Abstract
Valproic acid (VPA) inhibits histone deacetylase and has been reported to induce apoptosis in glioma. We report 44 heavily pretreated pediatric patients with high-grade glioma or diffuse intrinsic pontine glioma who received VPA as oral continues maintenance treatment with individual dose adaptation. The tumor status when starting the drug was: no measurable disease in 12, measurable but stable disease in 12, and measurable progressive disease in 22 patients. Average trough blood levels of VPA were 99 mg/l. The most frequent complaint was somnolence (three patients), but no severe toxicity was reported. One relapse patient responded, early progression of disease was observed in three frontline patients and in six relapsed patients. Median overall survival duration for all patients was 1.33 years, with large differences between first-line (5-year overall survival, 44%) and relapse therapy (5-year overall survival, 14%). This shows that valproate is safe in this patient population. The moderate tumor efficacy encourages studying the drug further as an element of multi-agent protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
This report describes a further piece of information considering the use of valproate (valproic acid, VPA) as novel caner drug in pediatric patients. VPA (di-n-propylacetic acid, MW 144.21) is a new anticancer agent, but the drug has been used for treatment of other diseases. It was first used as antiepileptic drug [1] and mood stabilizer [2], with very-well documented pharmacokinetic and toxicity profiles [3, 4]. Desired trough serum levels for antiepileptic efficacy range between 50–100 mg/l (equivalent to 0.3–0.6 mM). At therapeutic levels, the drug is teratogenic [5]. At higher drug doses, toxic effects such as thrombocytopenia, pancreatitis, hypothyroidism, and polycystic ovarian syndrome [6] are common side effects. In parallel to its antiepileptic mode of action, VPA was found to induce differentiation in the neuroectodermal [7] and hematopoetic systems [8, 9], and even an anti-tumor response was described in vitro [10, 11] and in patients [12, 13]. As an underlying mechanism for this observation inhibition of histone deacetylase by VPA was identified by Phiel [14]. Histone acetylation is a major mechanism of epigenetic regulation, loosening the DNA packing. Histone deacetylase down-regulates gene expression by wrapping the DNA tighter around the histone proteins, thus making genes less accessible to promoter binding proteins. Histone-deacetylase inhibition results in more acetylase groups binding to the histones and therefore promotes gene expression. Since this finding, a large body of literature describing details of VPA’s mechanism has been produced [15, 16] confirming that VPA induces tumor cell differentiation and inhibits proliferation as well as it increases the rate of apoptosis [17] and immunogenicity [18]. P21/WAF activation independent of p53 activation is one of the mechanisms of action [19] as well as ERalpha degradation [20] and inactivation of NF-KappaB [21], but the complete picture of intracellular events is far more complex including acetylation of p53 directly [22]. Furthermore, these effects are accompanied by the reduction of the tumor cells’ metastatic and angiogenic potency. Newly designed drugs with more specific functions, inhibiting certain types of histone deacetylases, have also been developed [18, 23–25]. As studies with histone deacetylase inhibitors move from preclinical models [26] to clinical trials [27], and studies in adult patients have shown tolerability [28], we asked if the drug can be given safely to heavily pretreated pediatric patients with high grade glioma and diffuse intrinsic pontine glioma.
Pediatric high-grade glioma (HGG) and diffuse intrinsic pontine glioma (DIPG) remain challenging diseases. Progress in developing treatments is slow because the diseases are rare and therefore are frequently combined in clinical studies [29, 30–34]. Radiation therapy is generally accepted as beneficial for both disease groups. Chemotherapy has been shown to be beneficial in children [17, 35] and in adults [36]; however, the overall results leave much room for improvement.
The HIT-GBM® study (acronym for “Hirntumor—glioblastoma multiforme”) is a prospective cohort-comparison study for this patient population [37] and a prospective registry part for control patients [29, 38, 39] following other treatment approaches. To elucidate whether Valproic acid (VPA) treatment contributes to long term survival in this pediatric patient population, VPA was added to the treatment protocol at the end of the intensive radiochemotherapy treatment protocol HIT-GBM-C as a rear-window. In addition, VPA was used as relapse treatment in other patients, who had relapsed on the intensive chemotherapy treatment before reaching the prescribed time to start VPA. This report describes our findings in both patient groups.
