Abstract
Chordoid meningioma is a rare variant of meningioma with histological features resembling those of chordoma. This tumour should have a greater risk of recurrence and aggressive growth (WHO grade II). So far, 92 such tumours have been described in the literature. We report two cases of chordoid meningioma occurring in adult female patients. In our two patients (aged 28 and 60 years with chordoid meningioma of the convexity and left-sided outer sphenoid wing, respectively) we centred on some rarely discussed aspects of the tumour. MRI scans showed no edema in the vicinity of either of the two meningiomas, whereas selective angiography of ACI and ACE revealed a dural type of vascular supply to the two neoplasms. In both cases, the tumour was removed by radical surgery (Simpson grade I resection) with a normal post-operative course. Both women (one 2 years post-surgery and one 4 years post-surgery) are now free from any signs of relapse on MRI and with normal neurological findings. The vascular endothelial growth factor (VEGF) expression was low in either case (5 and 40%, respectively). We regard the factors under consideration in our study (i.e. absence of edema, dural supply, low VEGF expression and radical Simpson grade I resection) as an important contribution to the discussion of the biological behaviour of chordoid meningioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “chordoid meningiomas” was first coined by Kepes et al. [1] as a histopathological classification of tumours removed in seven patients (aged 8–19 years) with Castleman’s syndrome. Kepes described the pathology as an independent entity in children with haematological involvement. Until June 2007, 92 chordoid meningiomas had been reported, including Kepes’ cohort. Additional to the three larger groups [1–3], the other publications are individual case reports. Epari et al. [3] reviewed 18 cases while 13 other cases have been analysed in articles published during the period July 2006 to June 2007 [4–10]. Oddly enough, leaving aside the seven cases reported by Kepes [1], chordoid meningioma has been reported in connection with Castleman’s syndrome in only two other patients. The tumour is currently classified as a variant of meningioma (WHO grade II) and presumed to be highly prone to recurrence [11]. Studies are mainly devoted to histopathological, immunohistochemical and cytological aspects, whereas interest in other factors regarding the risks of recurrence is largely missing from the relevant literature. Those factors include, among others, the radicality of meningioma resection, the presence or absence of peritumoral edema, the type of vascular supply and the vascular endothelial growth factor (VEGF) expression.
We report two cases of chordoid meningioma occurring in adult female patients that are intended to add to the discussion of the biological behaviour of chordoid meningiomas in response to Epari’s et al. article [3].
Case reports
Case I
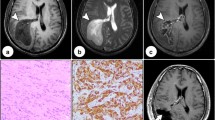
Our first patient was a 28-year-old woman with a negative family and personal history in week 34 of her pregnancy. The patient was free from any haematological or other systemic disease. For a number of weeks she had been complaining of headaches and for two weeks she had had left-sided hemiparesis developing gradually. MRI imaging was performed in 1.5 T General Electric EXCITE 2A suite. Imaging was perfomed in all three axes in T1 and T2 weighted-images and in T1 weighted-images after application of Gd-DPTA (the same MRI protocol was used in Case II as well). MRI scans revealed a meningioma of the convexity on the right, free from edema, there was no “dural tail” sign. Concerning this finding, birth by Caesarean section was indicated: 3 days thereafter she was admitted to our department. At the time of admission, she was fully conscious (GCS 15), her objective neurological picture dominated by moderate left-sided hemiparesis. Selective brain angiography (ACI and ACE) showed a dural type of blood supply to the meningioma (from ACE alone). During the surgical procedure (Feb. 24, 2004), she had a meningioma of the frontal convexity on the right radically removed (Simpson grade I-[12]). During and 7 days after surgery, corticoids (dexamethasone) were administered. The post-operative course was marked by gradual regression of the patient’s left-sided hemiparesis until it resolved ad integrum within 1 month. Methodology of histopathological examination: standard staining technique was used for Haematoxyllin-Eosin and Alcian blue (which was performed at pH 2.5). The following antibodies were used for imunohistochemical examination: progesteron receptor (PGR 636, RTU, DakoCytomation), vimentin (V 9, RTU, DakoCytomation), S 100 protein (polyclonal, RTU, DakoCytomation), VEGF (VG 1, 1:200, Zymed), CK 8 (35beta H 11, RTU, DakoCytomation), EMA (E 29, RTU, DakoCytomation), CD 31 (JC 70 A, RTU, DakoCytomation), LCA (2 B 11 + PD 7/26, 1:200, DakoCytomation), Ki 67 (MIB 1, 1:50, DakoCytomation).
Histopathological examination helped diagnose a chordoid meningioma with myxoid stroma (Fig. 1), positive alcian blue staining (Fig. 2), immunopositivity for vimentin (Fig. 3) and EMA (Fig. 4), immunonegativity for cytokeratin (CD 31) and GFAP. The proliferation index (Ki67) was less than 2% while the VEGF expression was very low (5%) (Fig. 5). We did not observe metachromatic decoration. There was a moderate vascularization using CD 31. Lymphoid infiltration was minimal (even using LCA antibody). Progesteron receptors were expressed in 80% of cells.
