Abstract
Forkhead box class O 3a (FOXO3a) is an important direct target of the phosphatidylinositol 3-kinase (PI3K)/protein B(Akt) pathway, mediating signal transduction in regulating cell survival and cell-cycle progression. Recent reports have shown that FOXO3a inhibits cell-cycle progression at the G1/S transition by controlling transcription of the cyclin-dependent kinase inhibitor p27kip1, which is frequently down-regulated in human cancers, including human glioma. In this study we investigated the status of FOXO3a expression and related signaling in human glioma in order to test its potential value as a therapeutic target for this disease. Immunohistochemistry, western blot, RT-PCR, and immunofluorescence staining analysis were performed on specimens from 70 cases of human glioma and on U87MG and T98G glioma cells. Our data showed FOXO3a expression is directly correlated with the malignant grade of glioma. More importantly, low expression of FOXO3a was associated with poor patient outcome. In vitro, FOXO3a modulated the cell cycle by transcriptional regulation of p27kip1. Administration of the PI3K pharmacological inhibitor LY294002 abrogated this effect by regulating FOXO3a expression and subcellular localization. Our results suggested that FOXO3a may be a favorable independent prognostic indicator of glioma. Gene therapeutic approaches aimed at PI3K or at pharmacological inhibitors of PI3K to down-regulate P-FOXO3a expression could be developed for management of glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is one of the most common primary tumors of the central nervous system. Despite recent advances in both diagnostic modalities and therapeutic strategies, glioma remains one of the deadliest human cancers. Five-year survival of patients with glioma is among the lowest for all cancers [1, 2]. In patients with glioblastoma multiforme, the median survival duration ranges from 9 to 12 months [2]. It is, therefore, necessary to intensify our efforts to better understand the mechanism of glioma development and to develop novel targeted approaches for management of this disease. An example of a target-specific molecular therapy of glioma is temozolomide, which alters levels of Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) proteins and results in increased Bax/Bcl-2 ratio [3]. Temozolomide, also, can be safely combined with several chemotherapeutic agents to increase time to progression, quality of life, and overall survival for some patients [4]. Truly, increasing knowledge of the genetic control of cellular proliferation and of modulation of the signaling pathways that are aberrant in glioma has the potential to provide an effective and better approach for its management.
The FOXO transcription factors play an evolutionarily conserved role in the control of metabolism, proliferation, survival, stress resistance, and longevity [5–7]. Expression of FOXO proteins can induce cell-cycle arrest in many cell types [5–7]. FOXO3a (previously termed FKHRL1), a member of the FOXO subclass of the forkhead transcription factors mediating signal transduction in regulating cell survival and cell-cycle progression, is an important direct target of the PI3K/Akt pathway. When PI3K and Akt are active, FOXO3a is directly phosphorylated by Akt [8]. By binding to the nuclear importer, active FOXO3a translocates to the nucleus, binds to DNA, and promotes the transcription of its target genes, for example p27kip1 and Bim [6]. However, activated Akt regulates transcription of FOXO3a target genes by modulation of FOXO3a activity by phosphorylating its three conserved serine/threonine residues (Thr-32, Ser-253, and Ser-315), leading to the release of FOXO3a from DNA and its translocation to the cytoplasm, which reduces transcription levels of p27kip1 [6, 9].
P27kip1, a member of the Cip/Kip family, can bind multiple cyclin-CDK complexes, including D-type cyclins CDK4 and CDK6 and E-type cyclin CDK2; thus, p27kip1 is a major regulator of G1–S transition in the cell cycle [10, 11]. The activity of p27kip1 is controlled by its concentration, distribution among different cellular complexes, and its cellular location [10, 11]. It has been shown that p27kip1 protein is frequently reduced in human cancers, including high-grade astrocytomas [12]. All these studies indicate that p27kip1 protein levels may be associated with the development of human cancers and seem to be an important marker of cancer progression.
However, to the best of our knowledge, the status of FOXO3a expression in human glioma, including its possible clinical significance and its correlation with p27kip1, has not been examined. In this study, therefore, we investigated the hypothesis that p27kip1 gene expression is regulated by FOXO3a, which itself is controlled by PI3K/Akt in human glioma. To evaluate the proposed hypothesis, we used immunohistochemical analysis to examine FOXO3a and p27kip1 protein levels in specimens of glioma and normal brain tissues and compared the findings with clinical outcomes. More importantly, we studied the effect of PI3K inhibitor LY294002 on the cell cycle in vitro to explore the potential value of activated FOXO3a as a therapeutic target for human glioma.
Materials and methods
Pathological samples
All the investigations described in this study were conducted after informed consent was obtained and in accordance with an institutional review board (IRB) protocol approved by the Partners Human Research Committee at the Affiliated Hospital of Nantong University. Fresh frozen primary human tissue samples of 70 WHO grade II, III, and IV astrocytomas were obtained from the pathology files of the Department of Pathology at the Affiliated Hospital of Nantong University from 2000–2006 under the auspices of an IRB-approved human subjects study protocol. All tumors were from patients with newly diagnosed glioma who had received no therapy before sample collection. Formalin-fixed, paraffin-embedded sections were prepared for all tissues and reviewed by a neuropathologist. Normal brain specimens were acquired from ten patients undergoing surgery for epilepsy and were reviewed to verify the absence of tumor.