Material and methods
Treatment protocol
Valproic acid treatment was a part of the complex HIT-GBM-C treatment protocol. Patients were eligible if they were between 3 and 18 years with newly diagnosed DIPG, or grade III or IV HGG. Eligibility required an informed consent approved by the Ethics Committee, as required by rules and regulations at that time and in accordance with the 1964 Declaration of Helsinki. Treatment started with standard fractionated focal radiation and simultaneous chemotherapy. The first chemotherapy cycle was the PEV cycle [40, 41], which consists of cisplatin, 20 mg/m2/day on days 1–5; etoposide, 100 mg/m2 on days 1–3; and vincristine, 1.5 mg/m2 only on day 5. During radiation, weekly vincristine injections were administered until the last week of radiation therapy, in which patients were given one cycle of PEIV [38, 41], combining PEV with ifosfamide, 1.5 g/m2/day on days 1–5. After simultaneous radiation-chemotherapy, chemotherapy continued with further cycles of PEIV in weeks 10, 14, 18, 22, 26, and 30, as well as vincristine in weeks 13, 17, 21, 25, and 29, using the same doses. Upon completion of eight intensive HIT-GBM-C chemotherapy cycles, VPA was given as additional maintenance treatment. If the tumor was stable by MRI criteria after the first two cycles of HIT-GBM-C chemotherapy, two more cycles of chemotherapy were recommended, but if the disease remained stable at cycle 4 or beyond, further chemotherapy cycles were skipped to start VPA treatment earlier. Also, in case of tumor progression during the chemotherapy, patients were offered valproic acid as relapse treatment.
VPA was given orally as a single agent, starting with 10 mg/kg/day in week 1 and 20 mg/kg/day in week 2. Serum trough levels were then analyzed and the dosage adjusted to reach target serum levels of 100–150 mg/l, unless unwanted side effects had already occurred. Before and after 6 weeks of VPA treatment, the tumor was evaluated by MRI. Tumor response was defined by one-dimensional measures on MRI images. Progressive disease was defined as 25% or more tumor growth. Partial response was defined as 50% or more tumor shrinkage. Measurement in between were defined as stable disease.
Statistics
In this report we extracted data from the HIT-GBM database, which documents 310 patients diagnosed with HGG or DIPG between 1995 and 2003. This population was recently described in detail [42]. To avoid selection bias and describe the real world of this patient population, only patients receiving VPA for epilepsy during chemotherapy were excluded, while patients treated with major violations during the chemotherapy protocol were not excluded. The analysis includes patients following the original HIT-GBM-C protocol and patients originally only registered as control patients but then treated following the relapse protocol with VPA. Patients who were treated following the protocol but did not reach therapeutic valproic acid level were not excluded. The results were analyzed using the statistical package SPSS 12.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Patients
Forty-four of 310 HIT-GBM database [42] patients received valproic acid for tumor treatment. The epidemiologic characteristics were as expected from this patient population; details are described in Table 1.
Toxicity and serum levels
Despite the heavily pretreated patient population, the toxicity of VPA was very mild. There was no toxic death, no SAE was reported. Adverse events only required reporting in case of grade III or IV. No such event was reported. However, minor toxic reactions were reported in few patients (Grade I or II). These included: somnolence, three patients; difficulties in concentrating, one; enuresis, one; increased sensitivity to light, one; thrombocytopenia, one; and anemia, one. Serum trough levels were reported in 23 patients. The mean level of 99 mg/l (standard deviation: 26 mg/l) was well in keeping with the target level. The range of 52–175 mg/l was wide, and toxicity was reported more frequently in the patients with lower drug levels. There was no detectable relationship between VPA level and outcome.
Outcome
Tumor status was evaluated after 6 weeks of VPA treatment. Of the 22 patients who started treatment according to protocol, 19 remained stable. Progressive disease occurred in 1 of 12 patients who started VPA therapy with no measurable tumor and 2 of ten patients who started VPA treatment with stable measurable tumors. Of the 22 patients who received VPA as relapse treatment, 14 had MRI examinations at the required time points before and 6 weeks after starting VPA. Of those one responded (PR), seven had stable disease, and six had further progression of their disease.
