Abstract
Gliomas are the most common type of primary tumor in the human central nervous system. STAT3, a signal transducer and activator of transcription 3, is over expressed in gliomas. Its involvement in tumorgenesis can be attributed to its ability to induce cell proliferation and inhibit apoptosis. Double-stranded decoy oligodeoxynucleotides (ODNs) which correspond closely to the STAT3 response element within the c-fos promoter are a potential tool for inhibiting a variety of tumor cell growth. To investigate its therapeutic potential in malignant gliomas, a 15-mer double-stranded decoy ODN mimicking STAT3-specific cis-elements was transfected into two glioma cell lines, U251 and A172. The STAT3 decoy ODN treatment specifically blocked STAT3 signaling and subsequently inhibited U251 and A172 cell proliferation by inducing apoptosis and cell-cycle arrest. The ODN treatment also decreased transcription and translation of downstream STAT3 target genes including c-myc, cyclin D1 and bcl-xl in both cell lines. Thus, targeted blockade of the STAT3 signaling pathway with a decoy ODN is a potential anti-glioma therapeutic approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas, the most common primary tumors in the human central nervous system, are clinically classified into four grades. Malignant gliomas, classified as grades 3 and 4, are highly invasive and aggressive tumors that have been resistant to conventional treatment including surgical resection, radiotherapy, and chemotherapy [1]. Despite the great advances made in treatments recently, the median survival for patients with high-grade gliomas is still less than one year [2]. With delineation of the molecular essence of malignant etiology, gene therapy has become an attractive treatment strategy. The discovery of effective targets and strategies will be key for development of gene therapies.
Experiments have suggested that the transcription factor STAT3 (signal transducer and activator of transcription 3) of the Jak-STAT signaling pathway may be an effective gene therapy target for treatment of a variety of cancers [3]. Endogenous STAT3 is involved in embryo development, physiologic regulation of cell growth, survival, and differentiation, and pathogenesis of malignancies. Aberrant STAT3 activation has been found in many malignancies, including primary malignant gliomas and numerous cancer-derived cell lines, including U87MG, LN-18, T98G, A172, U251, and U343MG [4–6]. The constitutive activation of STAT3 contributes to the transformation and progression of malignant gliomas by up-regulating genes encoding bcl-xl, bcl-2, c-myc, cyclin D1, and survivin, which inhibit apoptosis, promote cell proliferation, advance tumor angiogenesis, and enable evasion of immune surveillance [7, 8]. Rahaman et al. [7] used the Janus kinase inhibitor AG490 and a dominant-negative mutant protein of STAT3 to treat malignant glioma U251 cells. By inhibiting constitutive activation of STAT3, AG490 markedly reduced the proliferation rate of U251 cells. The association of persistently activated STAT3 and the proliferation and survival of malignant gliomas suggests that STAT3 acts as a promising molecular target of gene therapy for malignant gliomas.
Transcription factor decoy (TFD) oligodeoxynucleotides (ODNs) are a novel tool for targeting inhibition of the STAT3 signaling pathway. TFD ODNs have been shown to inhibit STAT3 pathway activation in squamous cell carcinoma of the head and neck (SCCHN) [9] and in human lung cancer [10]. The TFD ODN treatments were demonstrated to significantly attenuate the STAT3 signaling pathway, and this STAT3 blockade resulted in a depressed proliferation and increased apoptosis in both SCCHN and lung cancer cell lines. These findings revealed the therapeutic potential of STAT3 decoy ODN for malignant gliomas.
In the current study, decoy ODNs designed to specifically inhibit STAT3 signaling were employed to investigate the influence of this treatment on human malignant glioma cell lines U251 and A172. Our data showed that STAT3 decoy ODNs could be very efficiently transfected into glioma cells. The ODN treatment specifically blocked STAT3 signaling by inducing apoptosis and cell-cycle arrest, which resulted in significant inhibition of proliferation of glioma cells. Thus, targeted inhibition of STAT3 signaling with a decoy ODN is a novel anti-glioma therapeutic approach.
Materials and methods
Human samples
Human samples were collected from the archives of the Neurosurgery Institute of Shandong University Qilu Hospital (Jinan, China). The samples included 12 glioblastoma multiforme (GBM) specimens and four normal human neural tissue control samples which had been resected for intracranial decompression. This study was approved by the Institutional Review Board of Shandong University, and written informed consent forms were obtained from all participants.