Post-operative MRI follow-up checks were made every 6 months, each time with normal findings free from signs of relapse. The latest follow-up was in February 2007. The follow-up period of our first patient is four years.
Case II
Our other patient was a woman of 60 years with a negative family history. The patient was taking levothyroxin for many years to keep her hypothyroidism under control. Otherwise, the patient was without any haematological abnormalities or any other systemic disease. After 6 months of complaining of headaches, MRI scans indicated a non-swollen meningioma of the (sphenoid) wing on the left (outer variant) (Figs. 6, 7). For this finding, the patient was admitted to our department. On admission, the patient was fully conscious (GCS 15) and her neurological focal finding was negative. Selective cerebral angiography (ACI and ACE) revealed a dural type of blood supply to the meningioma (solely from the ACE) (Figs. 8, 9). During surgery (Feb. 12, 2005), she had a left-sided meningioma of the outer sphenoid wing which was radically removed (Simpson grade I). During surgery and for 7 consecutive days after surgery, the patient was treated with corticoids (dexamethasone). Her post-operative neurological picture was within the normal range.
The same protocol of histological examination was used as in Case I. Histopathological examination proved the presence of the choroid variant of meningioma similar to that in case 1. The proliferation index (Ki67) was less than 2% and the VEGF expression was low (40%). We did not observe metachromatic decoration. There was not increased vascularization even using CD 31. Lymphoid infiltration was negative. Progesteron receptors were expressed in 75% of cells.
Post-operative MRI scans, all of which were normal and without signs of relapse, were taken at 6-month intervals. The latest follow-up scan was taken in February 2007 (Fig. 10). This patient has been under follow-up care for 2 years now.
Discussion
Although chordoid meningioma was first described by Kepes et al. [1] exclusively in connection with Castleman’s syndrome, this combination has occurred in only 2 of the 85 cases reported until now, i.e. at a rate of 2.4%. Except for one 19-year-old woman, all other patients of the Kepes’ cohort were aged 18 years and younger. However, other authors have reported only seven more patients aged 18 years and younger, i.e. 8.2%. Although this incidence is higher than the usual rate of 1.5–2% for typical meningiomas [13], the reported figures are rather striking because chordoid meningioma was first regarded as a separate entity observed in children with haematological involvement [1]. The fact is that the chordoid variant of meningioma occurs at a rate exceeding 90% in the adult population unconnected with any haematological or other systemic pathology. We failed to find an explanation of this aspect of chordoid meningioma in the literature available to us.
Chordoid meningioma occurs very rarely, never exceeding 1% of surgically removed meningiomas, even in large cohorts [2, 3]. As a rule, it is localised supratentorially (at 81.5%) [3]. Unusual sites in the ventricular system, foramen jugulare and orbital area have been described in a number of case reports [5, 6, 10].
As follows from the literary data, the chordoid variant of meningioma is marked by a greater likelihood of recurrence, which is consistent with the tumours classification in the WHO grade II group and with an average MIB-1 proliferation index of 5.2% [2]. Relapses in larger cohorts with long follow-ups are frequent—(28.6% in Kepes’ series [1] and 42% in Couce’s series [2]). In our two patients with typical pathological and immunohistochemical characteristics of the chordoid variant of meningioma and with a low Ki-67 proliferation index no relapse has occurred 4 years (case 1) and 2 years (case 2) after surgery. Admittedly, our two patients’ follow-up periods are very short. We believe, however, that, taken together, the absence of edema, the dural type of vascular supply to the meningioma, its radical resection (classified as Simpson grade I) and a very low VEGF expression are factors predictive of a rather low probability of recurrence. Yet, in connection with the chordoid variant of meningioma, these factors fail to receive much attention in the current literature.
Peritumoral edema is closely related to the type of vascular supply to the meningioma, and to the VEGF expression. Meningiomas with a pial component of supply are expressive of VEGF and endothelial cells of the neighbouring cerebral capillaries have their matching receptors (VEGFR-1 and VEGFR-2), which provide the groundwork for tumour angiogenesis. The rate of VEGF expression closely correlates with the type of vascular supply, being practically low in the purely dural type and highest in the exclusively pial type of vascular supply to the tumour. The size of edema copies the growing expression of VEGF. Whereas in the dural type of blood supply there is no edema and VEGF expression is low; in the pial type the edema tends to be considerable and VEGF expression rather high [14–18]. The pial type indicates that the meningioma shares its blood supply with the surrounding brain tissue, from which it is not separated by any arachnoidal or CSF barrier, implying a significant limit for a safe radical extrapial resection of meningioma [19]. Moreover, radical removal of meningioma is often hampered by its localisation in the skull base region (cavernous sinus, suprasellar, petroclival), where there is the potential risk of injury to neural and vascular structures [13, 20, 21]. Of the 92 reported cases of chordoid meningioma, we found references to the absence or presence of peritumoral edema in only 14 (four cases did show its presence while in the remaining 10 cases no edema was discernible on CT or MRI scans) [3, 22, 23]. To our knowledge, the degree of radicality of chordoid meningioma resection, as based on Simpson’s classification, has not been described in the literature. According to Couce et al. [2], all relapsing meningiomas in their cohort (42%) were removed subtotally, except one. The use of selective ACI and ACE angiography for the purpose of establishing the dural or pial types of blood supply to meningioma and the degree of VEGF expression has not yet been published in any study of chordoid meningioma.