Antibodies
The antibodies used for immunohistochemistry in this study included: anti-p27kip1 (Santa Cruz Biotechnology, CA, USA), anti-FOXO3a (Cell Signaling Technology, Beverly, MA, USA), and anti-Ki-67 (Santa Cruz Biotechnology). Antibodies for western blotting, immunofluorescence, and immunoprecipitation included: anti-p27kip1 (Santa Cruz Biotechnology), anti-FOXO3a (Cell Signaling Technology), anti-phospho-FOXO3a (thr32) (Upstate Cell Signaling Solution, Lake, Placid, USA), anti-PCNA (Santa Cruz Biotechnology), anti-phospho-Akt (ser473) (Cell Signaling Technology), anti-CyclinD1 (Santa Cruz Biotechnology), anti-CDK4 (Santa Cruz Biotechnology), anti-CDK6 (Santa Cruz Biotechnology), anti-β-actin (Sigma Chemicals, St Louis, MO, USA), anti-α-tublin (Santa Cruz Biotechnology), anti-Lamin B (Santa Cruz Biotechnology), anti-PTEN (Abcam, Hong Kong, China), and anti-EGFR (ZSGB-Bio, China).
Immunohistochemistry
Serial consecutive sections 5 μm thick were mounted on glass slides coated with 10% polylysine. Sections were dewaxed in xylene and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked by immersion in 0.3% methanolic peroxide for 30 min. Immunoreactivity was enhanced by microwaving, by incubating the tissue sections for 10 min in 0.1 M citrate buffer. Immunostaining was performed using the avidin biotin peroxidase complex method and antigen–antibody reactions were visualized with the chromogen diaminobenzadine.
Evaluation of the results of immunohistochemical staining
Stained sections were observed under a microscope. Two independent pathologists (YW and LZ) evaluated the immunostaining. At least ten high-power fields were randomly chosen and at least 300 cells/field was counted in each section. FOXO3a, p27kip, and Ki-67 indexes were determined as the percentage of immunostained cells. PTEN staining was scored according to a previously established scale of 0–2 [13]. Tumor cells were graded “2” if their staining intensity was equal to or greater than that of vascular endothelium and “1” if their staining intensity was diminished relative to the endothelium. An expression score of “0” was given if staining was absent compared with control sections. EGFR was scored on a scale of 0–2 (0+, no staining; 1+, mild intensity cytoplasmic staining; and 2+, strong cytoplasmic staining).
Cell cultures
The human glioblastoma cell lines U87MG and T98G, which were purchased from Cell Library, China academy of Science, were cultured in DMEM (GibCo BRL, Grand Island, NY, USA) with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin–streptomycin mixture (GibCo BRL) at 37°C and 5% CO2.
Cell cycle analysis
For cell-cycle analysis, cells were fixed in 70% ethanol for 1 h at 4°C and then incubated with 1 mg/mL RNase A for 30 min at 37°C. Subsequently, cells were stained with propidium iodide (50 μg/mL PI; Becton–Dickinson, San Jose, CA, USA) in PBS, 0.5% Tween-20, and analyzed using a Becton–Dickinson BD FACScan flow cytometer and Cell Quest acquisition and analysis software. Gating was set to exclude cell debris, cell doublets, and cell clumps.
Cell fractionation, immunoblot analysis
Subcellular fractionation was performed as described. Briefly, cell pellets from a culture were incubated in a hypotonic buffer (10 mM HEPES [pH 7.2], 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 20 mM NaF, 100 μM Na3VO4, and protease inhibitor mixture) for 30 min at 4°C on a rocking platform. Cells were homogenized (Dounce, 30 strokes), and their nuclei were pelleted by centrifugation (10 min × 14,000 rcf, 4°C). The supernatant was saved as the cytosolic fraction, and nuclear pellets were incubated in nuclear lysis buffer (10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100) for 1 h at 4°C on a rocking platform. The nuclear fraction was collected by centrifugation (10 min × 14,000 rcf, 4°C).
Prior to immunoblotting, cells were washed twice with ice-cold PBS, resuspensed in 2 × lysis buffer (50 mM Tris–HCl, 120 mM NaCl, 0.5% Nonidet P-40, 100 mM NaF, 200 μM Na3VO4, and protease inhibitor mixture), and incubated for 20 min at 4°C while rocking. Lysates were cleared by centrifugation (10 min × 12,000 rpm, 4°C) and 50 μg total protein was resolved by SDS–PAGE and transferred on to a poly(vinylidene difluoride) membrane filter (Immbilon; Millipore). The membranes were first blocked and then incubated with the primary antibody described above for 2 h at room temperature. After washing three times, filters were incubated with horseradish peroxidase-conjugated human anti-mouse or anti-rabbit antibodies (Pierce) for 1 h at room temperature. Immunocomplexes were detected with an enhanced chemiluminescence system (NEN Life Science Products, Boston, MA, USA).
