treatment before medical therapy [86].
Nelson’s syndrome
A subset of Cushing’s patients do not achieve hormone normalization following microsurgery and radiosurgery, and require adrenalectomy as a “salvage” treatment for their disease. Although adrenalectomy is the definitive treatment for cortisol overproduction, a subset of patients may develop Nelson’s syndrome, characterized by rapid adenoma growth, hyperpigmentation and tumor invasion into the parasellar structures [87] (Table 5).
Our experience involves 23 patients with at least 6 months follow-up. Thirty percent of patients had a reduction in tumor size, and 60% had no change in size. Decrease in ACTH levels occurred in 67% of patients with elevated level before GKRS, but normalization only occurred in four patients [88]. Pollock and Young reported on 11 patients who underwent GKRS for Nelson’s syndrome. They reported control of tumor growth in 9 of 11 patients, with ACTH normalization in four patients (36%) [89].
There are relatively few studies detailing the results of radiosurgery for Nelson’s syndrome [1, 52, 58, 88–92]. These studies report a mean tumor dose from between 12 Gy to 28.7 Gy, and an endocrine remission rate ranging from 0% to 36%, although only a minority of these studies defined what was meant by endocrine remission. Even cases where endocrine remission was not achieved, tumor growth control rates were favorable, ranging from 82% to 100%.
Complications following radiosurgery for pituitary adenomas
The most common problem after radiosurgery is new found hypopituitarism. Well respected groups have reported a low incidence (0–36%) of pituitary dysfunction following radiosurgery [28, 36, 93, 94]. This incidence is likely higher when patients are followed long-term, with the Karolinska Institute reporting a 72% incidence of hypopituitarism when patients were followed longer than 10 years [56]. We have observed an overall risk of 20–30% for development of new hormone deficiency following radiosurgery without a significant difference across tumor pathologies. Recent studies using the Cyberknife for secretory adenomas, points to a significantly lower (9.5%) rate of endocrinopathy, although these studies are limited by follow-up of 12 months and less in some cases [66, 95, 96].
Ultimately, total dose prescribed and the prescription (margin) dose are likely the major factors determining the risk and onset of radiation induced hypopituitarism. Ultimately the sequence of hormone loss following pituitary radiosurgery are unknown, and little in the way of solid data supports the radiosensitivity. In spite of this however, some have argued that the GH axis is the most sensitive to the late effects of radiation, with the radiation induced defect likely occurring at the hypothalamic level [97, 98]. The gonadotropin and corticotrophin axes are also thought to be sensitive to radiation damage. Diabetes insipidus appears to be uncommon after radiosurgery with only sporadic case reports [99].
Cranial neuropathies following Gamma Knife surgery are exceedingly rare following the first procedure, although the incidence may increase on re-treatment [28]. Visual injury in general can be avoided if the dose to the optic apparatus is restricted to less than 8 Gy (see discussion above).
Injury to the cavernous segment of the carotid artery or brain parenchymal is uncommon following radiosurgery. The exact incidence of radiosurgical induced neoplasms is unknown at present, although we have not seen one in our series of pituitary patients treated with Gamma Knife. Induction of cavernous malformations following radiosurgery to the sellar region is also theoretically possible but thus far has not been reported.
Loeffler and colleagues recently reported on 6 patients, including 2 patients with pituitary adenomas who developed new tumors following radiosurgery [100]. They concluded that although the risk of new tumor formation after radiosurgery appears to be significantly less than that seen following fractionated radiotherapy, new tumors can develop in the full dose region as well as in the low-dose periphery of the radiosurgical field. The latency to new tumor formation in this small series (between 6 years and 20 years) was similar to that seen after conventional radiation therapy.
Prognosis and follow-up
Prognosis for pituitary adenoma patients is largely dependent upon the adenoma size and functionality as well as the patients’ pre-radiosurgical status. Patients being treated for pituitary adenomas must be followed long-term with serial clinical, ophthalmological, endocrine and radiological evaluations. In particular, height, weight and pubertal status must be carefully monitored in relevant age groups.
Serial visual field examinations and screening should be performed with questioning directed at assessing hormone function. In some cases serial testing of adrenal, thyroid function and GH status may be required as well. Patients receiving hormone replacement should have their replacement therapy adjusted as necessary.
