Abstract
Various radiation-induced tumors, including meningioma, glioma, and sarcoma, have been reported; however, metachronous intracranial double tumors induced by radiation therapy are extremely rare. A 1-year-old boy had undergone tumor removal and craniospinal radiation therapy (30 Gy) for cerebellar medulloblastoma. At 24 years old, parasagittal meningioma developed in the left parietal region and was totally removed. Six years later, an infiltrative tumor was newly found in the right fronto-temporal white matter. The patient underwent stereotactic biopsy, and the tumor was found to be an anaplastic astrocytoma. Chromosomal analysis by fluorescence in situ hybridization (FISH) revealed loss of heterozygosity (LOH) of 1p. As the patient had previously had craniospinal irradiation, no additional radiation therapy was delivered. He underwent chemotherapy with temozolomide and the disease is now stable. Since both secondary tumors were located within the area of previous radiation and the patient did not have any genetic disease predisposing him to tumors, radiation therapy was considered to be responsible for their tumorigenesis. To our knowledge, this case is the fourth case of radiation-induced double CNS tumors arising after radiotherapy to be described in the literature. Whenever radiation is administered to children or young adults, careful serial screening studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been well documented that irradiation induces neoplasm [1–18], and it is thought that the risk of developing radiation-induced intracranial tumors is approximately 1–3% [9]. Most frequently reported radiation-induced tumors are meningioma, glioma, and sarcoma [8, 9, 11]. Harrison reviewed radiation-induced meningioma in 296 cases collected from the literature [16], and Salvati reviewed 116 cases of radiation-induced glioma [5]. To the best of our knowledge, however, radiation-induced double intracranial tumors are extremely rare, and only three cases have been reported [2, 4, 7]. In this report, the authors described radiation-induced left parasagittal meningioma and right cerebral anaplastic astrocytoma appearing 23 and 29 years, respectively, after radiation therapy for cerebellar medulloblastoma.
Case report
In 1978, a 1-year-old boy was admitted to the hospital with symptoms of raised intracranial pressure for 1 month. On examination, the child had ataxia, hypotonia, and the anterior fontanel was tense, but he did not have papilledema. Plain skull X-ray showed suture diastasis. Right vertebral angiography revealed a tumor stain feeding from the left PICA. Computed tomography (CT) revealed a cerebellar vermian tumor with staining after the intravenous administration of contrast medium. The fourth ventricle was extremely compressed anteriorly and to the left. Severe hydrocephalus was observed. On February 27, 1978, a right ventriclo-peritoneal shunt was performed, and the patient then underwent a craniotomy with partial removal of the tumor on March 9, 1978. Microscopic examination demonstrated medulloblastoma (Fig. 1). The patient was subsequently treated with postoperative radiation therapy directed at the whole brain (30 Gy) and spinal axis (30 Gy). The patient tolerated radiotherapy well, but pituitary function deteriorated slightly. Therefore, he was treated with growth hormone replacement until he was 15 years old. Brain imaging via CT and MRI was obtained at regular intervals with stable changes consistent with a patient history of surgery and radiation, with no evidence of recurrence within the initial tumor bed region.
In 2001, at the age of 24, the patient noted the onset of mild headache. A neurological exam at that time did not reveal any other focal neurological deficits. When the patient was riding a bicycle, he was involved in a traffic accident. MRI demonstrated a left posterior parietal parasagittal mass (Fig. 2a). Left common carotid angiography revealed a tumor stain feeding from the left occipital artery. On March 5, 2001, total removal of the tumor was performed, and pathology demonstrated meningotheliomatous meningioma, WHO grade I (Fig. 2b). The patient did well following this resection with no neurological deficits. Follow-up MRI after 4 years did not show any tumor residue or recurrence.