RNA isolation and reverse transcriptase PCR (RT-PCR) analysis
Total RNA of U87MG cells were extracted using a Trizol extraction kit according to the manufacturer’s procedure. Total RNA was reverse-transcribed using the ThermoScript RT-PCR system (Invitrogen). Primer pairs for p27kip1 were sense, 5′-CAGAATCACAAACCCCTA-3′ and antisense, 5′-TGTTTTGAGTAGAAGAAT-3′. Cycling conditions were: 94°C for 45 s, 47.5°C for 45 s, 72°C for 30 s, and a total of 30 cycles. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control and was detected using the primers sense, 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ and antisense, 5′-TCCTTGGAGGCCATGTGGGCCAT-3′. Cycling conditions were: 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and a total of 28 cycles. After amplification, the products were separated on an agarose (1.5%) gel (cast in the presence of ethidium bromide) and visualized under UV light.
Immunofluorescence staining
The cells were washed with PBS, fixed with 4% paraformaldehyde (1 h), permeabilized with 0.1% Triton X-100 (15 min), and blocked for nonspecific binding in phosphate-buffered saline (PBS), 3% milk for 30 min. FOXO3a and p27kip1 in U87MG cells were stained with FOXO3a and p27kip1 antibody at 4°C overnight. After three washes with PBS, samples were treated with fluorescein isothiocyanate (FITC) conjugated goat-anti-rabbit IgG (The Jackson Laboratory) for 30 min at 37°C. For DNA staining, samples were incubated with Hoechst 33342 dye (1 μg/ml, 10 min) after incubation with secondary antibodies. The fluorescence was detected by use of a Leica fluorescence microscope (Germany). All assays were performed three times in duplicate.
Statistical analysis
The statistical significance of means was calculated by use of Student’s t test. The nonparametric Spearman’s rank correlation coefficient was used to evaluate the strength of the relationship between FOXO3a and Ki-67 expression. The χ 2 and Fisher exact tests were used to compare the expression of all proteins as groups (positive versus negative) with various clinical pathological parameters. Survival analysis was undertaken using the Kaplan–Meier method and curves were compared using log-rank test. P < 0.05 was required for statistical significance. All computations were carried out by use of SPSS13.0 statistical software.
Densitometric analyses
The density of specific bands was measured with a computer-assisted image-analysis system (Adobe Systems, San Jose, CA, USA) and normalized against GAPDH level. Differences between the control and treatment groups were calculated and expressed as relative increases setting the control as 1. Values were obtained from at least three independent reactions.
Results
Clinical significance of FOXO3a expression in glioma
Expression of FOXO3a directly correlates with the grade of glioma
We examined the expression and intracellular location of FOXO3a protein in 70 specimens of glioma. In 55 of the FOXO3a-positive tumors, FOXO3a immunoreactivity was predominantly located in the nuclear compartments; a weaker cytoplasmic reaction was observed in a variable number of glial cells (Fig. 1a). The percentage of FOXO3a-positive tumor cells ranged from 0.13 to 63.86% (mean ± SD, 23.53 ± 19.34%; Table 2). Using 5% as a cutoff, we found significantly higher levels of FOXO3a in low-grade astrocytomas (grade II) than in anaplastic astrocytomas (grade III; P < 0.001) and glioblastoma multiforme (grade IV; P < 0.001). Table 1 shows that 91.2% of grade II tumors were positive for FOXO3a expression. However, the percentages of FOXO3a-positive glial cells in grade III and grade IV tumors were 70.8 and 58.3%, respectively. Thus, FOXO3a positive expression was significantly associated with pathologic stage of the glioma (P < 0.05; Table 1). On other hand, no significant difference was seen regarding patients’ age (P = 0.796), gender (P = 0.834), KPS score (P = 0.508), tumor location (P = 0.456), type of surgery (P = 0.890), tumor diameter (P = 0.677), vessel density (P = 0.801), or necrosis (P = 0.446; Table 1).
Expression of FOXO3a and p27kip1 in human glioma. a Immunohistochemistry for FOXO3a, p27kip1, and Ki-67 were performed as described in Materials and methods. a–c High FOXO3a and p27kip1 expression in tumor cell nuclei was documented in grade II tumors, whereas Ki-67 staining showed the level was low. d–f High levels of proliferation and Ki-67 expression are seen in grade IV tumors. In contrast, low levels of Foxo3a and p27kip1 were observed in grade IV tumors. b Western blot analysis of FOXO3a, p27kip1, and PCNA in glioma tissues. Western blots of six samples of the respective grades of glioma tissues immunoblotted against FOXO3a, p27kip1, and PCNA. In all the samples tested, FOXO3a expression levels were significantly higher in low potential malignancy tissues than in malignant tissues. β-Actin was used as a control for protein load and integrity
To confirm the specificity of the immunohistochemical results, we performed western blot analysis for six glioma tissues (two cases of each stage group), for which freshly frozen materials were available. Expression of FOXO3a and p27kip1 were examined by western blot analysis, which showed accordant results with immunohistochemistry. As shown in Fig. 1b, an immunoreactive band of FOXO3a was seen in all six cases, FOXO3a expression was significantly higher in grade II tumors than in grade IV tumors. It has been well established that FOXO3a controls p27kip1 protein level in several cancers [13, 14]. As expected, a reduced level of p27kip1 protein was directly associated with malignancy of glioma. These results indicated that down-regulation of FOXO3a expression resulted in reduced p27kip1 expression in a subset of the glioma samples. Amount of β-actin, a housekeeping protein, was demonstrated to be rather constant among the samples. Three independent experiments were performed for each assayed variable.