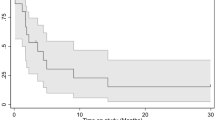
The overall median survival for all 44 patients after starting valproic acid was 1.33 years (SE, 0.41; 95% confidence interval, 0.98–3.60 years). The survival time largely depended on the tumor status when treatment was initiated, with the data confirming what appears intuitive: patients who started the treatment without documented tumor progression had better results (Fig. 1). Median survival durations were 4.05 years (95% CI, 2.91–5.19 years) for these patients, compared with 0.58 years (95% CI, 0.13–1.02) for patients who started valproic acid after relapse, and the 5-year overall survival rates were, respectively, 44% ± 12.7% SE compared with 14% ± 7% (P = 0.0013, log–rank test). However, even among patients who started valproate with a progressive tumor, there were three long-term survivors alive after 4.5, 4.95, and 5 years. Gender had no influence on survival (P = 0.7); the influences of tumor location [42], extent of resection [43], presence of metastases [44], and histologic type [42] were similar to those of previous analyses but because of small numbers were not significant.
Overall survival of pediatric patients with HGG or DIPG treated with valproic acid. Twenty-two patients started treatment as maintenance treatment in the protocol HIT-GBM-C without signs of tumor progression (black line), and 22 patients started Valproate as relapse treatment (gray line). Data are classified as censored, when the patient is alive at the last date of information
Discussion
Our analysis of the data showed that valproic acid treatment was well tolerated in 44 heavily pretreated pediatric HGG and DIPG patients when given orally as continuous treatment with individual dose adaptation. This is an important finding and will allow building this way to give valproate into multi-agent treatment protocols.
Valproic acid is one of the few drugs for which doses are frequently modulated according to drug levels in the blood. However, neither the tumor effect nor the side effects are closely related to drug levels in this study. In a reported case series of severe valproic acid intoxications [45], patients with peak valproic-acid concentrations above 450 mg/l were more likely to develop significant clinical effects and have longer hospital stays than were patients with lower concentrations. In that same study, peak valproic acid concentration above 850 mg/l was more likely than were lower concentrations to be associated with coma, respiratory depression, aspiration, or metabolic acidosis.
In adult patients intravenous valproate given over 5 days reached a maximal tolerated dose of 60 mg/kg/day with neurotoxicity being the dose limiting toxicity [28]. Those doses appear far higher, but they were only given for 5 days, resulting in fact in a lower total cumulative dose than given here. The comparison with our experience shows that the dose limiting toxicity was dependent of the route or schedule how the drug was given. In pediatric patients a feared rare side effect of antiepileptic VPA treatment is hepatopathia, which has an incidence of 1:5000 in the pediatric population. Worldwide, 250 fatal cases have been described, as reported by Koenig and colleagues [46]. In that study, the most recently described cases had a median drug level of only 78 mg/l. Hepatopathia is associated with increased liver enzyme (alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (gamma-GT)) levels in blood, decreased levels of fibrinogen, and increased levels of ammonia. Pre-existing metabolic diseases and co-medication with other antiepileptic drugs were frequent among these children [46]. Discontinuation of valproic acid and supportive care, including intravenous carnitine [47], is the recommended treatment if severe toxic reactions occur. In the 44 patients reviewed in our study, such toxic reactions did not occur despite the intensive chemotherapy that these children had prior to starting Valproate. Possibly the prior intensive chemotherapy functioned as a filter, preventing children with predispositioning disease from ever starting valproic acid treatment: Nevertheless, these data should not lead to a neglect of metabolic diagnostics before starting Valproate, particularly in fist-line treatment. The inverse relationship between drug levels and reported toxicity noted in our patients was caused by the dose recommendation of the study: doses were only increased in the absence of side effects. The data show that a wide range of inter-individual tolerance to the drug exists and that dosing by individual tolerability allows higher doses in some patients than does dosing by body weight or body surface area or drug levels.
When considering tumor efficacy one needs to acknowledge the particular poor prognostic patient group involved in this study (Table 1). Among the patients who received Valproate as the last part of the HIT-GBM-C protocol, the drug did not shrink any of the remaining heavily pretreated tumors. Still, findings of only three early progressive tumors among 22 patients and a projected 5-year overall survival of more than 40% are quite promising in this patient population [42]. This is confirmed by findings in our second subset of patients. One of these relapse patients responded. This patient had been described in details before [48]. We had also described another patient previously with recurrent HGG who responded to Valproate treatment [12], who was not a part of this study. Furthermore, one of us (P–H. D.) is aware of other patients with response to VPA outside of this data set, which are to be reported separately. However, even the fact that seven of the 14 patients prospectively enrolled in the database had stabilization of disease, and that the survival curve (Fig. 1) is strongly suggestive of a positive effect, and in particular that there were three long-term surviving patients confirm the impression that valproic acid has a stabilizing effect on pediatric HGG.