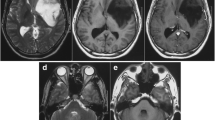
In 2006, at the age of 29, follow-up MRI showed a small intra-axial lesion in the right corona radiata (Fig. 3a), which was not enhanced after Gd-DTPA administration. After 10 months, MRI showed an enlarged infiltrative lesion in the white matter of the right fronto-temporal lobes, hypointense on T1-weighted image (WI), hyperintense on T2-WI, and mild enhancement by Gd-DTPA (Fig. 3b, c). A neurological exam did not reveal any focal neurological deficits. The patient underwent stereotactic biopsy to obtain diagnostic materials. Hematoxylin and eosin stain showed pleomorphic astrocytes and small, round, polygonal, and multinucleated cells. There was no definitive evidence of microvascular proliferation or necrosis (Fig. 4a). Tumor cells stained positive for GFAP and negative for synaptophysin (Fig. 4b). A moderate elevation in Ki-67 immunolabeling indicated an increase in the proliferative index of this tumor (MIB-1 index = 5.1%). Many p53-positive cells were observed (Fig. 4c). Chromosomal arm 1p and 19q deletion analysis was performed by fluorescence in situ hybridization (FISH), which revealed loss of heterozygosity (LOH) of 1p, but did not show 19q loss, regardless of the abnormal number of chromosome 19 (Fig. 5). According to these results, the tumor was found to be an anaplastic astrocytoma (WHO grade III).
(a) Axial T2-weighted MR image 28 years after irradiation showing a high intensity small lesion in the right corona radiate. (b) Axial T2-weighted MR image 29 years after irradiation showing the high intensity lesion extending from the corona radiating to the fronto-temporal subcortical region. (c) Axial T1-weighted MR image with gadolinium contrast showing mild enhancement in the lesion
(a) Photomicrographs of secondary anaplastic astrocytoma 29 years after initial irradiation showing pleomorphic astrocytes and small, round, polygonal, and multinucleated cells (H & E stain, original magnification ×200). (b) Immunohistochemically, the tumor cells were positive for GFAP. (c) p53 Protein was expressed in many of the tumor cells
FISH analysis in secondary anaplastic astrocytoma. (a) Dual probe hybridization for chromosome 1 showing a decreased number of red signals in the nucleus, indicating deletion of 1p36. (b) Dual probe hybridization for chromosome 19 showing no decreased number of red signals, indicating no deletion of 19q13
Neither the patient nor his family had any known genetic disease predisposing cancer. Germ line mutation of p53 gene was not found. As the patient previously had craniospinal irradiation, no additional radiation therapy was delivered, and he underwent chemotherapy with temozolomide. The disease is now stable.
Discussion
Radiation therapy is considered an integral part of the treatment of intracranial tumors; however, there are well-documented sequelae of radiation therapy, including radiation necrosis, leukoencephalopathy, arteritis, and postirradiation tumor development. The accepted criteria for incriminating radiation as the cause of human CNS tumors are well-defined [8] as follows: (1) the tumor was not present prior to irradiation; (2) the tumor must have arisen in the area included within the radiotherapeutic beam; (3) there was a reasonable interval between radiotherapy and the detection of the second tumor (usually several years); (4) a histological difference existed between the primary and subsequent tumors; (5) the patient must not suffer from pathologies favoring the development of tumors such as von Recklinghausen’s disease. In our case, secondary tumors of meningioma and anaplastic astrocytoma arose within the irradiation field, which did not exist prior to irradiation. In addition, the latency period between irradiation and tumor development was 23 and 29 years, respectively, and the patient did not have genetic or predisposing conditions for secondary malignancy; therefore, the tumors of our patient were diagnosed as radiation-induced intracranial tumors.
Concerning the risk of developing a second neoplasm, several factors, such as the radiation type and dose, tissue vulnerability, underlying diseases, and additional chemotherapy, may all play an important role. Patient age is also known to be an important factor. The majority of the patient with radiation-induced meningioma was irradiated during childhood, and the cumulative actuarial risk of a secondary meningioma following cranial irradiation in children at 25 years was 8.18% [6]. Ron and their colleagues emphasized that radiation doses on the order of 1–2 Gy in childhood can significantly increase the risk of neural tumors [9].
The latency period between irradiation and the development of meningioma ranged from 1.2 to 63 years, and the average latency period for high-dose radiotherapy (>20 Gy) is 19.5–24.6 years [1, 3, 16]. Ghim described that in 15 secondary meningioma patients treated with radiation at a young age, the mean latent period was 10.8 years [14]. Also, Strojan reviewed the literature and described that the latency period was significantly shorter in those who were aged 5 years or younger at the time of cranial irradiation than in others [6]. On the other hand, the latency period between irradiation and the development of glioma ranged from 1 to 61 years, and the average latency period with high-dose radiotherapy (>20 Gy) was 9.6 years [5]. There was no obvious difference in the mean latency period for patients who were younger than 5 years of age when irradiated (8.48 years) versus that for patients who were irradiated after 5 years of age (9.96 years) [5]. According to these analyses, the latency period correlated with the age at the time of cranial irradiation in meningioma rather than glioma.