Correlation between FOXO3a and different biological markers in glioma
p27kip1 is a potent regulator of the G1/S transition by inhibiting CDK activity [11, 12]. Several reports have revealed that FOXO3a-induced cell cycle arrest either correlates with, or in one case is dependent upon, p27kip1 expression [14–17]. To determine the physiological or pathological relationship among expression of FOXO3a, p27kip1, and proliferation index Ki-67 in glioma, we also examined the levels of p27kip1 and Ki-67 by immunohistochemical staining (Fig. 1a; Table 2). In most specimens, the proportion of p27kip1-positive tumor cells was similar to the proportion of FOXO3a-positive tumor cells but opposite to that of Ki-67-positive tumor cells. As continuous variables, FOXO3a expression was positively associated with p27kip1 (Spearman’s r = 0.845, P < 0.001) and was negatively associated with Ki-67 expression (Spearman’s r = −0.555, P < 0.001) in all cases of glioma analyzed. There was also a marked correlation between p27kip1 and Ki-67 (Spearman’s r = −0.647, P < 0.001) (Fig. 2c).
Both amplification of epidermal growth factor receptor (EGFR) and activation of PI3K feature prominently in glioma. Activation of PI3K that occurs as a consequence of EGFR amplification should respond to inhibitors of EGFR. PI3K may also be activated independently of EGFR through gain-of-function mutations in PI3K or by inactivation of the lipid phosphatase PTEN, a negative regulator of PI3K [18–20]. FOXO3a function, downstream of the PI3K signaling pathway, is as a direct substrate of the protein kinase Akt [21]. So we also detected the relationships between FOXO3a and PTEN and between FOXO3a and EGFR. Figure 3b graphically illustrates the existence of a direct relationship between FOXO3a level (%) and the intensity of PTEN expression (P = 0.001). However, there was no significant correlation between FOXO3a expression and EGFR status (P = 0.23).
Correlation between FOXO3a levels and PTEN intensity and between FOXO3a levels and EGFR intensity in glioma tissues. a Immunohistochemistry for PTEN and EGFR was performed as described in Materials and methods. a, d strongly cytoplasmic PTEN and EGFR immunostaining, b, e moderate cytoplasmic PTEN and EGFR immunostaining, c, f no immunostaining of PTEN and EGFR. b Boxplots depicting FOXO3a levels in glioma samples with PTEN and EGFR staining of different intensity. Black horizontal line, median value for each group; gray box, middle 50% of the values, lying between 25 and 75%; horizontal gray lines represent the minimum and maximum values observed in each group. Statistical analysis was performed by use of the Kruskal–Wallis test
Correlation between FOXO3a expression and patients’ survival
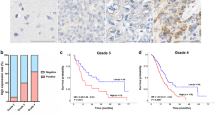
Currently, prognostic evaluation is mainly based on the traditional method including clinical stage, tumor site, and histopathologic grade. Recent studies have suggested that other factors, for example molecular and cellular characteristics of the primary tumors, may improve our ability to prognosticate. As Table 2 shows, mean amounts of FOXO3a and p27kip1 were 23.53 ± 19.34% and 28.84 ± 25.15%, respectively. Based on these mean amounts, patients were divided into two groups by FOXO3a: high FOXO3a expressers (>23.5%) and low FOXO3a expressers (≤23.5%). Survival analysis was restricted to 52 patients with available complete follow-up data and results of FOXO3a expression. By using Kaplan–Meier analysis, patients in the low expression FOXO3a group were significantly associated with poor overall survival (P < 0.001; Fig. 4a). Furthermore, by reviewing each grade of tumor separately we found FOXO3a index was similarly a significant predictor in grades II and III (P < 0.0001; Fig. 4b, c). In the grade IV group there were too few patients to perform any valid statistical comparisons in relation to FOXO3a status (P = 0.061; Fig. 4d). However, the trend was for better survival in patients with FOXO3a high expression tumors. Previous study has indicated that p27kip1 was an independent prognostic factor; thus, the combined phenotypes of two proteins were analyzed subsequently. Patients with the phenotype FOXO3a > 23.5% and p27kip1 > 28.8% had better overall survival than others (P = 0.010; Fig. 4e). Multivariate analysis using the Cox’s proportional hazards model showed that FOXO3a protein was an independent prognostic indicator for patients’ overall survival (P < 0.0001; Table 3).