Cell culture
Human glioma U251 and A172 cell lines from GBM tumors were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM medium (GIBCO/BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GIBCO/BRL), 100 IU/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37°C.
Antibodies and other reagents
Anti-STAT3, anti-phospho-specific STAT3 (Tyr705, Ser727), anti-bcl-xl, anti-cyclin D1, and anti-β-actin primary antibodies and horseradish peroxidase-conjugated second antibody were purchased from Cell Signaling Technology (New England BioLabs, USA). Annexin V-FITC Apoptosis Detection Kit was obtained from Bender (Austria). AG490 was purchased from Calbiochem (Merck, Germany).
STAT3 decoy ODN and mutant control decoy ODN
A double-stranded decoy oligonucleotide and a mutant control decoy oligonucleotide were synthesized as described previously [9, 10]. The STAT3 decoy ODN sequence was 5′-CATTTCCCGTAAATC-3′, 3′-GTAAAGGGCATTTAG-5′, and the mutant control decoy sequence was 5′-CATCTTGCCAATATC-3′, 3′-GTAGAACGGTTATAG-5′. They were synthesized, annealed, and purified by TaKaRa Biotechnology (Dalian, China).
Western blot analysis
Protein extracts were obtained from the human samples and cell lines by lysing the samples in 20 mmol/l Tris–HCl buffer (pH 7.4), 150 mmol/l NaCl, 1 mmol/l Na3VO4, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l PMSF, 50 mmol/l NaF, and 1% NP-40. The protein samples (30 μg/lane) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Millipore, Bedford, MA, USA). The membrane was blocked in Tris-buffered saline with 5% (w/v) nonfat dry milk, and then incubated with a primary antibody for 2 h at room temperature and horseradish peroxidase-conjugated secondary antibody for 45 min at room temperature. Immunoreactive proteins were visualized using an enhanced chemiluminescence (ECL) detection system (Pierce, Rockford, IL, USA). The bands were examined by densitometry using AlphaEaseFC software (Version 4.0.0, Alpha Innotech Corporation).
ODN transfection
Lipofectamine 2000 was obtained from Invitrogen (Carlsbad, CA, USA). Different concentrations (12.5, 25 and 50 nmol/l) of decoy ODN and mutant control decoy ODN were transfected into U251 and A172 glioma cells according to manufacturer’s instructions. The cells were incubated for 24 h in 5% CO2 at 37°C, and washed once with PBS.
Transfection efficiency assay
Flow cytometry was used to examine ODN transfection efficiency. The 5′ end of the decoy ODN was labeled with fluorescence isothiocyanate (FITC) (TaKaRa Biotechnology, Dalian, China). After transfection for 6 h, U251 and A172 cells were harvested by trypsinization, washed extensively with PBS, and examined by flow cytometry (FACS Calibur, BD Biosciences, CA, USA).
Luciferase reporter assay
A luciferase reporter assay was used to examine the specificity of the decoy ODN to the STAT3 signaling pathway. STAT6 was used as a control because its constitutive phosphorylation has been detected in some human malignancies, such as Reed-Sternberg cell cancer from patients with Hodgkin disease [11]. U251 and A172 cells were plated at a density of 1 × 104 cells per well in 96-well plates (Costar, Corning, NY, USA) and incubated for 24 h at 37°C. Increasing amounts of the STAT3 decoy or mutant control decoy ODNs were co-transfected with 50 ng of the pGL3-STAT3 luciferase reporter plasmid, or pGL3-STAT6 luciferase reporter plasmid, and 5 ng of pRL-TK (Promega, Madison, WI, USA) as an internal control for transfection efficiency. Six hours after transfection, the cells were incubated in fresh medium for an additional 24 h. The activities of firefly and renilla luciferases were measured by the Dual-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions.
Cell proliferation assay
Two methods were adopted to detect the inhibitory potency of STAT3 decoy ODNs in glioma cell proliferation. First, a cell-counting assay was used to examine both the time-dependent and dose-dependent inhibitory effect of STAT3 decoy ODNs on glioma cell proliferation. U251 and A172 cells were seeded in 24-well plates (Costar, Corning, NY, USA) at a density of 2.0 × 104 cells per well and 2.2 × 104 cells per well, respectively. After 24 h, the cells were treated with a range of doses of decoy ODN, mutant control decoy ODN, and TE (Tris-EDTA, vehicle). The cells were harvested at different time points (24, 48, and 72 h) and quantitated by trypan blue exclusion. Viability exceeding 90% was regarded as non-toxic.
