Abstract
Purpose To assess the frequency of chromosomes 1p and 19q deletions in gliomas and to correlate 1p deletion with prognosis in patients with grade 2 and grade 3 gliomas independently of histologic subtype. Methods We retrospectively evaluated 208 patients with WHO grade 2 and 3 gliomas who had 1p/19q molecular studies performed between 2000 and 2004. DNA was extracted from tumor tissue and germline material and evaluated by PCR using microsatellite markers for each chromosome. Results There were 113 men and 95 women with a median age at diagnosis of 40. Thirty-eight patients had a low-grade astrocytoma (A2), 58 low-grade oligodendroglioma (O2), 31 low-grade oligoastrocytoma (OA2), 21 anaplastic astrocytoma (A3), 37 anaplastic oligodendroglioma (O3), and 23 had an anaplastic oligoastrocytoma (OA3). Chromosome 1p analysis was performed in all patients and showed deletions in 105 patients (76% of O2, 42% of OA2, 21% of A2, 89% of O3, 17% of AO3, and 14% of A3). Chromosome 19q studies were performed in 118 patients and showed deletions in 46 (56% of O2, 45% of OA2, 27% of A2, 76% of O3, 11% of OA3 and 0% of A3). On multivariate analyses, chromosome 1p was a prognostic factor for prolonged PFS (HR = 1.75, P = 0.03) and OS (HR = 3.59, P = 0.02) in grade 2 gliomas but not for grade 3 (HR = 0.81, P = 0.7 for PFS; HR = 1.31, P = 0.7 for OS). Conclusion Chromosome 1p deletion is a significant positive prognostic marker in diffuse, grade 2 gliomas regardless of histologic subtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gliomas are the most common primary tumors of the central nervous system. The major subtypes of grade 2 (low-grade) and 3 (anaplastic) gliomas include astrocytomas, oligodendrogliomas and “mixed gliomas” or “oligoastrocytomas.” Chromosomes 1p and 19q deletions were initially described in oligodendrogliomas [1, 2] and later were associated with a better response to chemotherapy and prolonged survival in anaplastic oligodendrogliomas [3]. The longer survival of patients with anaplastic oligodendrogliomas and oligoastrocytomas with 1p and 19q deletions was confirmed by two large phase III clinical trials [4, 5]. More recently, two independent groups described a translocation t(1;19)(q10;p10) in such tumors, which is the genetic event that mediates the combined deletions of chromosomes 1p and 19q [6, 7].
Although 1p and 19q status has been extensively studied in anaplastic oligodendroglial gliomas (WHO grade 3), it has been less studied in low-grade gliomas (WHO grade 2) and astrocytic tumors [6, 8, 9]. Histopathological classification based on morphological criteria to differentiate astrocytic, oligodendroglial and mixed gliomas is frequently difficult and subject to variability among different pathologists [10]. The objective of this study was to assess the frequency of 1p and 19q deletions in grade 2 and 3 gliomas and to correlate 1p deletion with prognosis independently of the histological subtype.
Patients and methods
We retrospectively evaluated patients with supratentorial gliomas who had 1p/19q molecular studies performed at Memorial Sloan-Kettering Cancer Center (MSKCC) from January 2000 to December 2004. The inclusion criteria were: (1) initial pathological diagnosis of WHO grade 2 or 3 glioma; (2) detailed clinical information at diagnosis and during follow-up at MSKCC. All pathological slides were reviewed by a neuropathologist at MSKCC and classified according to the histological subtype and WHO grading system. Tumors included in this study under the rubric of grade 2 or 3 “oligoastrocytoma” exhibited either ambiguous, transitional histologic features or less commonly, regionally distinct components having the appearance of oligodendroglioma and astrocytoma. This study was approved by the Institutional Review Board.
Loss of heterozygosity analysis
DNA was extracted from tumor tissue and germline material (peripheral leukocytes or nail clipping) and evaluated by polymerase chain reaction using markers for each chromosome to assess loss of heterozygosity (LOH) as previously described [11]. An H&E-stained section was obtained before DNA extraction to evaluate the proportion of tumor in paraffin block. If tumor content was <90%, areas containing pure tumor were dissected. All cases were studied using at least three microsatellite markers of the chromosome 1p (tel-D1S468, D1S548, D1S1612, D1S1592, D1S552, D1S496-cent). Allelic losses of 19q were evaluated using three polymorphic markers mapped to a commonly deleted region at q13.3 (D1S219, D1S412, and PLA4C2G). Segmental loss was defined as evidence of interstitial or small terminal deletions, as manifested by LOH at one or more loci with retention of heterozygosity at the centromeric end of the evaluated region, with or without retention of heterozygosity at the telomeric end. LOH was scored as present when the tumor sample showed reduction in height of one of two peaks by 50%. This was compared with the ratio of peak heights of the two alleles in the corresponding germline DNA. The consistency of the significant findings was confirmed by repeat analysis. Our laboratory started performing 19q analyses a few years after initiating 1p analyses and we were unable to retrieve the pathology blocks or germline material to perform 19q analyses in all patients.