Still, the benefit these patients had from valproic acid was not overwhelming, and the golden key to cure this disease was not yet found by using VPA as a single agent. The future utility of the drug might be in combination with classic chemotherapeutic agents or radiation. Synergism was shown in preclinical models [15, 49–51], and preliminary clinical data suggests that the chemotherapy toxicity is not largely increased when Valproate is given as an antiepileptic during chemotherapy [52].
In conclusion, we found that valproic acid as a tumor treatment for pediatric patients with HGG and DIPG is safe when dosed by individual tolerance. Evidence for efficacy is provided. We recommend further clinical studies combining the molecule with chemotherapeutic agents, radiation, or both.
References
Lance JW, Anthony M (1975) Sodium valproate in the management of intractable epilepsy: comparison with clonazepam. Proc Aust Assoc Neurol 12:55–60
Post RM, Uhde TW (1983) Treatment of mood disorders with antiepileptic medications: clinical and theoretical implications. Epilepsia 24(Suppl 2):S97–S108. doi:10.1111/j.1528-1157.1983.tb04652.x
Anthony M, Hinterberger H, Lance JW (1977) Plasma sodium valproate levels and clinical response in epilepsy. Clin Exp Neurol 14:208–215
Limdi NA, Knowlton RK, Cofield SS, Ver Hoef LW, Paige AL, Dutta S et al (2007) Safety of rapid intravenous loading of valproate. Epilepsia 48:478–483. doi:10.1111/j.1528-1167.2007.00989.x
Brown NA, Kao J, Fabro S (1980) Teratogenic potential of valproic acid. Lancet 1:660–661. doi:10.1016/S0140-6736(80)91159-9
Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV (1993) Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med 329:1383–1388. doi:10.1056/NEJM199311043291904
Regan CM (1985) Therapeutic levels of sodium valproate inhibit mitotic indices in cells of neural origin. Brain Res 347:394–398. doi:10.1016/0006-8993(85)90207-0
Selby R, Nisbet-Brown E, Basran RK, Chang L, Olivieri NF (1997) Valproic acid and augmentation of fetal hemoglobin in individuals with and without sickle cell disease. Blood 90:891–893
Kieslich M, Schwabe D, Cinatl J Jr, Driever PH (2003) Increase of fetal hemoglobin synthesis indicating differentiation induction in children receiving valproic acid. Pediatr Hematol Oncol 20:15–22. doi:10.1080/713842215
Knupfer MM, Hernaiz-Driever P, Poppenborg H, Wolff JE, Cinatl J (1998) Valproic acid inhibits proliferation and changes expression of CD44 and CD56 of malignant glioma cells in vitro. Anticancer Res 18:3585–3589
Driever PH, Knupfer MM, Cinatl J, Wolff JE (1999) Valproic acid for the treatment of pediatric malignant glioma. Klin Padiatr 211:323–328
Witt O, Schweigerer L, Driever PH, Wolff J, Pekrun A (2004) Valproic acid treatment of glioblastoma multiforme in a child. Pediatr Blood Cancer 43:181. doi:10.1002/pbc.20083
Driever PH, Wagner S, Hofstadter F, Wolff JE (2004) Valproic acid induces differentiation of a supratentorial primitive neuroectodermal tumor. Pediatr Hematol Oncol 21:743–751. doi:10.1080/08880010490514985
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741. doi:10.1074/jbc.M101287200
Das CM, Aguilera D, Vasquez H, Prasad P, Zhang M, Wolff JE et al (2007) Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol 85:159–170. doi:10.1007/s11060-007-9402-7
Blaheta RA, Michaelis M, Driever PH, Cinatl J Jr (2005) Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev 25:383–397. doi:10.1002/med.20027
Dong G, Wang L, Wang CY, Yang T, Kumar MV, Dong Z (2008) Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J Pharmacol Exp Ther 325:978–984. doi:10.1124/jpet.108.137398
Johnson J, Pahuja A, Graham M, Hering B, Hancock WW, Bansal-Pakala P (2008) Effects of histone deacetylase inhibitor SAHA on effector and FOXP3+ regulatory T cells in rhesus macaques. Transplant Proc 40:459–461. doi:10.1016/j.transproceed.2008.01.039