Finally, serial MR imaging should be performed to assess for tumor recurrence. It is our practice to perform an initial post-radiosurgical MRI at 6 months after treatment with follow-up MRI’s yearly thereafter, unless otherwise indicated. Endocrine and ophthalmologic follow-up should typically occur at these same time points after discussion with the endocrinology team.
Conclusions
Stereotactic radiosurgery is useful in the treatment of both secretory and non-secretory pituitary adenomas. In most patients, radiosurgery controls adenoma growth. However, normalization of hormone overproduction can vary considerably depending on the patients’ presenting condition. Challenges for the future include delineating the optimal timing for the administration of antisecretory medications and identifying factors that can improve the response of adenomas to radiosurgery.
References
Laws ER Jr, Vance ML (1999) Radiosurgery for pituitary tumors and craniopharyngiomas. Neurosurg Clin N Am 10:327–336
Laws ER, Sheehan JP (2006) Pituitary surgery: a modern approach. Karger, Basel
Kovács K, Horvath E, Universities Associated for Research and Education in Pathology (1986) Tumors of the pituitary gland. Armed Forces Institute of Pathology: For sale by the Armed Forces Institute of Pathology, Washington, D.C., 20306-6000
Lyman JT, Phillips MH, Frankel KA, Levy RP, Fabrikant JI (1992) Radiation physics for particle beam radiosurgery. Neurosurg Clin N Am 3:1–8
Hopewell JW, Wright EA (1970) The nature of latent cerebral irradiation damage and its modification by hypertension. Br J Radiol 43:161–167
Laws ER Jr, Thapar K (1996) Recurrent pituitary adenomas. In: Landolt AM, Vance ML, Reilly PL (eds) Pituitary adenomas. Churchill-Livingtone, Edinburgh, pp 385–394
Nieman LK (2002) Medical therapy of Cushing’s disease. Pituitary 5:77–82. doi:10.1023/A:1022308429992
Vance ML (1998) Endocrinological evaluation of acromegaly. J Neurosurg 89:499–500
Kanter AS, Diallo AO, Jane JA Jr, Sheehan JP, Asthagiri AR, Oskouian RJ, Okonkwo DO, Sansur CA, Vance ML, Rogol AD, Laws ER Jr (2005) Single-center experience with pediatric Cushing’s disease. J Neurosurg 103:413–420
Jagannathan J, Dumont AS, Jane JA Jr, Laws ER Jr (2005) Pediatric sellar tumors: diagnostic procedures and management. Neurosurg Focus 18:6. doi:10.3171/foc.2005.18.6.7
Laws ER Jr, Ebersold MJ, Piepgras DG, Randall RV, Salassa RM (1985) The results of transsphenoidal surgery in specific clinical entities. In: Laws ER Jr, Randall RV, Kern EB et al (eds) Management of pituitary adenomas and related lesions with emphasis on transsphenoidal microsurgery. Appleton-Century-Crofts, New York, pp 277–305
Laws ER Jr, Fode NC, Redmond MJ (1985) Transsphenoidal surgery following unsuccessful prior therapy. An assessment of benefits and risks in 158 patients. J Neurosurg 63:823–829
Laws ER, Vance ML, Thapar K (2000) Pituitary surgery for the management of acromegaly. Horm Res 53(Suppl 3):71–75. doi:10.1159/000023538
Ciric I, Ragin A, Baumgartner C, Pierce D (1997) Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40:225–236 discussion 236–227
Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, Wellis G (2000) Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab 85:1287–1289. doi:10.1210/jc.85.3.1287
Landolt AM, Lomax N (2000) Gamma knife radiosurgery for prolactinomas. J Neurosurg 93(Suppl 3):14–18
Pouratian N, Sheehan J, Jagannathan J, Laws ER Jr, Steiner L, Vance ML (2006) Gamma knife radiosurgery for medically and surgically refractory prolactinomas. Neurosurgery 59:255–266 discussion 255–266
Pollock BE, Jacob JT, Brown PD, Nippoldt TB (2007) Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg 106:833–838. doi:10.3171/jns.2007.106.5.833
Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, Wellis G (1998) Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg 88:1002–1008
Hayashi M, Taira T, Chernov M, Fukuoka S, Liscak R, Yu CP, Ho RT, Regis J, Katayama Y, Kawakami Y, Hori T (2002) Gamma knife surgery for cancer pain-pituitary gland-stalk ablation: a multicenter prospective protocol since 2002. J Neurosurg 97:433–437
Levivier M, Massager N, Wikler D, Devriendt D, Goldman S (2007) Integration of functional imaging in radiosurgery: the example of PET scan. Prog Neurol Surg 20:68–81. doi:10.1159/000100096
Alexander E 3rd, Loeffler JS (1992) Radiosurgery using a modified linear accelerator. Neurosurg Clin N Am 3:167–190
Flickinger JC, Lunsford LD, Wu A, Maitz AH, Kalend AM (1990) Treatment planning for gamma knife radiosurgery with multiple isocenters. Int J Radiat Oncol Biol Phys 18:1495–1501
Wu A, Lindner G, Maitz AH, Kalend AM, Lunsford LD, Flickinger JC, Bloomer WD (1990) Physics of gamma knife approach on convergent beams in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 18:941–949
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl 3):219–222
Flickinger JC (1989) An integrated logistic formula for prediction of complications from radiosurgery. Int J Radiat Oncol Biol Phys 17:879–885
Flickinger JC, Lunsford LD, Kondziolka D (1992) Dose prescription and dose-volume effects in radiosurgery. Neurosurg Clin N Am 3:51–59
Jagannathan J, Sheehan JP, Pouratian N, Laws ER, Steiner L, Vance ML (2007) Gamma knife surgery for Cushing’s disease. J Neurosurg 106:980–987. doi:10.3171/jns.2007.106.6.980
Leber KA, Bergloff J, Langmann G, Mokry M, Schrottner O, Pendl G (1995) Radiation sensitivity of visual and oculomotor pathways. Stereotact Funct Neurosurg 64(Suppl 1):233–238
Leber KA, Bergloff J, Pendl G (1998) Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88:43–50
Chen JC, Giannotta SL, Yu C, Petrovich Z, Levy ML, Apuzzo ML (2001) Radiosurgical management of benign cavernous sinus tumors: dose profiles and acute complications. Neurosurgery 48:1022–1030 discussion 1030–1022
Lim YL, Leem W, Kim TS, Rhee BA, Kim GK (1998) Four years’ experiences in the treatment of pituitary adenomas with gamma knife radiosurgery. Stereotact Funct Neurosurg 70(Suppl 1):95–109. doi:10.1159/000056412
Sheehan JM, Vance ML, Sheehan JP, Ellegala DB, Laws ER Jr (2000) Radiosurgery for Cushing’s disease after failed transsphenoidal surgery. J Neurosurg 93:738–742
Witt TC, Kondziolka D, Flickinger JC, Lunsford LD (1998) Gamma knife radiosurgery for pituitary tumors. In: Lunsford LD, Kondziolka D, Flickinger J (eds) Gamma knife brain surgery progress in neurological surgery. Karger, Basel, pp 114–127
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E 3rd, Kooy HM, Flickinger JC (1993) Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys 27:215–221
Sheehan JP, Jagannathan J, Pouratian N, Steiner L (2006) Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res 34:185–205. doi:10.1159/000091581
Sheehan JP, Kondziolka D, Flickinger J, Lunsford LD (2002) Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg 97:408–414
Liu AL, Wang C, Sun S, Wang M, Liu P (2005) Gamma knife radiosurgery for tumors involving the cavernous sinus. Stereotact Funct Neurosurg 83:45–51. doi:10.1159/000085544
Kuo JS, Chen JC, Yu C, Zelman V, Giannotta SL, Petrovich Z, MacPherson D, Apuzzo ML (2004) Gamma knife radiosurgery for benign cavernous sinus tumors: quantitative analysis of treatment outcomes. Neurosurgery 54:1385–1393 discussion 1393–1384
Peker S, Kilic T, Sengoz M, Pamir MN (2004) Radiosurgical treatment of cavernous sinus cavernous haemangiomas. Acta Neurochir (Wien) 146:337–341 discussion 340
Nakamura N, Shin M, Tago M, Terahara A, Kurita H, Nakagawa K, Ohtomo K (2002) Gamma knife radiosurgery for cavernous hemangiomas in the cavernous sinus. Report of three cases. J Neurosurg 97:477–480
