Abstract
Survivin is a member of the inhibitor of apoptosis family, and is expressed in various malignant tumors. Survivin overexpression has been reported to be a poorer prognostic factor in various malignancies. However, the prognostic value of survivin expression in patients with glioblastoma is still controversial. Therefore, in this study the role of survivin as a predictor for survival was investigated in patients with glioblastoma. Tissue specimens were obtained from 66 patients with glioblastoma treated with radiotherapy. Survivin expression was detected by an immunohistochemical method. Nuclear and cytoplasm survivin scores were defined by using the cell positivity and staining intensity. The scores were defined as follows, 0 (no staining), 1 (less than 50% of cell positivity and any staining), 2 (more than 50% of cell positivity and weak to moderate intensity) and 3 (more than 50% of cell positivity and strong intensity). The correlation between survivin scores and the overall survival rate was evaluated. Nuclear and cytoplasm survivin staining were noted in 47 and 58 patients, respectively. The number of patients with nuclear survivin score of 0, 1, 2 and 3, were 19 (28.8%), 26 (39.4%), 9 (13.6%) and 12 (18.2%), respectively. The 3-year overall survival rate of the nuclear survivin score 3 was 0%, significantly lower than the 11.6% of the nuclear survivin score ≤2 (P = 0.0003). Cytoplasm survivin score did not correlate with the prognosis. Nuclear survivin expression may be a useful biomarker for predicting prognosis in patients with glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma, the most malignant brain tumor in adults, is highly resistant to radiotherapy n and chemotherapy. The recent standard treatments of this disease are considered to be surgical resection to the extent feasible, followed by radiotherapy and chemotherapy including temozolomide. The median survival time of the patients with glioblastoma has remained approximately 12 months over the last few decades, even though multimodal therapies have been applied.
Survivin is 16.5-kDa protein and a member of the inhibitors of apoptosis family. It is expressed in fetal tissues and tumor cells but not in normal adult differentiated tissues [1, 2]. Survivin is thought to inhibit the processing of procaspase-3 and procaspase-7 by direct binding [3]. Survivin overexpression is reported to be an unfavorable prognostic factor in various malignancies, including prostate cancer [4], colorectal [5], pancreatic [6] and non-small cell lung carcinomas [7, 8]. However, several reports have shown survivin expression to be associated with a favorable prognosis in gastric cancer [9], transitional cell carcinoma of bladder [10], breast cancer [11] and osteosarcoma [12]. Chakravarti et al. [13] reported that survivin expression in glioblastoma patients was an adverse prognostic factor, by using semiquantitative western blot analysis. However, their study did not distinguish between nuclear and cytoplasm survivin expression and the prognostic value of survivin expression by immunohistochemistry is still controversial. Therefore, we assessed the prognostic value of nuclear and cytoplasm survivin expression by immunohistochemistry in patients with glioblastoma.
Materials and methods
Patients and specimens
Available tissue specimens were obtained from all of the 66 patients with pathologically confirmed glioblastoma treated with radiotherapy at the Gunma University Hospital (Maebashi Japan), Takasaki National Hospital (Takasaki Japan) and Maebashi Red Cross Hospital (Maebashi Japan) between 1982 and 2005. The median follow-up period was 12 months. Sixty-one patients died due to tumor progression and 5 patients were alive with follow-up period ranging from 15 to 60 months. The mean and median of age were 55.1 ± 16.2 and 59 years old, respectively. There were 40 male and 26 female patients. Gross total resection was performed in 18 patients, partial resection in 43 patients and biopsy in 5 patients. The mean and median radiation doses were 56.9 ± 9.3 Gy and 60 Gy, respectively. In general, radiotherapy was performed by the conventional therapy (5 days × 2 Gy/week). However, some patients were performed by hyper- and hypo-fractionated radiotherapy. Therefore, we used the biological effective dose (BED) for analysis and set the α/β ratio as 10.0 Gy. The mean and median BED were 68.7 ± 10.9 and 72.0, respectively. Chemotherapy was performed in 45 patients as an initial treatment before and/or after irradiation. There were 64 patients with glioblastoma multiforme in 64 patients and 2 with giant cell glioblastoma, and the pathological diagnosis was based on the WHO Classification of Tumors of the Nervous System (2000) [14]. The pathological features, including the extent of necrosis, vascularity, cell density and existence of giant cells were evaluated.