Although there are many reported cases of radiation-induced single intracranial tumor, double intracranial neoplasm occurring after radiotherapy are extremely rare. To best of our knowledge, the present case is the fourth reported case of radiation-induced double tumors in patients treated with radiation (Table 1). The first case was a 22-year-old man treated for pituitary macroadenoma with craniotomy and external-beam radiation, who developed synchronous ependymona and meningioma 31 years after radiotherapy [7]. The second case was a 1-year-old girl with cerebellar ependymoma treated with surgery and craniospinal radiotherapy, who developed spinal meningioma at the age of 17, and subsequently developed cerebral glioblastoma at the age of 20 [4]. The third case was a 15-year-old boy treated for medulloblastoma with surgery and craniospinal irradiation, who developed meningioma 18 years after the initial treatment and subsequently anaplastic astrocytoma 23 years after the primary treatment [2]. Interestingly, meningiomas were found earlier than glioma (three cases) or found with glioma at the same time (one case). It is possible that radiation-induced meningioma may tend to develop earlier than glioma, when the same dose of radiation was delivered.
Several theories have been postulated to describe the occurrence mechanism of radiogenic tumors: (a) radiation can initiate genetic alterations by disrupting the deoxyribonucleic acid (DNA) of specific gene, such as PTEN or the p53 tumor suppressor gene [10, 13]; (b) karyotypic instability due to radiation-induced multiple chromosome aberrations may be responsible [19]; (c) vascular parenchymal alterations by irradiation may induce the release of oncogenic factors, such as platelet-derived and vascular endothelial growth factors [20], beta chain of fibroblast growth factor [18], and raised tissue level of basic fibroblast growth factor [21]. Tada identified an unusual somatic p53 mutation of a type specific to radiation-induced DNA damage in a secondary glioblastoma [10]. In our case, p53 was expressed in tumor cells of secondary anaplastic astrocytoma, suggesting that alteration of p53 occurred by irradiation.
Concurrent deletion of 1p and 19q constitutes a hallmark alteration in oligodendroglioma, being found in up to 80% of cases [22]. Most tumors show losses of one entire copy of 1p and 19q, while partial deletions are rare in oligodendroglioma [23]. In the present case, in addition to histological examination of the H & E stain, the findings that the LOH of 19q did not exist and GFAP was consistently expressed in tumor cells suggested the diagnosis of anaplastic astrocytoma.
It is not clear whether radiation caused the LOH of 1p in our patient. There is not the report that performed the analysis of LOH for chromosomal regions that are frequently deleted in radiation-induced glioma. In vivo analysis revealed frequent LOH on chromosomes 4, 12, and 19 in radiation-induced lymphomas of mice that are homologous to human chromosomes 1p, 12q, and 10q, respectively [24]. Also, Shoshan et al. reported that frequent allele losses were found on chromosomes 1p, 9p, 19q, and 18q in radiation-induced meningiomas [25]. Recently, Hata et al. described the case of radiation-induced anaplastic oligodendroglioma, which had the allelic loss of chromosomes 1p, 17p, and 19q [26]. From these reports, the LOH of 1p might tend to be induced by irradiation, but further studies are needed to elucidate the association between radiation and LOH of 1p. LOH of 1p in malignant glioma is known to be one of the important factors that predict better outcome in patients. However, radiation-induced oligodendroglioma with LOH of 1p rapidly progressed after surgery despite radiochemotherapy [26]. Therefore, we should carefully follow up the tumor of our patient.
Medulloblastomas require aggressive multimodality therapy, including surgery, radiation, and chemotherapy. The outlook for patients with medulloblastoma has improved dramatically during the past 20 years, primarily because of the use of craniospinal radiotherapy plus intensive chemotherapy. In light of the fact that improvements in surgical and other adjunctive treatments may lead to longer overall survival, the incidence of radiation-induced intracranial tumors will increase. Whenever radiation is administered to young children or adults, serial screening studies are suggested to enable earlier detection and treatment.