The second method was an MTT assay. U251 and A172 cells were plated in 96-well plates at a density of 4 × 103 cells per well and 6 × 103 cells per well, respectively. After 24 h, the cells were transfected with decoy ODN (0, 25, or 50 nmol/l), mutant control decoy ODN (50 nmol/l), or TE (50 nmol/l) and incubated in growth medium for 24, 48, or 72 h. Twenty microliters of MTT solution was added to each well and incubated at 37°C for 4 h. After the addition of 100 μl DMSO to each well, the optical density value at 540 nm (OD540) was measured. Cells from each line were also treated with the Janus kinase specific inhibitor AG490 (0, 25, or 50 μmol/l) under the same conditions as a control for the inhibition effect, and 0.1% DMSO was used as a blank control.
Cell cycle analysis by flow cytometry
U251 and A172 cells were seeded in six-well plates (Costar, Corning, NY, USA), then transfected with ODNs at a dose of 50 nmol/l. After 24 h, the cells were harvested with 0.25% trypsin (Sigma), washed twice in PBS, and fixed overnight with 70% (v/v) ethanol. Following incubation in 50 μg/ml Rnase A at 37°C for 30 min, cell nuclei were stained with propidium iodide (PI, Sigma) for 30 min. The resulting DNA histogram was examined and analyzed in a flow cytometer system (FACS Calibur, BD Biosciences, CA, USA) equipped with Modifit software.
Cell apoptosis assay
Twenty-four hours after transfection with 50 nmol/l STAT3 decoy ODN or mutant control decoy ODN, U251, and A172 cells were harvested, washed, and resuspended in 100 μl Annexin V binding buffer, and subsequently stained with Annexin V-FITC and propidium iodide (Annexin V-FITC Detection Kit, Bender, Austria) according to the manufacturer’s recommendations. Data acquisition and analysis were performed by flow cytometry using CellQuest software (BD Biosciences).
Reverse transcription polymerase chain reaction (RT-PCR) and primers
U251 and A172 cells were treated with 50 nmol/l ODNs for 24 h. Total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First-strand cDNA synthesis was carried out on 2 μg samples of total RNA using M-MLV reverse transcriptase according to the manufacturer’s procedure (Promega). PCR was performed using 4× dNTP mixture, MgCl2, Taq DNA polymerase with 10× reaction buffer, and upstream primer. PCR primers were synthesized by Shanghai Genecore Biotechnologies (Shanghai, China). Temperature conditions, primers, and product sizes are shown in Table 1. The PCR products were analyzed by electrophoresis and photographed using AlphaEaseFC (Multilmage light cabinet, Alpha Innotech).
Statistical analysis
Data were expressed as mean ± SD from at least three independent experiments. Statistical analysis was performed with SPSS software (version 13.0). P < 0.05 was considered statistically significant in all cases.
Results
Overexpression and constitutive activation of STAT3 in human malignant glioma samples and cell lines
Western blot analysis with anit-STAT3 and anti-phospho-specific STAT3 (Tyr705, Ser727) revealed more active STAT3 expression in human malignant glioma samples than in normal neural tissues (Fig. 1; other results are shown in the supplementary material). Tyr-705-phospho-STAT3 expression and Ser-727-phospho-STAT3 expression were observed in 75% (9 of 12) and 66.67% (8 of 12) of the human malignant glioma samples, respectively. Neither active form of STAT3 was observed in the normal neural tissue samples (Fig. 1; other results are shown in the supplementary material). Additionally, overexpressed STAT3 and its two active forms, Tyr-705-phospho-STAT3 and Ser-727-phospho-STAT3, were identified in both U251 and A172 cells (Fig. 1). This similarity between glioma tissue samples and GBM cell lines confirmed that the two cell lines should be considered appropriate in-vitro models in our line of research.