Conclusion
Unlike other authors who have described chordoid meningioma mainly as a histopathological entity, we centred on other aspects of this rare variety of meningioma in our two patients.
The research literature lacked information on peritumoral edema, vascular supply, and VEGF expression. Furthermore, in the literature the degree of radicality of resection is not always defined in terms of Simpson’s classification. In our opinion, standardisation of data on these factors might make it possible to have a closer look at the biological nature of chordoid meningiomas in connection with the emphasised risk of their recurrence.
References
Kepes JJ, Chen WY, Connors MH, Vogel FS (1988) “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer 62(2):391–406
Couce ME, Aker FV, Scheithauer BW (2000) Chordoid meningioma: a clinicopathologic study of 42 cases. Am Surg Pathol 24(7):899–905
Epari S, Sharma MC, Sarkar C, Garg A, Gupta A, Mehta VS (2006) Chordoid meningioma, an uncommon variant of meningioma: a clinicopathologic study of 12 cases. J Neurooncol 78(3):263–269
Hasegawa S, Yoshioka S, Urabe S, Kuratsu J (2006) Rapidly enlarging chordoid meningioma with abundant mucin production. Neuropathology 26(5):438–441
Takei H, Rivera A, Suzuki H, Bahrami A, Powell SZ (2006) Jugular foramen chordoid meningioma. Pathol Int 56(7):397–401
Takei H, Bhattacharjee MB, Adesina AM (2006) Chordoid glioma of the third ventricle: Report of a case with cytologic features and utility during intraoperative consultation. Acta Cytol 50(6):691–696
Lui PC, Chau TK, Wong SS, Lau PP, Tse GM, Thomas TM, Ng HK (2007) Cytology of chordoid meningioma: a series of 5 cases with emphasis on differential diagnoses. J Clin Pathol 60(9):1024–1028
Liu AJ, Wang FL, Li XH (2007) Chordoid meningioma: a report of two cases. Chin Med J 120(8):726–728
Braham E, Bellil S, Ben Hamouda K, Bettaieb I, Mekni A, Bellil K, Haouet S, Zitouna M, Kchir N (2007) Chordoid meningioma. Two cases. Neurochirurgie 53(1):39–42
Park SC, Oh DE, Suh YL, Kim YD (2007) Orbital chordoid meningioma: a rare subtype of meningioma. Ophthal Plast Reconstr Surg 23(3):246–248
Kleihues P, Cavenne WK (eds) (2000) World Health Organisation classification of tumours: Pathology and genetics of tumours of the nervous system. IARC Press, Lyon
Simpson D (1957) Recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Black P (1993) Meningiomas. Neurosurgery 32:643–657
Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PC (1999) Vascular endothelial growth factor expression, vascular volume, and capillary permeability in human brain tumors. Neurosurgery 44:732–740
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neurooncol 50:109–120
Bitzer M, Wockel L, Luft AR, Wakhloo AK, Petersen D, Opitz H et al (1997) The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J Neurosurg 87:368–373
Paek SH, Kim CY, Kim YY, Park IA, Kim MS, Kim DG, Jung HW (2002) Correlation of clinical and biological parameters with peritumoral edema in meningioma J. Neurooncol 60(3):235–245
Otsuka S, Tamiya T, Ono Y, Michiue H, Kurozumi K, Daido S, Kambara H, Date I, Ohmoto T (2004) The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol 70(3):349–357
Sindou MP, Alaywan M (1998) Most intracranial meningiomas are not cleavable tumors: anatomic-surgical evidence and angiographic predictibility. Neurosurgery 42:476–480
Al-Mefty O (1991) Meningiomas. Raven Press, New York
Philippon J (2003) Operability of intracranial meningiomas. Bull Acad Natl Med 187:591–598
Kobata H, Kondo A, Iwasaki K, Kusaka H, Ito H, Sawada S (1988) Chordoid meningioma in a child. Case report. J Neurosurg 88(2):319–323
Mitsuhashi T, Ono S, Inohara T, Otomo T, Aoki A, Ueki Y (2006) Chordoid meningioma-case report. Neurol Med Chir 46(1):37–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kozler, P., Beneš, V., Netuka, D. et al. Chordoid meningioma: presentation of two case reports, review of the literature, and plea for data standardisation. J Neurooncol 88, 115–120 (2008). https://doi.org/10.1007/s11060-008-9541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-008-9541-5