Statistics
Progression-free survival (PFS) was defined as the time from the radiological diagnosis to radiological or clinical progression of disease, death from any cause or date of last follow-up. Overall survival (OS) was defined as the time from the radiological diagnosis to death from any cause.
Kaplan–Meier and Cox regression analyses were performed to determine the correlations of clinical and molecular variables with PFS and OS in univariate analysis. Multivariate analyses were performed with Cox proportional-hazards regression model to identify variables that were independently predictive of outcome. Factors with P ≤ 0.1 on univariate analyses were entered as candidate variables and multivariate analysis was performed in a stepwise manner. Differences in frequency of types of treatment between 1p intact and deleted patients were calculated by chi-square tests. Two-sided P values ≤ 0.05 were considered significant.
Results
Among 325 patients with gliomas tested for 1p and/or 19q deletions between January 2000 to December 2004, 107 were excluded due to unavailable clinical information, and 10 due to initial diagnosis of glioblastoma multiforme. Therefore, we had both clinical and molecular pathology information in 208 patients with WHO grade 2 (n = 127) and grade 3 (n = 81) gliomas.
Demographics
There were 113 men and 95 women with a median age at diagnosis of 40 years (Table 1). Median follow-up was 67 months for grade 2 patients, 39 months for grade 3 patients. Eighty-three patients (65%) with grade 2 and 54 (67%) with grade 3 gliomas had progression of disease during this follow-up period. At the time of the study, 20 patients (16%) with grade 2 and 28 (35%) with grade 3 gliomas had died.
Histological subtype and frequency of 1p and 19q deletions
Thirty-eight patients had a low-grade astrocytoma (A2), 58 a low-grade oligodendroglioma (O2), 31 a low-grade “oligoastrocytoma” (OA2), 21 anaplastic astrocytoma (A3), 37 anaplastic oligodendroglioma (O3) and 23 had anaplastic “oligoastrocytoma” (OA3). Chromosome 1p analysis was performed in all tumors and showed deletions in 105 (88 complete and 17 partial deletions), an intact chromosome in 101 and uninformative results in two. Seventy-six percent of patients with O2, 42% of OA2, 21% of A2, 89% of O3, 17% of AO3, 14% of A3 had chromosome 1p deletion. Chromosome 19q studies were performed in 118 tumors and showed deletions in 46 (42 complete and four partial deletions), an intact chromosome in 66 and uninformative results in six. Fifty-six percent of O2 (14/25), 45% of OA2 (12/27), 27% of A2 (5/19), 76% of O3 (13/17), 11% of OA3 (2/19), 0% of A3 (0/11) had 19q deletions (Table 2). When tumor tissue for both chromosomes provided interpretable results, co-deletion of both 1p and 19q occurred in 56% O2 (14/25), 37% OA2 (9/24), 11% A2 (2/18), 81% O3 (13/16), 4% OA3 (1/19), and 0% A3 (0/9). Deletion of chromosome 1p was strongly associated with deletion of chromosome 19q (P < 0.0001).
Clinical and molecular prognostic factors
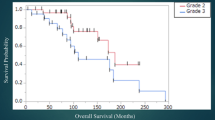
On the univariate analyses, the factors associated with prolonged PFS and OS in grade 2 gliomas were 1p deletion and 19q deletion. Seizure at presentation was also associated with prolonged OS in the grade 2 glioma group. Karnofsky performance scale (KPS) ≥80, seizure at presentation and 1p and 19q deletions were associated with longer PFS in grade 3 gliomas. Factors associated with prolonged OS in grade 3 gliomas were younger age, KPS ≥ 80, and deletions of chromosomes 1p and 19q (Table 3). On multivariate analyses, the only independent prognostic factor for prolonged PFS and OS in grade 2 gliomas was chromosome 1p deletion (HR = 1.75, 95% CI: 1.10–2.89, P = 0.03 for PFS and HR = 3.59, 95% CI: 1.25–10.3, P = 0.02 for OS). For grade 3 gliomas, the only other independent factor that predicted longer PFS was KPS ≥ 80 (HR = 8.20, 95% CI: 2.92–23.0, P < 0.0001) and age at diagnosis predicted longer OS for grade 3 gliomas (HR = 1.05, 95% CI: 1.00–1.09, P = 0.03) in a multivariate analysis. Chromosome 1p was not a prognostic factor for prolonged PFS (HR = 0.81, 95% CI: 0.29–2.26, P = 0.7) or OS (HR = 1.31, 95% CI: 0.32–5.35, P = 0.7) in grade 3 gliomas.