Huang L, Sowa Y, Sakai T, Pardee AB (2000) Activation of the p21WAF1/CIP1 promoter independent of p53 by the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through the Sp1 sites. Oncogene 19:5712–5719. doi:10.1038/sj.onc.1203963
Yi X, Wei W, Wang SY, Du ZY, Xu YJ, Yu XD (2008) Histone deacetylase inhibitor SAHA induces ERalpha degradation in breast cancer MCF-7 cells by CHIP-mediated ubiquitin pathway and inhibits survival signaling. Biochem Pharmacol 75:1697–1705. doi:10.1016/j.bcp. 2007.10.035
Domingo-Domènech J, Pippa R, Tápia M, Gascón P, Bachs O, Bosch M (2008) Inactivation of NF-kappaB by proteasome inhibition contributes to increased apoptosis induced by histone deacetylase inhibitors in human breast cancer cells. Breast Cancer Res Treat (in press)
Carlisi D, Vassallo B, Lauricella M, Emanuele S, D’Anneo A, Di Leonardo E et al (2008) Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. Int J Oncol 32:177–184
Hassig CA, Symons KT, Guo X, Nguyen PM, Annable T, Wash PL et al (2008) KD5170, a novel mercaptoketone-based histone deacetylase inhibitor that exhibits broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 7:1054–1065. doi:10.1158/1535-7163.MCT-07-2347
Fournel M, Bonfils C, Hou Y, Yan PT, Trachy-Bourget MC, Kalita A et al (2008) MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther 7:759–768. doi:10.1158/1535-7163.MCT-07-2026
Petrella A, D’Acunto CW, Rodriquez M, Festa M, Tosco A, Bruno I et al (2008) Effects of FR235222, a novel HDAC inhibitor, in proliferation and apoptosis of human leukaemia cell lines: role of annexin A1. Eur J Cancer 44:740–749. doi:10.1016/j.ejca.2008.01.023
Spiller SE, Ditzler SH, Pullar BJ, Olson JM (2008) Response of preclinical medulloblastoma models to combination therapy with 13-cis retinoic acid and suberoylanilide hydroxamic acid (SAHA). J Neurooncol 87:133–141. doi:10.1007/s11060-007-9505-1
Blumenschein GR Jr, Kies MS, Papadimitrakopoulou VA, Lu C, Kumar AJ, Ricker JL et al (2008) Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs 26:81–87. doi:10.1007/s10637-007-9075-2
Atmaca A, Al-Batran SE, Maurer A, Neumann A, Heinzel T, Hentsch B et al (2007) Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer 97:177–182. doi:10.1038/sj.bjc.6603851
Wolff JE, Boos J, Kuhl J (1996) HIT-GBM: multicenter study of treatment of children with malignant glioma. Klin Padiatr 208:193–196
Bouffet E, Khelfaoui F, Philip I, Biron P, Brunat-Mentigny M, Philip T (1997) High-dose carmustine for high-grade gliomas in childhood. Cancer Chemother Pharmacol 39:376–379. doi:10.1007/s002800050586
Estlin EJ, Lashford L, Ablett S, Price L, Gowing R, Gholkar A et al (1998) Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children’s Cancer Study Group. Br J Cancer 78:652–661
Liu L, Vapiwala N, Munoz LK, Winick NJ, Weitman S, Strauss LC et al (2001) A phase I study of cranial radiation therapy with concomitant continuous infusion paclitaxel in children with brain tumors. Med Pediatr Oncol 37:390–392. doi:10.1002/mpo.1215
Broniscer A, Gajjar A (2004) Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist 9:197–206. doi:10.1634/theoncologist.9-2-197
Donaldson SS, Laningham F, Fisher PG (2006) Advances toward an understanding of brainstem gliomas. J Clin Oncol 24:1266–1272. doi:10.1200/JCO.2005.04.6599
Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA et al (1989) The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol 7:165–177. doi:10.1007/BF00165101
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Wolff JE, Boos J, Krahling KH, Jurgens H (1996) Second temporal remission in a malignant glioma with trofosfamide and etoposide: a case report. Klin Padiatr 208:190–192
Wolff JE, Molenkamp G, Westphal S, Pietsch T, Gnekow A, Kortmann RD et al (2000) Oral trofosfamide and etoposide in pediatric patients with glioblastoma multiforme. Cancer 89:2131–2137. doi :10.1002/1097-0142(20001115)89:10<2131::AID-CNCR14>3.0.CO;2-J
Wolff JE, Westphal S, Molenkamp G, Gnekow A, Warmuth-Metz M, Rating D et al (2002) Treatment of paediatric pontine glioma with oral trophosphamide and etoposide. Br J Cancer 87:945–949. doi:10.1038/sj.bjc.6600552