Overall survival curves according to FOXO3a (a–d) and FOXO3a/p27kip1 (e) expression. On the basis of mean FOXO3a percentages, patients were divided into two groups: high FOXO3a expressers (>23.5%) and low FOXO3a expressers (≤23.5%). Patients were also divided into two groups according to p27kip1 expression: high p27kip1 expressers (>28.8%) and low p27kip1expressers (≤28.8%). a–d Patients in the low expression FOXO3a group were significantly associated with short overall survival. e Patients with the phenotype FOXO3a > 23.5% and p27kip1 > 28.8% had better cumulative survival than others
Biological significance of FOXO3a expression in glioma
FOXO3a regulates cell cycle by transcriptional regulation of p27kip1 in proliferating cells
Previous studies showed that ectopic expression of activated FOXO3a led to cell cycle arrest in which induction of p27kip1 by FOXO3a seemed to play an important role [17]. We examined the kinetics of FOXO3a during cell-cycle progression in glioma. U87MG and T98G cells were G1-arrested by serum starvation for 48 h, which increased cells in the G1 phase from 48 to 86% (date not shown). Western blot revealed that FOXO3a protein level increased after serum withdrawal and peaked at 48 h. At the same time, expression of p27kip1 was also up-regulated (Fig. 5a). Upon serum addition and release from G1, the abundance of FOXO3a and p27kip1 declined with similar kinetics and were reduced markedly 24 h after serum stimulation (Fig. 5b). Using RT-PCR analysis, we determined whether changes in p27kip1 protein could account for the effect of FOXO3a transcriptional regulation. In agreement with the findings of p27kip1 protein expression, p27kip1 mRNA expression increased with serum deprivation and declined after serum stimulation (Fig. 5c, d). In addition to the tight control of p27kip1 abundance, there is also important regulation at the level of p27kip1 subcellular localization. Following serum administration, FOXO3a and p27kip1 significantly decreased in the nuclear fraction in a time-dependent manner (Fig. 5e). These data suggested that FOXO3a regulated transcriptional expression of p27kip1 in proliferating glioma cells in vitro.
FOXO3a modulates serum-dependent transcriptional regulation of p27kip1 in glioma cells. a and c U87MG and T98G cells were synchronized by serum starvation for 0–72 h. Whole cell lysates or total mRNA was prepared as described in Materials and methods. FOXO3a and p27kip1 protein and p27kip1 mRNA expression were evaluated by western blotting (a) and RT-PCR (c), respectively. b and d U87MG and T98G cells were synchronized by serum starvation for 48 h. After the addition of medium containing 10% FBS for the indicated times, FOXO3a and p27kip1 protein (b) and p27kip1 mRNA (d) expression were determined. e U87MG cells were incubated in serum-free media as described in b. Nuclear and cytosolic proteins were immunoblotted for FOXO3a and p27kip1. The levels of lamin B and a-tubulin in the nuclear and cytosolic fractions, respectively, were also immunoblotted to confirm the purity of the subcellular fractions. Each immunoblot shown is representative of three experiments with similar results. Reduced expression of FOXO3a in the nucleus was observed 4 h after serum addition; in contrast, expression of FOXO3a was increased in cytoplasm. Similar results were observed for expression of p27kip1
Inhibition of the PI3K/Akt pathway leads to G1 arrest through retention of activated FOXO3a
FOXO3a is a major molecule in the PI3K/Akt signaling pathway which plays an important role in promoting cell survival and proliferation [22–24]. This transcription factor resides in the nucleus, where it is a positive regulator of gene expression. However, upon phosphorylation, FOXO3a is transported out of the nucleus and sequestered in the cytosol, where it is bound to the protein 14-3-3 [8]. In an attempt to investigate the mechanism of the PI3K/Akt pathway in the regulation of glioma cell proliferation, we examined the effect exerted by the PI3K pharmacologic inhibitor LY294002.
After synchronization by serum starvation for 48 h, U87MG cells were incubated in medium containing 10% FBS for the indicated times. On serum addition, the cells were transformed from the G1 phase to the S phase whereas exposure to 50 μM LY294002 for 24 h blocked proliferation and caused cells to accumulate in the G1 phase of the cell cycle, as assessed by flow cytometric analysis (Table 4). By western blot analysis, we observed that serine473-phosphorylation of Akt and threonine32-phosphorylation of FOXO3a were down-regulated after treatment with LY294002. Figure 6a, suggests that LY294002 could help to retain a greater amount of FOXO3a in the activated form to inhibit cancer cell growth. Therefore, we further investigated the location of FOXO3a by LY294002 treatment, which is related to its activation state.