Immunohistochemistry
The tissue specimens were cut into 4 μm sections on cylan-coated slides, then were deparaffinized and hydrated. For antigen retrieval, they were heated by microwave for 75 min in Dako Target Retrieval Solution (Dako, CA, U.S.A.) at 93.0°C and were cooled for 20 min. After washing with Phosphate Buffered Saline (PBS), nonspecific binding was blocked with peroxidase-blocking solution (Dako) for 15 min. The specimens were incubated overnight at 4.0°C with anti-survivin antibody (Novus Biologicals, CO, U.S.A.) diluted with antibody diluent (Dako) at 1:300. Then, they were washed with PBS. They were incubated with a labeled-polymer conjugated second antibody EnVision + kit (Dako) for 30 min. After washing in PBS, the specimens were developed with 3,3′-diaminobenzidine tetrahydrochloride (Dako) for 2 min. Specimens were slightly counterstained with hematoxylin, and finally dehydrated and mounted. As a positive control, a known positive control (breast cancer) specimen was handled in parallel. As a negative control, a specimen was treated without a primary antibody in the incubation step.
Evaluation
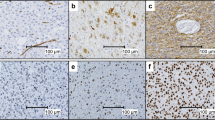
All immunohistochemical stained slides were pathologically assessed by one of the authors (K.O.), without knowledge of the clinical data. The cell nuclei and cytoplasm of more than 500 tumor cells were evaluated in each specimen. Only the viable glioblastoma areas were evaluated, while the necrotic and non-viable areas were excluded. Nuclear and cytoplasm survivin scores were evaluated with cell positivity and staining intensity. Scores were defined as follows, 0 (no staining), 1 (less than 50% of cell positivity and any intensity), 2 (more than 50% of cell positivity and weak to moderate intensity) and 3 (more than 50% of cell positivity and strong intensity). The correlation between nuclear and cytoplasmic survivin scores and overall survival were evaluated.
Statistical analysis
The overall survival period was calculated from the date of operation to the date of death or last follow-up. The Kaplan–Meier method was used for the overall survival curves, and differences were statistically analyzed with the Log-rank test. The multivariate analysis for overall survival was applied with the Cox proportional hazard model with a 95.0% CI. The differences were considered statistically significant at P < 0.05. All analyses were performed with SPSS 11.0 for Windows.
Results
The overall survival rates of all patients at 3 and 5 years were 11.1% and 5.9%, respectively (Fig. 1). The median survival period was 12 months. The overall survival based on clinical characteristics and survivin scores were shown in Table 1. The 3-year survival rates of patients aged <60 and ≥60 were 11.5% and 10.3%, respectively (P = 0.26). The 3-year survival rates of male and female were 5.4% and 19.2%, respectively (P = 0.71). As for the extent of surgery, the 3-year survival rates of patients performed gross total resection was 22.2%, significantly higher than 6.7% performed partial resection and biopsy (P = 0.02). The 3-year survival rates of patients irradiated with BED ≤72.0 and with BED >72.0 were 9.7% and 13.6%, respectively (P = 0.40). The 3-year survival rates of patients on which chemotherapy was performed was 11.6%, significantly higher than 10.0% patients on which chemotherapy was not performed (P = 0.04).
Nuclear and cytoplasm survivin staining were noted in 47 and 58 patients, respectively (Fig. 2). The nuclear survivin score 0, 1, 2 and 3, were 19 patients (28.8%), 26 patients (39.4%), 9 patients (13.6%) and 12 patients (18.2%), respectively. The 3-year overall survival rate of the nuclear survivin score 3 was 0%, significantly lower than 11.6% of the nuclear survivin score ≤2 (P = 0.0003, Fig. 3). The cytoplasm survivin score 0, 1, 2 and 3, were 8 patients (12.1%), 47 patients (71.2%), 6 patients (9.1%) and 5 patients (7.6%), respectively. These cytoplasm survivin scores did not correlate with the prognosis. With regard to histology, the 3-year overall survival rates in glioblastoma multiforme and giant cell glioblastoma were 11.4% and 0 %, respectively (P = 0.90). The other pathological features, including the extent of necrosis, vascularity, cell density and existence of giant cells were not significant prognostic factors (P = 0.94, P = 0.71, P = 0.13 and P = 0.64, respectively).
The results of multivariate survival analysis for the clinical characteristics and the nuclear survivin score were given in Table 2. The factor with the strongest impact on overall survival was nuclear survivin score (P = 0.003, hazard ratio = 0.35).
