Abstract
The aim of the study was to investigate the ability of 1HMRS to reflect proliferative activity of diffuse low-grade gliomas (WHO grade II). Between November 2002 and March 2007, a prospective study was performed on consecutive patients with suspected supratentorial hemispheric diffuse low-grade tumors. All the patients underwent MR examination using uniform procedures, and then surgical resection or biopsy within 2 weeks of the MR examination. Proliferative activity of the tumors was assessed by Ki-67 immunochemistry (Mb-1) on paraffin embedded tumor sections. Spectroscopic data was compared with Ki-67 labeling index and other histologic data such as histological subtype, cellular atypia, cellular density using univariate and multivariate analysis. 82 of 97 consecutive patients had histologically confirmed WHO grade 2 gliomas. Ki-67 proliferation index (PI) was correlated with specific spectral patterns: (1) low PI (<4%) was associated with increased Cho/Cr and absence of both free lipids or lactates; (2) intermediate PI (4–8%) was associated with resonance of lactates; and (3) high PI (>8%) was characterized by a resonance of free lipids. On multivariate analysis, resonance of lactates and resonance of free lipids appeared as independent predictors of intermediate PI (P < 0.001) and high PI (P < 0.001), respectively; moreover, free lipids resonance was correlated with cellular atypia (P < 0.05). This study suggests that 1HMRS is a reliable tool to evaluate the proliferation activity of WHO grade 2 glioma and to identify potentially more aggressive clinical behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proton Magnetic Resonance Spectroscopic (1HMRS) examination is a non-invasive tool for investigating the spatial distribution of metabolic changes in brain lesions. However, at this time, no tumor-specific metabolite has been detected by 1HMRS, even if specific patterns in the changes of metabolic concentrations compared with the normal brain have been established by several studies [1, 2]. Thus, increased levels of choline containing compounds (Cho) and a reduction of the resonance of N-acetylaspartate (Naa), and sometimes of Creatine (Cr), have been reported in human brain tumors. Cho is composed of choline, phosphocholine, and glycerophosphocholine, and is thought to be a marker of increased membrane turnover or higher cellular density [3, 4]. Naa is regarded as a neuronal marker, owing to the fact that it is mainly contained within neurons [5]. The Cr peak is the signal from both Cr and phosphocreatine and plays a role in tissue energy metabolism [6]. The range of Cho increase and Naa decrease is compatible with the range of tumor infiltration [7, 8]. For pathologic conditions that appear similar to brain tumors on conventional MR imaging, variations of these three metabolites and others (myo-inositol, lactate, lipids, glutamine + glutamate, alanine) can be used for differential diagnosis [7, 9]. It has recently been suggested by some studies that 1HMRS may allow the differentiation of the degree of malignancy of brain gliomas [10, 11], as well as its degree of infiltration [7]. However, to our knowledge, no study has hypothesized that 1HMRS may point out any predictive factor of aggressivity within the specific field of World Health Organization (WHO) grade 2 gliomas.
At some point, all low-grade gliomas (LGGs) may transform to upper grade gliomas, with variable progression rates [12]. Then, the challenge in their management is to obtain a non-invasive tool for predicting the potential aggressivity of a given lesion at the time of examination. By definition, histological analysis does not provide sufficient information on the natural course of LGG towards malignant transformation. Immunochemistry and molecular biology may give additional information on that aspect [13, 14], but also require an invasive act, e.g. at least a biopsy.
Here, the following question arises: is there a possibility to obtain from 1HMRS, a non-invasive procedure, pertinent information on the natural course of LGG (malignant transformation) prior to any biopsy or any treatment orientation?
In this study, we report a population of 82 patients presenting with histologically WHO grade 2 cerebral gliomas, in order to match pathological criteria to the data obtained from 1HMRS. For the first time, as far as we know, a close relationship between Ki-67 labeling index and specific spectral patterns could be demonstrated in a prospective study.
Patients and methods
Patients
Between November 2002 and March 2007, a prospective study on consecutive adult patients with suspected supratentorial hemispheric diffuse LGGs was performed.
All the patients underwent MR examination, and then underwent surgery or biopsy within 2 weeks of the MR examination.
Inclusion criteria
-
1.
Patients with a histologic confirmed WHO grade II glioma.
-
2.
None of them underwent any oncological or steroid treatment before MR examination.
-
3.