Inhibition of PI3K induces FOXO3a activation and translocation in U87MG cells. a and b After synchronization by serum starvation for 48 h, U87MG cells were treated with serum, DMSO, or 50 μM LY294002 and cells were collected at the times indicated for western blot and/or RT-PCR. Details of the experiments are given in Materials and methods. After incubation with LY294002 for 24 h we found a significant decrease in the percentage of S phase, and P-Akt, P-FOXO3a, FOXO3a, p27kip1, and β-actin were visualized by western blot. Results from RT-PCR analysis are mean ± standard deviation from three independent experiments; *P < 0.05. c U87MG cells were incubated in the absence and presence of LY294002 for 24 h. Samples of cytoplasmic or nuclear extracts, isolated at the times indicated, were subjected to immunoblot analysis for FOXO3a and p27kip1. The levels of lamin B and α-tubulin in the nuclear and cytosolic fractions, respectively, also were immunoblotted to confirm the purity of the subcellular fractions. Each immunoblot shown is representative of three experiments with similar results. d Immunofluorescence gave a result similar to that from western blot. After treatment with LY294002 the proteins FOXO3a and p27kip1 were accumulated in the nucleus. e Following incubation of cells with LY294002 for 24 h, levels of the proteins CDK4, CDK6, and cyclin D1 were assessed by western blot analysis
In response to LY294002 treatment for 24 h, FOXO3a was largely redistributed to the nucleus and the amount in the cytoplasm declined. The levels of compartment-specific lamin B in the nucleus and tubulin in the cytosol were not changed. To confirm these results, we also tested the subcellular distribution of FOXO3a by immunofluorescence analysis in U87MG cells either in the absence or presence of LY294002. Similarly, expression of FOXO3a seemed to be mainly nuclear in location after administration of LY294002 for 24 h (Fig. 6d).There is broad consensus that activated FOXO3a resides in the nucleus and could function to promote the transcription of cell-cycle inhibitors [9, 15]. As expected, it also caused a parallel change in the distribution of p27kip1 (Fig. 6c, d). These findings suggested that the PI3K/Akt pathway controlled glioma cell proliferation by regulating FOXO3a expression and subcellular localization.
Progression through the G1-S transition is mainly regulated by sequential activation of CDK4, CDK6, and, later, CDK2 by D-type cyclins in mid-to-late G1 [25]. The Cip/Kip family of cyclin kinase inhibitors bind all CDKs and may prevent their activation or directly inhibit their kinase activity [25]. Therefore, we evaluated the effect of inhibition of PI3K on these important cell-cycle regulatory molecules. Inhibition of the PI3K/Akt pathway by LY294002 resulted in significant accumulation of p27kip1, at both the mRNA and protein levels (Fig. 6a, b), and sequentially induced a striking depletion of CDK4, CDK6, and cyclin D1 protein levels in U87MG cells (Fig. 6d). These events were consistent with the observed accumulation of cells in G1 phase of the cell cycle (Table 3) and up-regulation of FOXO3a expression (Fig. 6a).
Taken together, our data suggested that targeted inhibition PI3K in human glioma cancer cells caused activation of transcription factor FOXO3a, which, in turn, activated transcription of target gene p27kip1 which is involved in cell-cycle regulation.
Discussion
It is conceivable that neoplasms occur as a result of acquired alterations in oncogenes and tumor suppressor genes regulating signal transduction pathways involved in cell proliferation and differentiation, and cell-cycle control. The CKI p27kip1, which belongs to the second group of cyclin-dependent kinase inhibitors (CDKI), the Cip/Kip family, is one of the most important cell-cycle inhibitors, and its expression has the character of a tumor-suppressor gene. Loss of p27kip1 protein expression may result in tumor development and/or progression [26]. Regulation of p27kip1 levels was thought to occur predominantly at the post-transcriptional level, through the regulation of ubiquitin-mediated degradation. Recent data show FOXO3a directly regulates p27kip1 transcription [14, 16, 17], suggesting that reduced p27kip1 levels after a proliferative stimulus may also be associated with FOXO3a.
FOXO3a is a member of a large family of forkhead transcription factors. About 40 different forkhead transcription factors have been identified to date in mammalian cells. Forkhead proteins have been assigned to 17 subfamilies ranging from FOXA to FOXQ [27]. Of these, the FOXO factors are the only ones known to date to be regulated by the PKB/Akt pathway, and others clearly function in distinct, cellular processes. FOXO transcription factors have been implicated in a remarkable number of diverse cellular processes from development and metabolism, to stress and aging, to proliferation and programmed cell death [28, 29]. The ability of FOXOs to control cell survival and cell death suggests that FOXOs may function as tumor suppressors. Indeed, loss of FOXO function has been observed in a number of human cancers and may be a common feature of carcinogenesis. Accili et al. [5] had found that FOXO3a is a key tumor suppressor in breast cancer. Recently, Hu and colleagues [30] showed that down-regulation of FOXO3a by FOXO3a-siRNA in human breast cancer MDAMB cell lines induced cell proliferation and tumorigenesis in nude mice and that ectopic expression of FOXO3a in the cells inhibited tumorigenesis in nude mice, suggesting that FOXO3a has general suppression effects for tumorigenesis in vivo. In this study, we provided a novel insight that FOXO3a is a critical prognosis variable for human glioma.
First, we used immunohistochemical analysis to assess FOXO3a expression in glioma cases. FOXO3a was detected in 78.6% of all cases if a 5% cutoff was used to define positivity. Our data also showed FOXO3a levels were significantly higher in grade II tumors than in grade IV tumors. These findings suggested that alteration of expression of FOXO3a protein could affect tumor development. Reduced expression of FOXO3a was closely associated with the glioma malignancy. Malignant conversion of tumors is a complex process that may be regulated, at least in part, by reduced expression of FOXO3a. In all cases of glioma analyzed, FOXO3a expression was positively correlated with p27kip1 expression but inversely associated with cell proliferation identified by Ki-67 expression, a marker of cell proliferation expressed specifically in the cell nucleus from late G1 to S phase (Fig. 2a, b). These results were consistent with a previous study on breast cancer [5]. Taken together, our observations supported the concept that FOXO3a, as a regulator of p27kip1, might contribute to the progression of glioma.