Overexpression and constitutive activation of STAT3 in human malignant glioma specimens and cell lines. Protein extracts from human specimens, U251 cells and A172 cells, were examined by Western blot using anti-STAT3, anti-phospho-specific STAT3 (Tyr705, Ser727), and β-actin antibodies. NHN: normal human neural tissue
Incorporation of STAT3 decoy ODN into malignant glioma cells
Flow cytometry of glioma cells collected 6 h after transfection with FITC-labeled STAT3 decoy ODN showed that the decoy ODN was introducted into U251 and A172 cells efficiently in a dose-dependent manner (Figs. 2a, b). The MFI means of U251 and A172 cells reached a maximum of 64.03 and 56.22, respectively, at 50 nmol/l decoy ODN.
Transfection of STAT3 decoy ODN into U251 and A172 cells. U251 (a) and A172 (b) cells were treated with FITC-labeled STAT3 decoy ODN, mediated by Lipofectamine 2000, at 0, 6.25, 12.5, 25, or 50 nmol/l. After a 24-h incubation, the transfection efficiencies were determined by flow cytometry. MFI: mean fluorescence intensity
Decoy ODN specifically blocked the STAT3 signaling pathway in malignant glioma cells
As shown in Fig. 3a, transfection with STAT3 decoy ODN substantially reduced transcription activity of STAT3 in U251 and A172 cells. However, the transfection did not cause a similar decrease in STAT6 transcription activity. Western blot analysis further showed that the STAT3 decoy ODN did not have significant effect on the levels of phosphorylated STAT3 (Tyr705 and Ser727) in U251 and A172 cells (Fig. 3b). These data confirmed that STAT3 decoy ODN could specifically block the STAT3 signaling pathway.
Specific blockage of STAT3 signaling pathway by decoy ODN in malignant glioma cells. (a) U251 and A172 cells treated with STAT3 decoy ODN or mutant control decoy ODN were co-transfected with pGL3-STAT3-TK-Luciferase construct, pGL3-STAT6-TK-Luciferase construct, or pGL3-TK-Luciferase vector. After transfecting for 6 h, cells were incubated for 24 h and harvested. Luciferase activity was measured, and the firefly luciferase activity was normalized relative to renilla luciferase activity. The polylines represent the ratios between individual normalized luciferase activity and that of pGL3-TK-Luciferase reporter plasmid. Values are expressed as the mean ± SD of three independent experiments. * P < 0.05 compared with the control and mutant control decoy groups. (b) Whole-cell extracts (30 μg/lane) from human malignant glioma cells treated with 50 nmol/l STAT3 decoy ODN for 0, 24, 48, and 72 h. Western blot analysis was performed using anti-STAT3, anti-phospho-specific STAT3 (Tyr705, Ser727), or β-actin antibodies
STAT3 decoy ODN inhibited malignant glioma cell proliferation
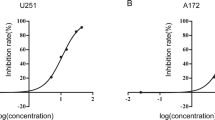
The cell-counting assay demonstrated that the growth rates of both U251 and A172 cells were inhibited after treatment with STAT3 decoy ODN for 48 h or 72 h (P < 0.05). This inhibition was both time-dependent (Fig. 4a) and dose-dependent (Fig. 4b). The inhibition rate detected by MTT assay confirmed that STAT3 decoy ODN treatment caused potent growth inhibition in the two cell lines in a time and dose-dependent manner (Fig.4c), with maximum inhibition of 57.74% in U251 cells and 49.33% in A172 cells. In contrast, the inhibition efficiency of malignant glioma cells treated with mutant control decoy ODN was negligible compared with that in cells treated with the decoy ODN. The Janus kinase specific inhibitor AG490 (positive control) resulted in even more pronounced inhibition of glioma cell proliferation (66.67% in U251 and 63.67% in A172) (Fig. 4d).