Informed consent was obtained from each of them.
Exclusion criteria
-
1.
Age under 18
-
2.
Any chemotherapy, radiotherapy or surgery before treatment
-
3.
WHO grade other than 2
-
4.
Contra-indication to MRI investigation
Magnetic resonance methods
All the patients underwent pre-therapeutic MR examination according to the same method using a whole body MR system (Signa 1.5T General Electric Healthcare, Milwaukee, Wisconsin, USA).
-
All slices were acquired a 5 mm slice thickness, 1.5 mm gap. Other parameters were: for T2-weighted coronal images, TR/TE 4,500/100, matrix of 320 × 224; for T2-FLAIR axial images, TI/TR/TE 2,200/8,800/140, matrix of 228 × 224; for T1-weighted axial images TR/TE 400/9, matrix of 512 × 224. T1-weighted acquisition was repeated in axial and coronal planes after intravenous administration of gadolinium.
-
MR spectroscopic methods
All the spectroscopic data was obtained before intravenous administration of gadolinium using the same sequence, e.g. Point Resolved Single voxel Spectroscopy (PRESS), with TR = 1,500 ms and two different TEs of 35 and 135 ms, 96 scans. T2-FLAIR axial images were used for positioning the excitation box.
Depending on the size of the lesion, in order to obtain as complete as possible a spectroscopic sampling, one to five voxels (each of them ranging from 3 to 8 cm3) were positioned into the tumor, defined by the limits of signal abnormalities on T2-FLAIR images. For each tumor, only the spectrum presenting the most pejorative parameters was used in the analysis, for instance the most elevated Choline to Naa Index (CNI) and eventual resonance of lactate and/or lipids. An additional voxel has been positioned in the controlateral hemisphere at the level of the tumor location (TR = 1,500/TE = 135 ms). Raw spectroscopic data was processed under Spectral Analysis General Electric (SAGE) software for accurate quantification, using normalization of Cr resonance on the controlateral side. The following metabolites were assessed at short echo time: free lipids (.8–1.2 ppm), Myo inositol (Mi, 3.56 ppm). The following metabolites were assessed at long echo time; lactates (1.36 ppm), Naa (2.02 ppm), Cr, (3.03 ppm), Choline (3.2 ppm).
Histopathologic evaluation
Histologic examination (cellular density and characteristics) was performed on wax embedded (5 μm cut) and H–E stained samples. Immunohistochemistry used Ki-67 (Mib-1).
All glioma specimens were histologically examined by an experienced neuropathologist “blind” to other results and graded according to the WHO classification of tumors of the nervous system [15]. Tumor cells were identified by using formalin-fixed and paraffin-embedded sections stained with hematoxylin–eosin and immunostained for MIB-1 (Dako). MIB-1 positive cells were counted and cell density was determined under high magnification. The proportion of labeled cells was evaluated in 10 HPF selected among the most cellular dense areas of the tumor, selecting the highest value.
Parameters classification
-
Histological subtypes: oligodendroglioma, astrocytoma, mixed glioma
-
Cellular density was classified as low, moderate or high, according to WHO criteria.
-
Cellular atypia was classified as present or absent.
-
Ki-67 groups were classified as groups 1, 2 or 3 according to the range of the percentage of proliferating tumor cells <4%, ≥4% and <8%, and ≥8% respectively.
-
Spectroscopic MR data included the determination of following ratios: the Mi/Cr, Cho/Cr, Naa/Cr, Lactates/Cr, Lipids/Cr and Cho/Naa.
Statistical analysis
The qualitative and quantitative variables tested included the baseline characteristic variables of the patients, and immunohistology and spectroscopy MR variables. Baseline characteristics of the patients were age, sex and tumor location. Immunohistochemical variable was the Ki-67 index, histological parameters were cellular density, cellular atypia. Spectroscopy MR data included Cho/Cr, Naa/Cr, Cho/Naa, Lactates/Cr, Lipids/Cr and Mi/Cr ratios.
Variables were tested by means of a univariate analysis, using the Fisher exact test or Wilcoxon rank-sum test or Kruskal–Wallis test for qualitative variables and the Wilcoxon signed-rank test for quantitative variables with a P-value <0.05 considered as indicative of a statistically significant difference.