Treatment
Treatment was not standardized in either grade 2 or grade 3 glioma subgroups. Upfront treatment for grade 2 glioma patients included resection (68%), radiation therapy (17%) and chemotherapy (10%). Twenty-six percent of patients with grade 2 gliomas were followed after radiological diagnosis or biopsy. There was no difference in the frequency of resection, radiation or chemotherapy between patients with chromosome 1p intact or deleted.
Initial treatment of grade 3 glioma patients included resection (83%), radiation therapy (48%) and chemotherapy (47%). There was also no difference in the frequency of resection, radiation or chemotherapy between patients with chromosome 1p intact or deleted.
There was also no significant difference in the mean number of resections, extent of resection or chemotherapeutic regimens during the follow-up period between patients with chromosome 1p intact or deleted.
Discussion
Our study suggests that chromosome 1p status is a significant prognostic marker in diffuse grade 2 gliomas regardless of histologic subtype. A retrospective study of 131 grade 2 gliomas (32 astrocytomas, 30 mixed gliomas and 69 oligodendrogliomas) also found that histologic subtype was not associated with differences in PFS and chromosome 1p status was the only independent prognostic factor for longer PFS [9]. However, the larger number of oligodendrogliomas which have a higher frequency of deletion of chromosome 1p, in ours and their series, could have skewed the results in both studies. Another retrospective study of 79 grade 2 gliomas (59 astrocytomas, 14 mixed and 6 oligodendrogliomas) found that deletions of chromosome 1p and 19q were associated with prolonged overall survival for the whole group and pure oligodendroglioma histology was also an independent factor for prolonged survival [8].
Among grade 3 gliomas, deletions of chromosome 1p and 19q are well established prognostic factors based on two randomized phase III trials in oligodendrogliomas and oligoastrocytomas [4, 5]. We could not show that 1p or 19q deletion was an independent prognostic factor due to the relatively small number of patients with grade 3 gliomas in our study.
As shown in previous studies, 1p and 19q deletions are more frequent in pure oligodendroglial tumors, and 1p/19q co-deletion is considered a molecular hallmark of such histological subtype. The frequency of 1p deletion among the three histological subtypes found in our series is in the range of previously reported studies. Previous studies have shown 1p deletions in 70–85% of pure oligodendrogliomas, 30–50% of oligoastrocytomas, and 5–15% of astrocytomas [4–6, 9, 12–14]. 1p/19q co-deletion has been reported rarely in astrocytomas and we found only two such cases in our series [15]. Our data support the concept that some astrocytic tumors have also deletion of 1p chromosome (21% in our series). However, the prognostic importance of this deletion in astrocytomas can only be determined in larger prospective series.
Fluorescent in situ hybridization (FISH) and LOH through PCR are the most commonly used techniques to detect chromosome 1p deletion. They seem to be equivalent to differentiate deletion from intact chromosomes [16, 17] but the cut-off values used for FISH and PCR analyses are not standardized in the clinical practice and this may obviously affect the results. LOH PCR is considered by some as the gold standard for detection of allelic losses because it compares PCR products of corresponding genetic regions in both tumor and normal DNA. FISH analyzes the absolute copy number and not the allele status and can miss mitotic recombination [16]. However, FISH has the advantage of not requiring germline material and of being more sensitive than PCR when detecting deletions in specimens of mixed cellularity and in tumor cell populations making up as little as 15% of all cells in the specimen, compared with the requirement of 60–90% tumor cell content for LOH PCR studies. Only more detailed PCR studies but not FISH can discriminate between partial or complete 1p deletions and clarify the significance of a partial deletion of chromosomes 1p or 19q. Two prior studies showed a higher frequency of partial 1p deletion in astrocytic tumors [11, 12]; one study suggested that partial deletion of chromosome 1p was associated with a worse prognosis when compared to intact 1p [12]. However, all studies, including ours, had only a small number of tumors with partial 1p deletions so the prognostic significance of partial deletion requires further study.