Discussion
Sasaki et al. [15] reported that survivin expression increased in parallel with malignant grade in astrocytic tumors. Kajiwara et al. [16] also reported that survivin expression was significantly associated with malignant grade, and they concluded that survivin might play an important role in the oncogenesis and progression of astrocytic tumors. They mentioned that the median survival period for patients with survivin positive tumors was shorter than that for patients with survivin negative tumors in astrocytic tumors [16]. Saito et al. [17] reported that simultaneous survivin expression in both nucleus and cytoplasm was worth prognosis in high-grade astrocytomas. Uematsu et al. [18] compared the survivin index and MIB-1 index, and investigated a positive linear correlation of them was weak. And they mentioned that survivin index was more sensitive marker to predict survival than MIB-1 index in low grade gliomas.
Previously 4 studies have been attempted among the immunohistochemical analyses of glioblastoma. Das et al. [19] firstly reported that the survivin was highly expressed in glioblastoma, but did not discuss the relationship to prognosis. Xie et al. [20] reported a difference in the survivin expression between primary glioblastoma and secondary glioblastoma, but also did not mention prognosis. Preusser et al. [21] reported that survivin did not seem to be useful as a prognostic factor in 104 patients with glioblastoma. Recently, Mellai et al. [22] showed that a positive linear correlation was found between survivin expression and proliferation, whereas inverse correlation did not found between survivin expression and apoptosis. However, they did not indicate the correlation between survivin expression and survival. The present study is the first report to show that the nuclear survivin score correlate with a worse prognosis in the patients with glioblastoma.
Fortugno et al. [23] reported that survivin existed in 2 distinct nuclear and cytoplasmic subcellular pools in human cervical carcinoma HeLa cells. Nuclear survivin has been proposed to serve in the maintenance of the integrity of the mitotic spindle in HeLa cells [24]. In many immunohistochemical studies, nuclear survivin expression has been shown to be an unfavorable factor for prognosis, including prostate cancer [4], rectal cancer [25], oesophageal squamous cell carcinoma [26], colorectal carcinoma [27], soft-tissue sarcomas [28], breast cancer [29], laryngeal squamous cell carcinoma [30], hepatocellular carcinoma [31], ovarian carcinoma [32] and non-small cell lung carcinoma [7, 8]. Shinohara et al. [7] suggested that strong nuclear staining of survivin may represent increased mitotic events, hence resulting in poor survival. Recently, it was reported that survivin has 3 splicing variants, survivin-ΔEx3, survivin-2B, and survivin wild type [33, 34]. Survivin-ΔEx3 exists in the nucleus, and nuclear survivin has been considered active in the maintenance of the integrity of the mitotic spindle. Yamada et al. [35] reported that survivin-ΔEx3 was significantly more increased in malignant brain tumors than benign brain tumors in vitro setting. Both survivin-2B and survivin wild type exist in the cytoplasm, and cytoplasmic survivin has been considered to be anti-apoptotic factor [36]. However, Islam et al. [37] reported that survivin wild type can inhibit apoptosis and survivin-2B does not. Immunohistochemically, a few studies have reported cytoplasmic survivin expression to be an unfavorable factor in patients with colorectal cancer [5], pancreatic cancer [6], and oral squamous cell carcinoma [38]. Previously, we reported that cytoplasmic survivin expression was an unfavorable factor in patients with cervical squamous cell carcinomas [39]. However in this study cytoplasmic expression is not a prognostic factor for survival in patients with glioblastoma. Shinohara et al. [7] suggested that cytoplasmic staining might represent the combined level of 2 functionally opposing variants of survivin wild type and survivin-2B, and therefore, as a result, cytoplasmic survivin expression was not a significant prognostic marker. Cytoplasmic survivin score was tended to be higher in relatively large cells (alike a giant cell; data not shown). In this report, we showed correlations between prognosis and the pathological features, including the existence of giant cells. We additionally analyzed the correlation between cytoplasmic survivin score and the existence of giant cells. Then, higher survivin score was significantly correlated with the existence of giant cells (Pearson test; P = 0.048). Though the existence of giant cells does not correlate to tumor phenotype exactly, cytoplasmic survivin score might have a correlation with tumor phenotypes.
Survivin has been expected to serve as a treatment target in various malignancies. Yang et al. [40] reported that apoptosis was induced when the activity of survivin was blocked by the expression of dominant-negative mutant survivin in various tumors, including pancreatic cancer cell lines, breast cancer cell lines, and a colon carcinoma cell line, but not normal cell lines. Ansell et al. [41] reported that cell growth was significantly inhibited by using an antisense oligonucleotide approach in aggressive non-Hodgkin’s lymphomas. Van Houdt et al. [42] reported that transcriptional targeting therapy of the survivin promoter significantly inhibited the growth of glioma xenografts in vivo.