References
Choudhary A, Pradhan S, Huda MF et al (2006) Radiation-induced meningioma with a short latent period following high dose cranial irradiation—case report and literature review. J Neurooncol 77:73–77
Hope AJ, Mansur DB, Tu PH et al (2006) Metachronous secondary atypical meningioma and anaplastic astrocytoma after postoperative craniospinal irradiation for medulloblastoma. Childs Nerv Syst 22:1201–1207
Al-Mefty O, Topsakal C, Pravdenkova S et al (2004) Radiation-induced meningiomas: clinical, pathological, cytokinetic, and cytogenetic characteristics. J Neurosurg 100:1002–1013
Amirjamshidi A, Abbassioun K (2004) Radiation-induced tumors of the central nervous system occurring in childhood and adolescence. Four unusual lesions in three patients and a review of the literature. Childs Nerv Syst 16:390–397
Salvati M, Frati A, Russo N et al (2003) Radiation-induced gliomas: report of 10 cases and review of the literature. Surg Neurol 60:60–67
Strojan P, Popović M, Jereb B (2000) Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys 48:65–73
Alexander MJ, DeSalles AA, Tomiyasu U (1998) Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. J Neurosurg 88:111–115
Nishio S, Morioka T, Inamura T et al (1998) Radiation-induced brain tumours: potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien) 140:763–770
Ron E, Modan B, Boice JD Jr et al (1998) Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med 319:1033–1039
Tada M, Sawamura Y, Abe H et al (1997) Homozygous p53 gene mutation in a radiation-induced glioblastoma 10 years after treatment for an intracranial germ cell tumor: case report. Neurosurgery 40:393–396
Goldstein AM, Yuen J, Tucker MA (1997) Second cancers after medulloblastoma: population-based results from the United States and Sweden. Cancer Causes Control 8:865–871
Kaschten B, Flandroy P, Reznik M et al (1995) Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg 83:154–162
Chang SM, Barker FG II, Larson DA et al (1995) Sarcomas subsequent to cranial irradiation. Neurosurgery 36:685–690
Ghim TT, Seo JJ, O’Brien M et al (1993) Childhood intracranial meningiomas after high-dose irradiation. Cancer 71:4091–4095
Brada M, Ford D, Ashley S et al (1992) Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ 304:1343–1346
Harrison MJ, Wolfe DE, Lau TS et al (1991) Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg 75:564–574
Shapiro S, Mealey J Jr, Sartorius C (1989) Radiation-induced intracranial malignant liomas. J Neurosurg 71:77–82
Liwnicz BH, Berger TS, Liwnicz RG et al (1985) Radiation-associated gliomas: a report of four cases and analysis of postradiation tumors of the central nervous system. Neurosurgery 17:436–445
Sachs RK, Hlatky LR, Trask BJ (2000) Radiation-produced chromosome aberrations. Trends Genet 16:143–146
Li M, Jendrossek V, Belka C (2007) The role of PDGF in radiation oncology. Radiat Oncol 11:2–5
Rothbart D, Awad IA, Lee J et al (1996) Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery 38:915–924
Jeuken JW, von Deimling A, Wesseling P (2004) Molecular pathogenesis of oligodendroglial tumors. J Neurooncol 70:161–181
Griffin CA, Burger P, Morsberger L et al (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol 65:988–994
Okumoto M, Park YG, Song CW et al (1999) Frequent loss of heterozygosity on chromosomes 4, 12 and 19 in radiation-induced lymphomas in mice. Cancer Lett 135:223–228
Shoshan Y, Chernova O, Juen SS et al (2000) Radiation-induced meningioma: a distinct molecular genetic pattern? J Neuropathol Exp Neurol 59:614–620
Hata N, Shono T, Mizoguchi M et al (2007) Loss of heterozygosity analysis in an anaplastic oligodendroglioma arising after radiation therapy. Neurol Res 29:723–726
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasayama, T., Nishihara, M., Tanaka, K. et al. Two metachronous tumors induced by radiation therapy: case report and review of the literature. J Neurooncol 88, 315–320 (2008). https://doi.org/10.1007/s11060-008-9570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9570-0