Abstract
Upfront temozolomide (TMZ) is often proposed for elderly patients with malignant gliomas as an alternative to radiotherapy (RT). A recent randomized trial showed that RT provides a survival benefit in elderly glioblastoma patients (≥70 years) with good performance status (KPS ≥ 70) compared with supportive care alone (median survival (MS) = 29.1 vs. 16.9 weeks). We retrospectively analyzed all patients who were eligible for this trial, but who refused to participate and were finally treated with TMZ alone. Thirty-nine eligible patients (median age: 75 years (range 70–83), median KPS: 70 (range 70–80), histologically proven glioblastomas) were treated up-front with oral TMZ for 1–12 cycles (mean = 5). One complete response and 10 partial responses were observed. Overall median survival (MS) was 36 weeks and median progression-free survival (PFS) was 20 weeks for the whole group. MS was 27.4 weeks and PFS was 19.5 weeks for the 27 patients that did not receive second-line treatment at progression. Eight grade III/IV toxicities (seven hematologic, one gastro-intestinal) were seen, but no treatment-related deaths were observed. These preliminary results support further randomized studies comparing TMZ with RT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite the increased incidence of malignant gliomas in the elderly over the last two decades [1], the standard of care for these patients was nonexistent until recently, in part because of very poor expected survival (4–6 months) [2, 3]. A recent phase III trial showed that addition of radiotherapy to supportive care prolongs survival of elderly patients (≥70 years) with glioblastoma and a good performance status (KPS ≥ 70) and reported no deleterious effect on quality of life or cognitive function, establishing a new standard of care in this population [4].
Because upfront temozolomide (TMZ) is sometimes proposed for elderly patients as an alternative to radiotherapy [5, 6], we decided to review a cohort of eligible patients who were treated with TMZ alone because they refused to participate to the radiotherapy trial.
Methods
Patients
We retrospectively reviewed all patients from four institutions (Lyon, Marseille, Nancy, Paris) who met the inclusion criteria for the NCT00430911 trial (age ≥ 70 years, histologically proven, newly diagnosed WHO GBM and Karnofsky performance score (KPS) ≥ 70) who were proposed for participation in the study but refused and received upfront chemotherapy with TMZ. This study was permitted by the ethical committee of the Pitié-Salpêtrière Hospital.
Treatment
After surgery, patients were treated with temozolomide mostly with the conventional regimen of 150–200 mg/m2 for five days every four weeks, except patients from Marseille who received an adjusted dose-intensity regimen (150–200 mg/m2 temozolomide for 6, 7 or 8 days every 28 days) [7]. Response was estimated according to the McDonald criteria [8]. Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.
Histology and MGMT detection
Histological analysis was performed by a local pathologist according to the WHO classification.
MGMT (O6-methylguanine–DNA methyltransferase) immunohistochemistry was performed as previously described [9]. Briefly, tissue sections were incubated overnight with an anti-MGMT mouse monoclonal antibody (470P803C; 200 μg/ml) diluted 1:40 in PBS. Isolated cytoplasmic or granular nuclear reactivity were not considered positive. We chose the threshold of 10% of positive cell nuclei [10] to discriminate low and high MGMT expression.
Statistical analysis
OS was defined as the time from histological diagnosis until death or last follow-up. PFS was defined as the time from histological diagnosis until tumor progression or last follow-up. The log rank test was used to test for equality of OS and PFS distribution as estimated by the Kaplan–Meier method. Two-sided P values less than 0.05 were considered significant.
Results
Patients
Patient’s characteristics are reported in Table 1. From February 2001 to January 2005, 39 eligible patients who declined to participate to the NCT00430911 trial were treated up-front with oral TMZ. The median age was 75 years (range 70–83) and the median KPS was 70 (range 70–90). Glioblastoma was histologically proven in all cases. Twenty-one of the 39 patients (54%) had a biopsy, 18 (46%) had undergone surgery with partial resection in 14 cases (36%) and total debulking in four cases (10%). The median time between surgery and temozolomide initiation was 28 days (range: 11–58 days). In cases of tumor resection, evaluation of the measurable residual disease was assessed by means of a new MRI. The patient population was very similar to the randomized trial population in terms of age (median 75 years (range 70–83), vs. 75 years (range 70–84)), performance status (median 70 (range 70–90), vs. 75 (range 70–90)), and extent of resection (50% vs. 48%).