Wolff JE, Wagner S, Sindichakis M, Pietsch T, Gnekow A, Kortmann RD et al (2002) Simultaneous radiochemotherapy in pediatric patients with high-grade glioma: a phase I study. Anticancer Res 22:3569–3572
Wolff JE, Wagner S, Reinert C, Gnekow A, Kortmann RD, Kuhl J et al (2006) Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J Neurooncol 79:315–321. doi:10.1007/s11060-006-9147-8
Wolff JE, Classen CF, Wagner S, Kortmann RD, Palla SL, Pietsch T, Kühl J, Gnekow A, Kramm CM (2008) Subpopulations of malignant gliomas in pediatric patients: analysis of the HIT-GBM database. J Neurooncol 87(2):155–164
Kramm CM, Wagner S, Van Gool S, Schmid H, Strater R, Gnekow A et al (2006) Improved survival after gross total resection of malignant gliomas in pediatric patients from the HIT-GBM studies. Anticancer Res 26:3773–3779
Benesch M, Wagner S, Berthold F, Wolff JE (2005) Primary dissemination of high-grade gliomas in children: experiences from four studies of the Pediatric Oncology and Hematology Society of the German Language Group (GPOH). J Neurooncol 72:179–183. doi:10.1007/s11060-004-3546-5
Spiller HA, Krenzelok EP, Klein-Schwartz W, Winter ML, Weber JA, Sollee DR et al (2000) Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol 38:755–760
Koenig SA, Buesing D, Longin E, Oehring R, Haussermann P, Kluger G et al (2006) Valproic acid-induced hepatopathy: nine new fatalities in Germany from 1994 to 2003. Epilepsia 47:2027–2031. doi:10.1111/j.1528-1167.2006.00846.x
Bohan TP, Helton E, McDonald I, Konig S, Gazitt S, Sugimoto T et al (2001) Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology 56:1405–1409
Wagner S, Warmuth-Metz M, Emser A, Gnekow AK, Strater R, Rutkowski S et al (2006) Treatment options in childhood pontine gliomas. J Neurooncol 79:281–287. doi:10.1007/s11060-006-9133-1
Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA et al (2005) Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 114:380–386. doi:10.1002/ijc.20774
Zaskodova D, Rezacova M, Vavrova J, Vokurkova D, Tichy A (2006) Effect of valproic acid, a histone deacetylase inhibitor, on cell death and molecular changes caused by low-dose irradiation. Ann N Y Acad Sci 1091:385–398. doi:10.1196/annals.1378.082
Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN (2005) In vivo synergy between topoisomerase II and histone deacetylase inhibitors: predictive correlates. Mol Cancer Ther 4:1993–2000. doi:10.1158/1535-7163.MCT-05-0194
Masoudi A, Elopre M, Amini E, Nagel ME, Ater JL, Gopalakrishnan V, Wolff JEA (2008) Influence of Valproic acid on outcome of high-grade gliomas in children. AntiCancer Res (accepted)
Wagner S, Reinert C, Schmid HJ, Liebeskind AK, Jorch N, Langler A et al (2005) High-dose methotrexate prior to simultaneous radiochemotherapy in children with malignant high-grade gliomas. Anticancer Res 25:2583–2587
Wagner S, Csatary CM, Gosztonyi G, Koch HC, Hartmann C, Peters O et al (2006) Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. APMIS 114:731–743. doi:10.1111/j.1600-0463.2006.apm_516.x
Acknowledgment
Supported by Deutsche Kinderkrebsstiftung, Germany and Core Grant CA 16672. The original HIT-GBM® Database has been used before as a source for analyses with different focuses and different subsets of data [39, 42–44, 53, 54]. This analysis only uses the data generated prospectively for including patients formally enrolled on the treatment study and the registry or at least the registry until 2003 with follow up updated in 2006. The other HIT-GBM publications have been based on overlapping but not identical patient populations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolff, J.E.A., Kramm, C., Kortmann, RD. et al. Valproic acid was well tolerated in heavily pretreated pediatric patients with high-grade glioma. J Neurooncol 90, 309–314 (2008). https://doi.org/10.1007/s11060-008-9662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9662-x