The possible role of FOXO3a protein on glioma patients’ prognosis, is still unclear. In our preliminary survival analysis, patients with low expression of FOXO3a were significantly associated with poor overall survival. In recent years, a growing body of literature has revealed reduced levels of p27kip1 protein in various tumor types; this has been characterized as an independent factor of poor prognosis [31]. When FOXO3a and p27kip1 were combined, patients with the phenotype of FOXO3a (high)/p27kip1 (high) showed more favorable overall survival than other phenotypes of FOXO3a/p27kip1 (P < 0.001; Fig. 4). Collectively, our results indicated that FOXO3a aberrations in glioma portended particularly aggressive clinical behavior. But this early finding needed to be confirmed with a larger group of patients.
Oncogenic signaling cascades and molecular events contribute to loss of FOXO function. FOXOs can be deregulated through genetic defects on the one hand and posttranslational modifications on the other hand. Thus, each type of cancer might harbor an individual signature of FOXO deregulation determining its unique phenotype. For example, inhibitory phosphorylation of FOXOs by mitogenic signaling cascades, e.g. PI3K/Akt, has been observed in various human cancers.
Akt is a critical determinant of tumorigenesis, affecting the growth and survival of cancer cells by phosphorylating a series of substrates [32]. There is broad consensus that Akt-dependent phosphorylation is crucial to preventing FOXO3a displacement to the nucleus where they would otherwise function to promote the transcription of cell cycle inhibitors, for example p27kip1 [9, 15]. From our series of studies, we found that LY294002 significantly inhibited the phosphorylation and activation of Akt, resulting in lower levels of P-FOXO3a (Fig. 6a). More important, LY294002-treatment caused FOXO3a nuclear accumulation compared with control groups. These results suggested that LY294002 regulated the activity of FOXO3a by regulating its phosphorylation and subcellular location. It is known that FOXO3a regulates the transcription of p27kip1 by binding to its promoter [33]. Indeed, we found that p27kip1 expression was increased by LY294002 treatment, whereas alteration of the level of p27kip1 protein by LY294002 treatment could also be because of the altered activity of proteasome as observed in other agent treatments [34] and, as such, requires further investigation.
According to these results, we believe that chemotherapeutics such as PI3K inhibitor or PI3K siRNA which save FOXO3a from inactivating phosphorylation were likely to have broad applications for treating patients with glioma. The possibly best-established example is treatment of chronic myelogenous leukemia (CML) with the tyrosine kinase inhibitor STI571 (gleevec), which inhibits PI3K/Akt and thus FOXO3a phosphorylation and restores its transcriptional activity as reflected by Bim upregulation [35, 36]. Similarly, it has been shown that treatment of human osteosarcoma cells with the PI3K/Akt inhibitor grifolin reduces the fraction of phosphorylated and inactivated FOXOs, thereby increasing the rate of apoptosis [37, 38].
Besides the PI3K/Akt pathway, additional mitogenic cascades have been found to impair FOXO function in cancer. Inactivation of FOXOs because of overexpressed Ikkβ was reported in breast cancers lacking activation of the PI3K/Akt cascade. Hu et al. [30] and Hu and Hung [39] reported that the level of Ikkβ/NF-κB activation was directly correlated with the amount of phosphorylated FOXO3a in the cytoplasm. Furthermore, both the transcriptional activity and protein expression of FOXO3a have been shown to be impaired after ERK activation. Overexpression of ERK in the human hepatoma cell line Hep-3BX significantly reduced the nuclear content of FOXO3a, and its total cellular expression. ERK phosphorylates FOXO3a on Ser294, Ser344, and Ser425, which results in FOXO3a translocation into the cytosol and MDM2-mediated ubiquitination. It is still possible that other signaling pathways regulate FOXO3a function in glioma cells as reported in other cell lines; further studies will be needed to address this finding more completely.
In conclusion, we showed large variations of FOXO3a expression and showed a close correlation with p27kip1 and cell proliferation in glioma. FOXO3a was an independent prognostic factor for a subset of patients with glioma. So expression of FOXO3a is likely to be a useful tool for both diagnostic and possibly prognostic applications in glioma. Gene therapeutic approaches aimed at FOXO3a may be developed for management of glioma.