Lim YJ, Leem W, Park JT, Kim TS, Rhee BA, Kim GK (1999) Cerebral infarction with ICA occlusion after Gamma Knife radiosurgery for pituitary adenoma: a case report. Stereotact Funct Neurosurg 72(Suppl 1):132–139. doi:10.1159/000056449
Mampalam TJ, Tyrrell JB, Wilson CB (1988) Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med 109:487–493
Martinez R, Bravo G, Burzaco J, Rey G (1998) Pituitary tumors and gamma knife surgery. Clinical experience with more than two years of follow-up. Stereotact Funct Neurosurg 70(Suppl 1):110–118. doi:10.1159/000056413
Shin M, Kurita H, Sasaki T, Tago M, Morita A, Ueki K, Kirino T (2000) Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg 93(Suppl 3):2–5
Mingione V, Yen CP, Vance ML, Steiner M, Sheehan J, Laws ER, Steiner L (2006) Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg 104:876–883. doi:10.3171/jns.2006.104.6.876
Pamir MN, Peker S (2006) Microvascular decompression for trigeminal neuralgia: a long-term follow-up study. Minim Invasive Neurosurg 49:342–346. doi:10.1055/s-2006-960487
Inoue HK, Kohga H, Hirato M, Sasaki T, Ishihara J, Shibazaki T, Ohye C, Andou Y (1999) Pituitary adenomas treated by microsurgery with or without Gamma Knife surgery: experience in 122 cases. Stereotact Funct Neurosurg 72(Suppl 1):125–131. doi:10.1159/000056448
Izawa M, Hayashi M, Nakaya K, Satoh H, Ochiai T, Hori T, Takakura K (2000) Gamma knife radiosurgery for pituitary adenomas. J Neurosurg 93(Suppl 3):19–22
Jackson IM, Noren G (1999) Gamma knife radiosurgery for pituitary tumours. Best Pract Res Clin Endocrinol Metab 13:461–469. doi:10.1053/beem.1999.0033
Feigl GC, Bonelli CM, Berghold A, Mokry M (2002) Effects of gamma knife radiosurgery of pituitary adenomas on pituitary function. J Neurosurg 97:415–421
Ganz JC, Backlund EO, Thorsen FA (1993) The effects of Gamma Knife surgery of pituitary adenomas on tumor growth and endocrinopathies. Stereotact Funct Neurosurg 61(Suppl 1):30–37. doi:10.1159/000100656
Hayashi M, Izawa M, Hiyama H, Nakamura S, Atsuchi S, Sato H, Nakaya K, Sasaki K, Ochiai T, Kubo O, Hori T, Takakura K (1999) Gamma Knife radiosurgery for pituitary adenomas. Stereotact Funct Neurosurg 72(Suppl 1):111–118. doi:10.1159/000056446
Losa M, Valle M, Mortini P, Franzin A, da Passano CF, Cenzato M, Bianchi S, Picozzi P, Giovanelli M (2004) Gamma knife surgery for treatment of residual nonfunctioning pituitary adenomas after surgical debulking. J Neurosurg 100:438–444
Kim SH, Huh R, Chang JW, Park YG, Chung SS (1999) Gamma Knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg 72(Suppl 1):101–110. doi:10.1159/000056445
Hoybye C, Grenback E, Rahn T, Degerblad M, Thoren M, Hulting AL (2001) Adrenocorticotropic hormone-producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery 49:284–291 discussion 291–282
Ikeda H, Jokura H, Yoshimoto T (2001) Transsphenoidal surgery and adjuvant gamma knife treatment for growth hormone-secreting pituitary adenoma. J Neurosurg 95:285–291
Kobayashi T, Kida Y, Mori Y (2002) Gamma knife radiosurgery in the treatment of Cushing disease: long-term results. J Neurosurg 97:422–428
Mokry M, Ramschak-Schwarzer S, Simbrunner J, Ganz JC, Pendl G (1999) A six year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg 72(Suppl 1):88–100. doi:10.1159/000056444
Wowra B, Stummer W (2002) Efficacy of gamma knife radiosurgery for nonfunctioning pituitary adenomas: a quantitative follow up with magnetic resonance imaging-based volumetric analysis. J Neurosurg 97:429–432
Yoon SC, Suh TS, Jang HS, Chung SM, Kim YS, Ryu MR, Choi KH, Son HY, Kim MC, Shinn KS (1998) Clinical results of 24 pituitary macroadenomas with linac-based stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 41:849–853. doi:10.1016/S0360-3016(98)00124-2