Our work included a large group of patients with prolonged followup and our results give support to the growing evidence that 1p and 19 q chromosome status is relevant to grade 2 gliomas and not only to anaplastic tumors. However, our data has inherent bias associated to retrospective analyses, use of non-standardized treatment regimens and analyses of 19q chromosome status performed in only 57% of patients. The existing preponderance of oligodendroglial tumors over other subtypes of grade 2 gliomas in our study together with a relatively smaller number of patients in each other subgroup precludes a separate analysis of chromosome 1p status for each histological subtype. Although our study has limitations in providing these types of analysis, further molecular characterization of gliomas, including probing for 1p/19q translocation, could eventually supplant traditional histologic diagnosis as the main eligibility criterion for glioma clinical trials, or at the very least, molecular features are likely to become important stratification variables in such trials. An ongoing European Organization for Research and Treatment of Cancer (EORTC) and National Cancer Institute Canada (NCIC) phase III randomized study of radiotherapy versus temozolomide in patients with low-grade gliomas stratified according to 1p status will provide more definite information in the future.
References
Ransom DT, Ritland SR, Kimmel DW, Moertel CA, Dahl RJ, Scheithauer BW et al (1992) Cytogenetic and loss of heterozygosity studies in ependymomas, pilocytic astrocytomas, and oligodendrogliomas. Genes Chromosomes Cancer 5:348–356
Bello MJ, Vaquero J, de Campos JM, Kusak ME, Sarasa JL, Saez-Castresana J et al (1994) Molecular analysis of chromosome 1 abnormalities in human gliomas reveals frequent loss of 1p in oligodendroglial tumors. Int J Cancer 57:172–175
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ et al (2006) Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European organisation for research and treatment of cancer phase III trial. J Clin Oncol 24:2715–2722
Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, Brachman D et al (2006) Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: intergroup radiation therapy oncology group trial 9402. J Clin Oncol 24:2707–2714
Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M et al (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66:9852–9861
Griffin CA, Burger P, Morsberger L, Yonescu R, Swierczynski S, Weingart JD et al (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol 65:988–994
Mariani L, Deiana G, Vassella E, Fathi AR, Murtin C, Arnold M et al (2006) Loss of heterozygosity 1p36 and 19q13 is a prognostic factor for overall survival in patients with diffuse WHO grade 2 gliomas treated without chemotherapy. J Clin Oncol 24:4758–4763
Kujas M, Lejeune J, Benouaich-Amiel A, Criniere E, Laigle-Donadey F, Marie Y et al (2005) Chromosome 1p loss: a favorable prognostic factor in low-grade gliomas. Ann Neurol 58:322–326
Giannini C, Scheithauer BW, Weaver AL, Burger PC, Kros JM, Mork S et al (2001) Oligodendrogliomas: reproducibility and prognostic value of histologic diagnosis and grading. J Neuropathol Exp Neurol 60:248–262
Barbashina V, Salazar P, Holland EC, Rosenblum MK, Ladanyi M (2005) Allelic losses at 1p36 and 19q13 in gliomas: correlation with histologic classification, definition of a 150-kb minimal deleted region on 1p36, and evaluation of CAMTA1 as a candidate tumor suppressor gene. Clin Cancer Res 11:1119–1128
Idbaih A, Marie Y, Pierron G, Brennetot C, Hoang-Xuan K, Kujas M et al (2005) Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol 58:483–487
Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM et al (2000) Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 18:636–645
Walker C, du Plessis DG, Joyce KA, Fildes D, Gee A, Haylock B et al (2005) Molecular pathology and clinical characteristics of oligodendroglial neoplasms. Ann Neurol 57:855–865
Okamoto Y, Di Patre PL, Burkhard C, Horstmann S, Jourde B, Fahey M et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol (Berl) 108:49–56
Scheie D, Andresen PA, Cvancarova M, Bo AS, Helseth E, Skullerud K et al (2006) Fluorescence in situ hybridization (FISH) on touch preparations: a reliable method for detecting loss of heterozygosity at 1p and 19q in oligodendroglial tumors. Am J Surg Pathol 30:828–837
Smith JS, Alderete B, Minn Y, Borell TJ, Perry A, Mohapatra G et al (1999) Localization of common deletion regions on 1p and 19q in human gliomas and their association with histological subtype. Oncogene 18:4144–4452
Acknowledgements
We thank Drs. Lisa M. DeAngelis and Marc Ladanyi for their advice and critical reading.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwamoto, F.M., Nicolardi, L., Demopoulos, A. et al. Clinical relevance of 1p and 19q deletion for patients with WHO grade 2 and 3 gliomas. J Neurooncol 88, 293–298 (2008). https://doi.org/10.1007/s11060-008-9563-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9563-z