Abbreviations
- FOXO3a:
-
Forkhead box class O 3a
- PI3K:
-
Phosphatidylinositol 3-kinase
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome 10
- EGFR:
-
Epidermal growth factor receptor
- CDK4:
-
Cyclin-dependent kinase 4
- CDK6:
-
Cyclin-dependent kinase 6
- PCNA:
-
Proliferating cell nuclear antigen
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PI:
-
Propidium iodide
- FITC:
-
Fluorescein isothiocyanate
- CML:
-
Myelogenous leukemia
- DMSO:
-
Dimethyl sulfoxide
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC et al (2005) Cancer statistics. CA Cancer J Clin 55:10–30
Surawicz TS, Davis F, Freels S et al (1998) Brain tumor survival: results from the national cancer data base. J Neurooncol 40:151–160
Das A, Banik NL, Patel SJ, Ray SK (2004) Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax: Bcl-2 ratio and preventing proteolytic activities. Mol Cancer 3:36
Choi JW, Lee MM, Kim IA, Kim JH et al (2008) The outcomes of concomitant chemoradiotherapy followed by adjuvant chemotherapy with temozolomide for newly diagnosed high grade gliomas: the preliminary results of single center prospective study. J Korean Neurosurg Soc 44:222–227
Accili D, Arden KC (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117:421–426
Burgering BM, Kops GJ (2002) Cell cycle and death control: long live forkheads. Trends Biochem Sci 27:352–360
Tran H, Brunet A, Griffith EC, Greenberg ME (2003) The many forks in FOXO’s road. Sci STKE 2003: RE5
Van Der Heide LP, Hoekman MF, Smidt MP (2004) The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380:297–309
Brunet A, Bonni A, Zigmond MJ et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96:857–868
Polyak K, Lee MH, Erdjument-Bromage H et al (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59–66
Toyoshima H, Hunter T (1994) p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78:67–74
Kirla RM, Haapasalo HK, Kalimo H, Salminen EK (2003) Low expression of p27 indicates a poor prognosis in patients with high-grade astrocytomas. Cancer 97:644–648
Choe G, Horvath S, Cloughesy TF, Crosby K et al (2003) Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res 63:2742–2746
Dijkers PF, Medema RH, Pals C, Banerji L et al (2000) Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol Cell Biol 20:9138–9148
Nakamura N, Ramaswamy S, Vazquez F et al (2000) Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol 20:8969–8982
Stahl M, Dijkers PF, Kops GJ, Lens SM (2002) The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol 168:5024–5031
Medema RH, Kops GJ, Bos JL, Burgering BM (2000) AFX-like forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782–787
Vogt PK, Bader AG, Kang S (2006) PI 3-kinases: hidden potentials revealed. Cell Cycle 5:946–949
Zhao JJ, Roberts TM (2006) PI3 kinases in cancer: from oncogene artifact to leading cancer target. Sci STKE 2006: pe52
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619
Lam EW, Francis RE, Petkovic M (2006) FOXO transcription factors: key regulators of cell fate. Biochem Soc Trans 34:722–726
Kennedy SG, Wagner AJ, Conzen SD et al (1997) The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 11:701–713
Songyang Z, Baltimore D, Cantley LC et al (1997) Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci USA 94:11345–11350
Downward J (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10:262–267
Reed SI (1997) Control of the G1/S transition. Cancer Surv 29:7–23
Lloyd RV, Erickson LA, Jin L et al (1999) p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 154:313–323
Kops GJ, Burgering BM (1999) Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med 77:656–665
Carlsson P, Mahlapuu M (2002) Forkhead transcription factors: key players in development and metabolism. Dev Biol 250:1–23
Lehmann OJ, Sowden JC, Carlsson P et al (2003) Fox’s in development and disease. Trends Genet 19:339–344
Hu MC, Lee DF, Xia W, Golfman LS et al (2004) IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117:225–237
Burgering BM, Medema RH (2003) Decisions on life and death: FOXO forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol 73:689–701
Plas DR, Thompson CB (2003) Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem 278:12361–12366
Yang L, Xie S, Jamaluddin MS, Altuwaijri S et al (2005) Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem 280:33558–33565
Nam S, Smith DM, Dou QP (2001) Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem 276:13322–13330
Essafi A, Fernandez de Mattos S et al (2005) Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene 24:2317–2329
Fernandez de Mattos S, Essafi A, Soeiro I et al (2004) FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol 24:10058–10071
Alexia C, Bras M, Fallot G, Vadrot N et al (2006) Pleiotropic effects of PI-3′ kinase/Akt signaling in human hepatoma cell proliferation and drug-induced apoptosis. Ann NY Acad Sci 1090:1–17
Jin S, Pang RP, Shen JN, Huang G, Wang J, Zhou JG (2007) Grifolin induces apoptosis via inhibition of PI3K/AKT signalling pathway in human osteosarcoma cells. Apoptosis 12:1317–1326
Hu MC, Hung MC (2005) Role of IkappaB kinase in tumorigenesis. Future Oncol 1:67–78
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (grants 30300099 and 30770488), the Natural Scientific Foundation of Jiangsu Province (grants BK2003035 and BK2006547), the College and University Natural Scientific Research Program of Jiangsu Province (grants 03KJB180109 and 04KJB320114), the Technology Guidance Plan for Social Development of Jiangsu Province (grant BS2004526), and the Health Project of Jiangsu Province (grant H200632).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jinlong Shi and Li Zhang both contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, J., Zhang, L., Shen, A. et al. Clinical and biological significance of forkhead class box O 3a expression in glioma: mediation of glioma malignancy by transcriptional regulation of p27kip1 . J Neurooncol 98, 57–69 (2010). https://doi.org/10.1007/s11060-009-0045-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-0045-8