Abe T, Yamamoto M, Taniyama M, Tanioka D, Izumiyama H, Matsumoto K (2002) Early palliation of oculomotor nerve palsy following gamma knife radiosurgery for pituitary adenoma. Eur Neurol 47:61–63. doi:10.1159/000047951
Hayashi M, Taira T, Ochiai T, Chernov M, Takasu Y, Izawa M, Kouyama N, Tomida M, Tokumaru O, Katayama Y, Kawakami Y, Hori T, Takakura K (2005) Gamma knife surgery of the pituitary: new treatment for thalamic pain syndrome. J Neurosurg 102:38–41 Suppl
Chang SD, Murphy M, Geis P, Martin DP, Hancock SL, Doty JR, Adler JR Jr (1998) Clinical experience with image-guided robotic radiosurgery (the Cyberknife) in the treatment of brain and spinal cord tumors. Neurol Med Chir (Tokyo) 38:780–783. doi:10.2176/nmc.38.780
Adler JR Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL (1997) The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg 69:124–128. doi:10.1159/000099863
Kajiwara K, Saito K, Yoshikawa K, Kato S, Akimura T, Nomura S, Ishihara H, Suzuki M (2005) Image-guided stereotactic radiosurgery with the CyberKnife for pituitary adenomas. Minim Invasive Neurosurg 48:91–96. doi:10.1055/s-2004-830261
Kim MS, Lee SI, Sim JH (1999) Gamma Knife radiosurgery for functioning pituitary microadenoma. Stereotact Funct Neurosurg 72(Suppl 1):119–124. doi:10.1159/000056447
Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Veldhuis J, Wass J, Von Werder K, Melmed S (2000) Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 85:526–529. doi:10.1210/jc.85.2.526
Jagannathan JSJ, Pouratien N, Laws ER Jr, Steiner L, Vance ML (2008) Gamma Knife radiosurgery for acromegaly: outcomes after failed transsphenoidal surgery. Neurosurgery 62:1262–1270
Buchfelder M, Fahlbusch R, Schott W, Honegger J (1991) Long-term follow-up results in hormonally active pituitary adenomas after primary successful transsphenoidal surgery. Acta Neurochir Suppl (Wien) 53:72–76
Cozzi R, Barausse M, Asnaghi D, Dallabonzana D, Lodrini S, Attanasio R (2001) Failure of radiotherapy in acromegaly. Eur J Endocrinol 145:717–726. doi:10.1530/eje.0.1450717
Freda PU (2003) How effective are current therapies for acromegaly? Growth Horm IGF Res 13(Suppl A):S144–S151
Fukuoka S, Ito T, Takanashi M, Hojo A, Nakamura H (2001) Gamma knife radiosurgery for growth hormone-secreting pituitary adenomas invading the cavernous sinus. Stereotact Funct Neurosurg 76:213–217. doi:10.1159/000066721
Horvath E, Kovacs K, Scheithauer BW, Randall RV, Laws ER Jr, Thorner MO, Tindall GT, Barrow DL (1983) Pituitary adenomas producing growth hormone, prolactin, and one or more glycoprotein hormones: a histologic, immunohistochemical, and ultrastructural study of four surgically removed tumors. Ultrastruct Pathol 5:171–183. doi:10.3109/01913128309141837
Jezkova J, Marek J, Hana V, Krsek M, Weiss V, Vladyka V, Lisak R, Vymazal J, Pecen L (2006) Gamma knife radiosurgery for acromegaly – long-term experience. Clin Endocrinol (Oxf) 64:588–595. doi:10.1111/j.1365-2265.2006.02513.x
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602. doi:10.1210/jc.2003-030871
Chu JW, Matthias DF, Belanoff J, Schatzberg A, Hoffman AR, Feldman D (2001) Successful long-term treatment of refractory Cushing’s disease with high-dose mifepristone (RU 486). J Clin Endocrinol Metab 86:3568–3573. doi:10.1210/jc.86.8.3568
Morange-Ramos I, Regis J, Dufour H, Andrieu JM, Grisoli F, Jaquet P, Peragut JC (1998) Gamma-knife surgery for secreting pituitary adenomas. Acta Neurochir (Wien) 140:437–443. doi:10.1007/s007010050121
Petrovich Z, Yu C, Giannotta SL, Zee CS, Apuzzo ML (2003) Gamma knife radiosurgery for pituitary adenoma: early results. Neurosurgery 53:51–59 discussion 59–61
Mahmoud-Ahmed AS, Suh JH (2002) Radiation therapy for Cushing’s disease: a review. Pituitary 5:175–180. doi:10.1023/A:1023365200437