STAT3 decoy ODN inhibition of malignant glioma cell proliferation. (a) U251 and A172 cells were transfected with STAT3 decoy ODN or mutant control decoy ODN at 50 nmol/l for 24, 48, or 72 h. Cells were counted by trypan blue exclusion. (b) U251 and A172 cells were transfected with STAT3 decoy ODN at concentrations of 0, 6.25, 12.5, 25, and 50 nmol/l. After incubation for 24, 48, or 72 h, the cells were counted. (c) As described in (b), the inhibition rates were detected and counted via MTT assay. (d) U251 and A172 cells were treated with AG490. After incubation for 24, 48, or 72 h, the inhibition rates were detected and counted via MTT assay. Values are expressed as the mean ± SD of three independent experiments. * P < 0.05 compared with the control and mutant control decoy groups
STAT3 decoy ODN induced cell-cycle arrest in malignant glioma cells
As shown in Table 1, 24 h after treatment with 50 nmol/l STAT3 decoy ODN, the portion of U251 cells in G0/G1 phase increased from 49.58 ± 1.09% to 54.89 ± 1.35% (P < 0.05), while the portion of cells in S phase decreased from 42.36 ± 1.50% to 37.89 ± 0.67% (P < 0.05). Meanwhile, more than 10% and 7% of A172 cells similarly shifted with respect to the percentages in G0/G1 phase and S phase (Table 2). The cells treated with the mutant control decoy ODN showed no obvious alterations. These findings demonstrated that the arrest of the cell cycle induced by STAT3 decoy ODN played an essential role in the inhibition of malignant glioma cell growth.
STAT3 decoy ODN promoted apoptosis in malignant glioma cells
As shown in Figs. 5a, b, the results of the Annexin V/PI staining assay for apoptosis revealed that STAT3 decoy ODN treatment led to increased apoptosis. The portions of apoptotic U251 cells and A172 cells increased from 5.44% to 23.57% and from 5.20% to 22.48%, respectively, following the STAT3 decoy ODN treatment (P < 0.05). In contrast, after mutant control decoy ODN treatment only 6.81% of U251 cells and 6.16% of A172 cells were apoptotic (P > 0.05 vs. no treatment). These data demonstrated that STAT3 decoy ODN interfered with the anti-apoptotic program in malignant glioma cells.
Apoptosis induced by STAT3 decoy ODN in malignant glioma cells. After transfection with 50 nmol/l STAT3 decoy ODN for 24 h, U251 (a) and A172 (b) cells were detected by flow cytometry using double staining with FITC-AnnexinV and PI antibodies. The right histogram shows the percentage of apoptotic cells. Values are expressed as the mean ± SD from three independent experiments. * P < 0.05 compared with the control and mutant control decoy groups
STAT3 decoy ODN down-regulated STAT3-targeted genes in malignant glioma cells
The decoy ODN strategy is based on the competition between the endogenous cis-elements within the regulatory regions of target genes and exogenously added molecules mimicking the specific cis-elements [12]. Transfection of STAT3 decoy ODN into cells will competitively inhibit the binding of STAT3 with its endogenous cis-elements, resulting in subsequent alteration of downstream targeted genes expression. To examine the consequences of the decoy ODN on STAT3-targeted genes, we detected anti-apoptotic gene bcl-xl, and cell cycle control genes cyclin D1 and c-myc on the transcription and translation levels. Following STAT3 decoy ODN treatment (50 nmol/l), the mRNA levels of c-myc, cyclin D1, and bcl-xl in U251 cells were decreased by 52%, 50% and 27.67%, respectively (Fig. 6a; P < 0.05 vs. TE). In A172 cells, the mRNA levels of c-myc, cyclin D1, and bcl-xl were decreased by 46.67, 40.67, and 28.87%, respectively (Fig. 6b; P < 0.05 vs. TE). Meanwhile, the mutant control decoy ODN treatment did not affect mRNA levels of the malignant glioma cells.
Down-regulation of STAT3-targeted genes by STAT3 decoy ODN in malignant glioma cells. After transfection with 50 nmol/l STAT3 decoy ODN for 24 h, U251 (a) and A172 (b) cells were harvested and mRNA levels of c-myc, cyclin D1, and bcl-xl were examined by RT-PCR. The right histogram shows the densitometry values of each gene after normalization to its corresponding internal control. U251(c) and A172 (d) cells were transfected with 50 nmol/l STAT3 decoy ODN for 24 h, whole-cell extracts were obtained and protein expression levels were examined. The right histogram represents the relative expression levels of bcl-xl and cyclin D1 as determined by Western blot. Values are expressed as the mean ± SD from three independent experiments. * P < 0.05 compared with the control and mutant control decoy groups
Following STAT3 decoy ODN treatment, the protein expression levels of cyclin D1 and bcl-xl in U251 cells decreased by 47.33 and 85.66%, respectively, relative to controls (Fig. 6c). Consistent with the findings in U251 cells, the protein expression levels of cyclin D1 and bcl-xl in A172 cells were attenuated by 38.25 and 3.63%, respectively (Fig. 6d). Hence, STAT3 decoy ODN treatment-induced inhibition of the proliferation of human malignant glioma cells was associated with down-regulation of the expression of STAT3-targeted genes.