Survivin expression was associated with radiation resistance in various malignancies. Chakravarti et al. reported that survivin enhanced double-strand DNA break repair and tumor cell metabolism, and thereby survivin suppressed radiation-induced cell death in primary human glioblastoma [43]. Saito et al. [44] showed that survivin suppression by small interfering RNA (siRNA) enhanced radiosensitivity in glioma cells. And they analyzed the cell death induced by radiation was mitotic cell death correlated with chromosome instability. Rodel et al. [25] reported that the attenuation of survivin mRNA and protein by siRNA enhanced radiation sensitivity in colorectal cancer cell lines. They suggested that anti-survivin strategies improved the radiation response, and may be ultimately correlated with the better outcome [25, 43, 44].
In conclusion, we suggest that nuclear survivin score is a useful biomarker to predict survival outcome in patients with glioblastoma.
References
Li F, Ambrosini G, Chu EY et al (1998) Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 396:580–584. doi:10.1038/25141
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921. doi:10.1038/nm0897-917
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58:5315–5320
Shariat SF, Lotan Y, Saboorian H et al (2004) Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer 100:751–757. doi:10.1002/cncr.20039
Kawasaki H, Altieri DC, Lu CD et al (1998) Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58:5071–5074
Kami K, Doi R, Koizumi M et al (2004) Survivin expression is a prognostic marker in pancreatic cancer patients. Surgery 136:443–448. doi:10.1016/j.surg.2004.05.023
Shinohara ET, Gonzalez A, Massion PP et al (2005) Nuclear survivin predicts recurrence and poor survival in patients with resected nonsmall cell lung carcinoma. Cancer 103:1685–1692. doi:10.1002/cncr.20951
Lu B, Gonzalez A, Massion PP et al (2004) Nuclear survivin as a biomarker for non-small-cell lung cancer. Br J Cancer 91:537–540. doi:10.1038/sj.bjc.6602027
Okada E, Murai Y, Matsui K et al (2001) Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett 163:109–116. doi:10.1016/S0304-3835(00)00677-7
Lehner R, Lucia MS, Jarboe EA et al (2002) Immunohistochemical localization of the IAP protein survivin in bladder mucosa and transitional cell carcinoma. Appl Immunohistochem Mol Morphol 10:134–138. doi:10.1097/00022744-200206000-00007
Kennedy SM, O’Driscoll L, Purcell R et al (2003) Prognostic importance of survivin in breast cancer. Br J Cancer 88:1077–1083. doi:10.1038/sj.bjc.6600776
Trieb K, Lehner R, Stulnig T et al (2003) Survivin expression in human osteosarcoma is a marker for survival. Eur J Surg Oncol 29:379–382. doi:10.1053/ejso.2002.1415
Chakravarti A, Noll E, Black PM et al (2002) Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20:1063–1068. doi:10.1200/JCO.20.4.1063
Kleihues P, Burger PC, Scheithauer BW (1993) Histological typing of tumours of the central nervous system. World Health Organization international histological classification of tumours, 2nd edn. Springer-Verlag, Berlin
Sasaki T, Lopes MB, Hankins GR et al (2002) Expression of survivin, an inhibitor of apoptosis protein, in tumors of the nervous system. Acta Neuropathol 104:105–109. doi:10.1007/s00401-002-0532-x
Kajiwara Y, Yamasaki F, Hama S et al (2003) Expression of survivin in astrocytic tumors correlation with malignant grade and prognosis. Cancer 97:1077–1083. doi:10.1002/cncr.11122
Saito T, Arifin MT, Hama S et al (2007) Survivin subcellular localization in high-grade astrocytomas: simultaneous expression in both nucleus and cytoplasm is negative prognostic marker. J Neurooncol 82:193–198. doi:10.1007/s11060-006-9267-1
Uematsu M, Ohsawa I, Aokage T et al (2005) Prognostic significance of the immunohistochemical index of survivin in glioma: a comparative study with the MIB-1 index. J Neurooncol 72:231–238. doi:10.1007/s11060-004-2353-3
Das A, Tan WL, Teo J et al (2002) Expression of survivin in primary glioblastomas. J Cancer Res Clin Oncol 128:302–306. doi:10.1007/s00432-002-0343-4
Xie D, Zeng YX, Wang HJ et al (2006) Expression of cytoplasmic and nuclear survivin in primary and secondary human glioblastoma. Br J Cancer 94:108–114. doi:10.1038/sj.bjc.6602904