Rahn T, Thoren M, Hall K, Backlund EO (1980) Stereotactic radiosurgery in Cushing’s syndrome: acute radiation effects. Surg Neurol 14:85–92
Post KD, Habas JE (1990) Comparison of long term results between prolactin secreting adenomas and ACTH secreting adenomas. Can J Neurol Sci 17:74–77
Yildiz F, Zorlu F, Erbas T, Atahan L (1999) Radiotherapy in the management of giant pituitary adenomas. Radiother Oncol 52:233–237. doi:10.1016/S0167-8140(99)00098-5
Witt TC (2003) Stereotactic radiosurgery for pituitary tumors. Neurosurg Focus 14:e10. doi:10.3171/foc.2003.14.5.11
Pan L, Zhang N, Wang EM, Wang BJ, Dai JZ, Cai PW (2000) Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg 93(Suppl 3):10–13
Molitch ME (1992) Pathologic hyperprolactinemia. Endocrinol Metab Clin North Am 21:877–901
Nagesser SK, van Seters AP, Kievit J, Hermans J, Krans HM, van de Velde CJ (2000) Long-term results of total adrenalectomy for Cushing’s disease. World J Surg 24:108–113. doi:10.1007/s002689910020
Mauermann WJ, Sheehan JP, Chernavvsky DR, Laws ER, Steiner L, Vance ML (2007) Gamma Knife surgery for adrenocorticotropic hormone-producing pituitary adenomas after bilateral adrenalectomy. J Neurosurg 106:988–993. doi:10.3171/jns.2007.106.6.988
Pollock BE, Young WF Jr (2002) Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int J Radiat Oncol Biol Phys 54:839–841. doi:10.1016/S0360-3016(02)02975-9
Wolffenbuttel BH, Kitz K, Beuls EM (1998) Beneficial gamma-knife radiosurgery in a patient with Nelson’s syndrome. Clin Neurol Neurosurg 100:60–63. doi:10.1016/S0303-8467(97)00124-8
Levy RP, Fabrikant JI, Frankel KA, Phillips MH, Lyman JT, Lawrence JH, Tobias CA (1991) Heavy-charged-particle radiosurgery of the pituitary gland: clinical results of 840 patients. Stereotact Funct Neurosurg 57:22–35. doi:10.1159/000099553
Ganz JC (2002) Gamma knife radiosurgery and its possible relationship to malignancy: a review. J Neurosurg 97:644–652
Pollock BE, Kondziolka D, Lunsford LD, Flickinger JC (1994) Stereotactic radiosurgery for pituitary adenomas: imaging, visual and endocrine results. Acta Neurochir Suppl (Wien) 62:33–38
Jane JA Jr, Vance ML, Woodburn CJ, Laws ER Jr (2003) Stereotactic radiosurgery for hypersecreting pituitary tumors: part of a multimodality approach. Neurosurg Focus 14:e12. doi:10.3171/foc.2003.14.5.13
Adler JR Jr, Gibbs IC, Puataweepong P, Chang SD (2006) Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery 59:244–254 discussion 244–254
Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler JR Jr (2004) Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery 54:799–810 discussion 810–812
Shalet SM (1993) Radiation and pituitary dysfunction. N Engl J Med 328:131–133. doi:10.1056/NEJM199301143280211
Blacklay A, Grossman A, Ross RJ, Savage MO, Davies PS, Plowman PN, Coy DH, Besser GM (1986) Cranial irradiation for cerebral and nasopharyngeal tumours in children: evidence for the production of a hypothalamic defect in growth hormone release. J Endocrinol 108:25–29
Piedra MP, Brown PD, Carpenter PC, Link MJ (2004) Resolution of diabetes insipidus following gamma knife surgery for a solitary metastasis to the pituitary stalk. Case report. J Neurosurg 101:1053–1056
Loeffler JS, Niemierko A, Chapman PH (2003) Second tumors after radiosurgery: tip of the iceberg or a bump in the road? Neurosurgery 52:1436–1440 discussion 1440–1432
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jagannathan, J., Yen, CP., Pouratian, N. et al. Stereotactic radiosurgery for pituitary adenomas: a comprehensive review of indications, techniques and long-term results using the Gamma Knife. J Neurooncol 92, 345–356 (2009). https://doi.org/10.1007/s11060-009-9832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9832-5