Discussion
Human gliomas arise from either neuroepithelial cells or their progenitor cells, which are regulated by various extracellular stimuli, including cytokines such as interleukin-6 (IL-6) and interferons, and growth factors such as platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) [4]. The factors specifically bind to their receptors on the cell surface and trigger the intrinsic tyrosine kinase activity to activate the tyrosine kinase Jaks, which phosphorylates the tyrosine residue (705) of STAT3 [13]. Upon activation, STAT3 dimerizes via the reciprocal phosphotyrosine-src homology domain 2 (phosphtyrosine-SH2), and subsequently translocates into the nucleus, binds to its specific DNA response elements within the promoter region of target genes, and activates their transcription [14]. The downstream target genes of STAT3 include bcl-2, c-myc, cyclin D1, survivin, mcl-1 [15–18], VEGF, IL-10, and TGF-β [19–21], which can protect apoptosis, enhance cell proliferation, promote angiogenesis, and evade immune surveillance [22]. With the elucidation of important signal transduction cascades, aberrant STAT3 activation was found to play a critical role in the transformation and progression of various malignancies [17–19, 23–25].
The idea that STAT3 signaling is critical to malignant glioma generation came from the observation that aberrant STAT3 is frequently detected in many primary malignant gliomas and numerous cancer-derived cell lines [4–6]. Subsequent studies further confirmed that constitutive activation of STAT3 contributes to the transformation and progression of malignant glioma by inhibiting apoptosis, promoting cell proliferation, advancing tumor angiogenesis, and evading immune surveillance [7, 8]. Based on these observations, experimental studies to inhibit malignant glioma cell growth by targeted inhibition of the STAT3 signaling pathway have been carried out. RNA interference (RNAi) has been used to treat gliomas. Konnikova et al. [26] reported that knockdown of STAT3 expression could induce morphologic and biochemical changes in glioblastoma A172 cells consistent with apoptosis. Iwamaru et al. [27] induced apoptosis in malignant glioma cells both in vitro and in vivo. Other strategies of targeted inhibition of the aberrant STAT3 signaling pathway, including inhibition of upstream kinases [5, 7, 28], and dominant-negative mutants [7], also resulted in the depressed proliferation, elevated apoptosis, and modulation of STAT3 target genes in several malignant glioma cell lines. Such findings indicate that STAT3 may be an ideal molecular target for gene therapy in malignant gliomas. Thus, STAT3 inhibition in malignant glioma has been gaining increasing attention.
We examined the existence of aberrant STAT3 expression and detected the frequency of phosphorylated-STAT3 in human malignant glioma samples and cell lines using Western blot analysis. We found that STAT3 was overexpressed and constitutively activated in human malignant glioma samples.
Previous research indicated that decoy ODNs work via competitive inhibition [12]. That is, transfected “decoy” ODN is expected to competitively inhibit the binding of activated STAT3 and endogenous cis-elements in its downstream target genes. The present observations provided critical direct evidence that decoy ODN can specifically block the binding of activated STAT3 and the endogenous cis-elements. Through luciferase reporter gene assay, it was demonstrated that STAT3 decoy ODN could specifically block STAT3 transactivation in U251 and A172 cells that had been transfected with STAT3-Luciferase construct. Following the results, the inhibition happens after STAT3 activation, so decoy ODN could not alter the expression of active STAT3. Subsequent Western blot analysis confirmed that STAT3 decoy ODN did not change the expression level of active STAT3 in U251 and A172 cells. The results indicate that decoy ODN specifically blocks the STAT3 signaling pathway via the target blockage of the binding of activated STAT3 and endogenous cis-elements existing in its downstream target genes.
Because STAT3 decoy ODN is only a small molecule of 15 oligonucleotides, it was relatively easy to transfect into malignant giloma cells. The transfection produced specific blockade of the STAT3 signal pathway, followed by marked down-regulation of STAT3 downstream oncogenes, including anti-apoptosis gene bcl-xl and cell cycle control genes c-myc and cyclin D1, at both transcription and translation levels. These resulted in cell_cycle progression arrested from G0/G1 to S phase and promoted cell apoptosis, which synergistically contributed to malignant glioma cell-growth suppression.