Preusser M, Gelpi E, Matej R et al (2005) No prognostic impact of survivin expression in glioblastoma. Acta Neuropathol 109:534–538. doi:10.1007/s00401-005-0992-x
Mellai M, Caldera V, Patrucco A et al (2008) Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res 28:109–118
Fortugno P, Wall NR, Giodini A et al (2002) Survivin exists in immunochemically distinct subcellular pools and is involved in spindle microtubule function. J Cell Sci 115:575–585
Uren AG, Wong L, Pakusch M et al (2000) Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol 10:1319–1328. doi:10.1016/S0960-9822(00)00769-7
Rodel F, Hoffmann J, Distel L et al (2005) Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res 65:4881–4887. doi:10.1158/0008-5472.CAN-04-3028
Grabowski P, Kuhnel T, Muhr-Wilkenshoff F et al (2003) Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer 88:115–119. doi:10.1038/sj.bjc.6600696
Sarela AI, Macadam RC, Farmery SM et al (2000) Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 46:645–650. doi:10.1136/gut.46.5.645
Kappler M, Kotzsch M, Bartel F et al (2003) Elevated expression level of survivin protein in soft-tissue sarcomas is a strong independent predictor of survival. Clin Cancer Res 9:1098–1104
Span PN, Sweep FC, Wiegerinck ET et al (2004) Survivin is an independent prognostic marker for risk stratification of breast cancer patients. Clin Chem 50:1986–1993. doi:10.1373/clinchem.2004.039149
Pizem J, Cor A, Gale N (2004) Survivin expression is a negative prognostic marker in laryngeal squamous cell carcinoma and is associated with p53 accumulation. Histopathology 45:180–186. doi:10.1111/j.1365-2559.2004.01925.x
Ito T, Shiraki K, Sugimoto K et al (2000) Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology 31:1080–1085. doi:10.1053/he.2000.6496
Cohen C, Lohmann CM, Cotsonis G et al (2003) Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol 16:574–583. doi:10.1097/01.MP.0000073868.31297.B0
Mahotka C, Liebmann J, Wenzel M et al (2002) Differential subcellular localization of functionally divergent survivin splice variants. Cell Death Differ 9:1334–1342. doi:10.1038/sj.cdd.4401091
Mahotka C, Wenzel M, Springer E et al (1999) Survivin-deltaEx3 and survivin-2B: two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res 59:6097–6102
Yamada Y, Kuroiwa T, Nakagawa T et al (2003) Transcriptional expression of survivin and its splice variants in brain tumors in humans. J Neurosurg 99:738–745
O’Connor DS, Wall NR, Porter AC et al (2002) A p34cdc2 survival checkpoint in cancer. Cancer Cell 2:43–54. doi:10.1016/S1535-6108(02)00084-3
Islam A, Kageyama H, Hashizume K et al (2000) Role of survivin, whose gene is mapped to 17q25, in human neuroblastoma and identification of a novel dominant-negative isoform, survivin-beta/2B. Med Pediatr Oncol 35:550–553. doi:10.1002/1096-911X(20001201)35:6<550::AID-MPO12>3.0.CO;2-Y
Lo ML, Pannone G, Staibano S et al (2003) Survivin expression in oral squamous cell carcinoma. Br J Cancer 89:2244–2248. doi:10.1038/sj.bjc.6601402
Suzuki Y, Oka T, Yoshida D et al (2006) Correlation between survivin expression and prognosis in cervical squamous cell carcinomas treated with radiation therapy. Gynecol Oncol 104:642–646. doi:10.1016/j.ygyno.2006.10.005
Yang L, Cao Z, Yan H et al (2003) Coexistence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res 63:6815–6824
Ansell SM, Arendt BK, Grote DM et al (2004) Inhibition of survivin expression suppresses the growth of aggressive non-Hodgkin’s lymphoma. Leukemia 18:616–623. doi:10.1038/sj.leu.2403281
Van Houdt WJ, Haviv YS, Lu B et al (2006) The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg 104:583–592. doi:10.3171/jns.2006.104.4.583
Chakravarti A, Zhai GG, Zhang M et al (2004) Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene 23:7494–7506. doi:10.1038/sj.onc.1208049
Saito T, Hama S, Izumi H et al (2008) Centrosome amplification induced by survivin suppression enhances both chromosome instability and radiosensitivity in glioma cells. Br J Cancer 98:345–355. doi:10.1038/sj.bjc.6604160
Acknowledgements
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirai, K., Suzuki, Y., Oka, K. et al. Nuclear survivin expression predicts poorer prognosis in glioblastoma. J Neurooncol 91, 353–358 (2009). https://doi.org/10.1007/s11060-008-9720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9720-4