Treatment
Patients received 1 to 12 TMZ cycles (median = 5); initially, all patients received the planned five days regimen (150–200 mg/m2) every four weeks. Fourteen patients (all in Marseille center) were switched to an adjusted dose-intensity regimen over 6–8 days from cycle 2 or later. Patients received 1 to 12 cycles of chemotherapy (mean = 5). Treatment was discontinued because of disease progression in all cases except two because of toxicities (see above). Seven grade III/IV hematological toxicities (18%), one grade II cutaneous toxicity (2%), and one grade IV gastro-intestinal toxicity were observed (2%). No treatment-related death was observed. After disease progression, supportive care was given in 27 cases (69%), eight patients received second-line chemotherapy (seven went on a nitrosourea-based regimen and one on vepeside), three patients had radiotherapy, and one patient was re-operated upon.
Survival and progression
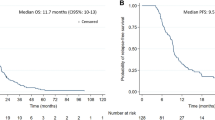
One complete response (2%), ten partial responses (26%) (Figs. 1, 2), 12 stable diseases (31%), and 13 progressive diseases (33%) were observed. The radiographic responses were confirmed on follow-up MRI scans performed 1–2 months later, according to the McDonald criteria. The dose of corticosteroids remained stable or decreased in these patients. Three patients could not be evaluated for response. At the time of analysis (December 2008), 37 patients were dead, and two patients were lost for follow-up. Overall median survival (MS) was 36 weeks and median progression-free survival (PFS) was 20 weeks. PFS and OS were not related to KPS (KPS = 70 or KPS > 70), age (<76 vs. >76 years) or extent of surgery (biopsy vs. tumor removal), but were related to response (MS = 54 weeks for responders vs. 29.8 for patients with SD and 12.4 for patients with progressive disease; P < 0.03; median PFS = 38.8 weeks for responders vs. 20 for patients with SD and 7.7 for patients with progressive disease; P < 10−4).
When only considering the 27 patients who did not receive second-line therapy at recurrence, MS was 27.4 weeks and PFS was 19.5 weeks.
MGMT analysis
Twenty-eight of 39 tumor samples were available for MGMT analysis. MGMT immunohistochemistry was negative in 15 patients and positive in the other 13 (range: 10–60%). No correlation was found between MGMT status and OS or PFS in our population and between MGMT status and response rate or disease control rate.
Discussion
Radiotherapy has been shown to prolong survival of elderly GBM patients with good performance status, but the benefit remains modest [4]. Oral temozolomide is a well-tolerated treatment that has been shown in retrospective studies to induce objective responses and improve neurologic status in up to 50% of elderly patients [6]. Therefore, upfront temozolomide has been considered by many authors as a valuable alternative to RT. However, to date no comparative study has been performed and a phase III randomized study remains warranted. Despite the limits and bias of retrospective studies, it is worth noting that our patient population is clinically quite similar to the randomized trial population in terms of age, performance status, and extent of resection; moreover, the survival of our TMZ-treated patients who did not receive second-line chemotherapy (27.4 weeks) was in the range of the RT arm of the NCT00430911 trial (29 weeks) [4].
We found that MGMT expression was not predictive of outcome. MGMT inactivation makes the cell more sensitive to alkylating agents, and by promoting genetic instability and mutations, it may also have a negative prognostic impact [11–13]. In addition, the most reliable technique for MGMT testing remains a controversial issue. Furthermore, the limited number of tested patients may explain why MGMT expression was not predictive of outcome in our study.
In conclusion, chemotherapy with oral TMZ is an attractive alternative to radiotherapy. Other therapeutic options have been proposed for elderly patients: an abbreviated course of radiotherapy (40 Gy over 3 weeks) as an alternative to standard therapy [14, 15] or radiation and concomitant TMZ [14–17]. Despite more favorable OS (median ranging from 10.6 to 13.7 months) and PFS (median ranging from 7 to 9.5 months), substantial deterioration of mental status was observed with this modality. Another option that could be discussed is therapeutic abstention, which depends on the poor performance status of the patient and their wishes.