In conclusion, targeted blocking of the STAT3 signaling pathway with a decoy ODN has the potential to be employed as an effective anti-glioma therapy. Future investigations, including experimental neoplastic model experiments and clinical trials, are needed in order to propel further progression of this potential targeted cancer therapy.
References
Wechsler-Reya R, Scott MP (2001) The developmental biology of brain tumors (review). Annu Rev Neurosci 24:385–428
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA (2001) Malignant glioma: genetics and biology of a grave matter. Genes Dev 15:1311–1333
Ihle JN (1996) STATs: signal transducers and activators of transcription (review). Cell 84:331–334
Schaefer LK, Menter DG, Schaefer TS (2000) Activation of Stat3 and Stat1 DNA binding and transcriptional activity in human brain tumour cell lines by gp130 cytokines. Cell Signal 12:143–151
Akasaki Y, Liu G, Matundan H, Ng H, Yuan X, Zeng Z, Black K, Yu JS (2006) A peroxisome proliferator-activated receptor-agonist, troglitazone, facilitates caspase-8 and-9 activities by increasing the enzymatic activity of Protein-tyrosine phosphatase-1β on human glioma cell. J Biochem 281:6165–6174
Rahaman SO, Vogelbaum MA, Haque SJ (2005) Aberrant stat3 signaling by interleukin-4 in malignant glioma cells: Involvement of IL-13Ra2. Cancer Res 65:2956–2963
Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ (2002) Inhibition of constitutively active STAT3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene 21:8404–8413
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE Jr (1999) STAT3 as an oncogene. Cell 98:295–303
Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, Grandis JR (2003) Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci USA 100:4138–4143
Zhang X, Zhang J, Wang L, Wei H, Tian Z (2007) Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer 7:149
Mustafa B, Maria RB, Heinz B, Meir W (2003) Signal transducer and activator of transcription proteins in leukemias. Blood 101:2940–2954
Morishita R, Tomita N, Kaneda Y, Ogihara T (2004) Molecular therapy to inhibit NFkappaB activation by transcription factor decoy oligonucleotides. Curr Opin Pharmacol 4:139–146
Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R (2002) Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 62:6659–6666
Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P (2001) DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem 276:6675–6688
Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19:2474–2488
Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, Sekikawa A, Kawada M, Suzuki K, Kayahara T, Fukui H, Sawada M, Chiba T (2004) STAT3 is constitutively activated and supports cell survival in association with surviving expression in gastric cancer cells. Oncogene 23:4921–4929
Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R (2006) Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther 5:621–629
Kube D, Holtick U, Vockerodt M, Ahmadi T, Haier B, Behrmann I, Heinrich PC, Diehl V, Tesch H (2001) STAT3 is constitutively activated in hodgkin cell lines. Blood 98:762–770
Qiu Z, Huang C, Sun J, Qiu W, Zhang J, Li H, Jiang T, Huang K, Cao J (2007) RNA interference-mediated signal transducers and activators of transcription 3 gene silencing inhibits invasion and metastasis of human pancreatic cancer cells. Cancer Sci 98:1099–1106
Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A (2006) Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 25:2520–2530
Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A (2006) Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta1. J Exp Med 203:1021–1031
Yu H, Jove R (2004) The STATs of cancer-new molecular targets come of age. Nat Rev Cancer 4:97–105
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513
Alvarez JV, Grenlinch H, Sellers WR, Meyerson M, Frank DA (2006) Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res 66:3162–3168
Nilsson MB, Langley RR, Fidler IJ (2005) Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res 65:10794–10800
Konnikova L, Kotecki M, Kruger MM, Cochran BH (2003) Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer 3:23
Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26:2435–2444
Ren W, Duan Y, Yang Y, Ji Y, Chen F (2008) Down-regulation of Stat3 induces apoptosis of human glioma cell: a potential method to treat brain cancer. Neurol Res 22 (in press)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, J., Li, G., Sun, T. et al. Blockage of the STAT3 signaling pathway with a decoy oligonucleotide suppresses growth of human malignant glioma cells. J Neurooncol 89, 9–17 (2008). https://doi.org/10.1007/s11060-008-9590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9590-9