These results support randomized studies comparing these different therapeutic modalities that are currently under investigation. The “Nordic trial” is a randomized trial conducted by the Nordic Clinical Brain Tumor Study Group evaluating RT alone with TMZ chemotherapy alone in elderly patients. An NCIC (National Cancer Institute of Canada)—EORTC prospective randomized trial is also ongoing, studying hypofractionated RT in comparison with the same RT plus temozolomide.
References
Fleury A, Menegoz F, Grosclaude P, Daures JP, Henry-Amar M, Raverdy N, Schaffer P, Poisson M, Delattre JY (1997) Descriptive epidemiology of cerebral gliomas in France. Cancer 79:1195–1202
Kita D, Ciernik IF, Vaccarella S, Franceschi S, Kleihues P, Lütolf UM, Ohgaki H (2009) Age as predictive factor in glioblastomas: population-based study. Neuroepidemiology 33:17–22
Barnholtz-Sloan JS, Williams VL, Maldonado JL, Shahani D, Stockwell HG, Chamberlain M, Sloan AE (2008) Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg 108:642–648
Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin P, Bondiau PY, Meneï P, Loiseau H, Bernier V, Honnorat J, Barrié M, Mokhtari K, Mazeron JJ, Bissery A, Delattre JY (2007) Association of French-speaking neuro-oncologists. Radiotherapy for glioblastoma in the elderly. N Engl J Med 356:1527–1535
Glantz M, Chamberlain M, Liu Q, Litofsky NS, Recht LD (2003) Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer 97:2262–2266
Chinot OL, Barrie M, Frauger E, Dufour H, Figarella-Branger D, Palmari J, Braguer D, Hoang-Xuan K, Moktari K, Peragut JC, Martin PM, Grisoli F (2004) Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly populations. Cancer 100:2208–2214
Chamberlain MC, Chalmers L (2007) A pilot study of primary temozolomide chemotherapy and deferred radiotherapy in elderly patients with glioblastoma. J Neurooncol 82:207–209
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Mollemann M, Wolter M, Felsberg J, Collins VP, Reifenberger G (2005) Frequent promoter hypermethylation and low expression of the MGMT gene in oligodendroglial tumors. Int J Cancer 113:379–385
Chinot OL, Barrie M, Fuentes S, Eudes N, Lancelot S, Metellus P, Muracciole X, Braguer D, Ouafik L, Martin PM, Dufour H, Figarella-Branger D (2007) Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol 25:1470–1475
Komine C, Watanabe T, Katayama Y, Yoshino A, Yokoyama T, Fukushima T (2003) Promoter hypermethylation of the DNA repair gene O6-methylguanine-DNA methyltransferase is an independent predictor of shortened progression free survival in patients with low-grade diffuse astrocytomas. Brain Pathol 13:176–184
Criniere E, Kaloshi G, Laigle-Donadey F, Lejeune J, Auger N, Benouaich-Amiel A, Everhard S, Mokhtari K, Polivka M, Delattre JY, Hoang-Xuan K, Thillet J, Sanson M (2007) MGMT prognostic impact on glioblastoma is dependent on therapeutic modalities. J Neurooncol 83:173–179
Chinot OL, Barrié M, Fuentes S, Eudes N, Lancelot S, Metellus P, Muracciole X, Braguer D, Ouafik L, Martin PM, Dufour H, Figarella-Branger D (2007) Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol 25:1470–1475
Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22:1583–1588
Idbaih A, Taillibert S, Simon JM, Psimaras D, Schneble HM, Lopez S, Lang P, Toubiana T, Feuvret L, Delattre JY, Mazeron JJ (2008) Short course of radiation therapy in elderly patients with glioblastoma multiforme. Cancer Radiother 12:788–792
Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M (2009) Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer 115:3512–3518
Combs SE, Wagner J, Bischof M, Welzel T, Wagner F, Debus J, Schulz-Ertner D (2008) Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys 70:987–992
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laigle-Donadey, F., Figarella-Branger, D., Chinot, O. et al. Up-front temozolomide in elderly patients with glioblastoma. J Neurooncol 99, 89–94 (2010). https://doi.org/10.1007/s11060-009-0110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-0110-3