Independent predictors of cellular atypia and Ki-67 groups variables were identified by means of a multivariate analysis, using binary logistic regression (nominal variables) (LOGXACT; Cytel Software, Cambridge, Massachusetts MA, USA) with a significance level of 0.05. These predictive factors were identified among the spectroscopy MR variables previously screened with univariate analysis with a level of P-values <0.2.
Values expressed as means were associated with the Standard Deviation (±SD) of the mean.
Results
Patient’s characteristics
Eighty-two of the 97 consecutive patients fulfilled the inclusion criteria and were enrolled in the study, including 38 (46%) women and 45 (54%) men. The mean age was 37.6 years (range 20–66 years).
Pathological and immunohistochemical findings
All of them had a histopathologically proven WHO grade 2 supratentorial gliomas. The tumor samples were obtained for 14 patients by biopsy, and by surgical resection for 68 patients. They included 23 astrocytomas (28%), 30 oligodendrogliomas (36.6%), and 29 mixed gliomas (35.4%). Cellular densities were classified as weak (n = 7, 8.5%), moderate (n = 38, 46.4%) and high (n = 37, 45.1%). Cellular atypia was found in 35 tumors (42.5%). Ki-67 immunopositivity was classified into three subgroups according to the proliferation index (PI): group 1 with low PI included 48 (58.5%) patients, group 2 with intermediate PI included 22 (26.8%) patients and group 3 with high PI included 12 (14.6%) patients.
MR imaging findings
All the patients demonstrated hyper intense and slightly heterogeneous lesions on T2-weighted images which were hypointense on T1-weighted images. Twenty-four of them presented at least one area of mild T1 signal enhancement after intravenous administration of gadolinium. In each case, at least one spectroscopic voxel encompassed the area of post-gadolinium enhancement.
MR spectroscopic findings
For each of the 82 patients, quantification of the following metabolite ratios: Cho/Naa, Cho/Cr, Naa/Cr, Mi/Cr, Lactates/Cr and Lipids/Cr, has been performed. Results are expressed in Table 1 as mean value of each ratio ± SD for total population, and groups 1, 2, 3 of PI respectively. We observed parallel increases of Cho/Cr and Cho/Naa as well as progressive decrease of Naa/Cr from one group to the next. It is noticeable that no resonances of lactate or free lipids were observable in group 1, while they could be observed in the two other groups. Lastly, no evolution of the Mi/Cr ratio could be observed from one group to another.
Statistical results
The statistical study undertaken on the whole data showed various correlations between cellular atypia and MRS data following the three groups of Ki-67.
On univariate analysis, variations of CNI and Cho/Cr index were correlated (P = 0.022).
As showed in Table 1, specific spectral patterns appeared to correlate with three ranges of Ki-67 proliferative indices:
-
A Ki-67 < 4% correlated with the absence of free lipids or lactate resonance.
-
A 4% ≤ Ki-67 < 8% correlated with a resonance of lactates and absence of resonance of free lipids.
-
A 8% ≤ Ki-67 correlated with increased CNI and a resonance of free lipids and a lower Naa/Cr (than in the two other groups).
Additionally, the presence of cellular atypia correlated with increased CNI, decreased Naa/Cr and free lipids resonance (Table 2), while a Ki-67 ≥ 8% correlated with the presence of cellular atypia (Table 3). Conversely, the absence of cellular atypia correlated with a Ki-67 < 4%. A trend towards correlation was found between cellular density and spectral patterns of group 1 or 2, without significance (P = .089).
On the other hand, no correlation could be found between MRS data and post-gadolinium enhancement or histological subtype.
Besides, no correlation between Mi/Cr values and any immunohistological data was found.
On multivariate analysis, a resonance of lactates arose as an independent predictive factor of a 4% ≤ Ki-67 < 8% (P < 0.001), while a resonance of free lipids arose as an independent predictive factor of a Ki-67 > 8% (P < 0.001). Conversely, no independent spectroscopic predictive factor of a Ki-67 < 4% emerged.
Discussion
Until now, the major issue of MRS was to improve the accuracy of distinction between low- and high-grade gliomas, and to assess their infiltration of the brain using multivoxel and/or monovoxel spectroscopy [16–18]. This study was specifically devoted to WHO grade 2 gliomas in which we attempted to investigate if 1HMRS was able to non-invasively assess their proper aggressivity. We compared spectral profiles and histological and immunohistochemical data of the tumors. The variability of biological markers of grade 2 gliomas has already been stressed [19–21], as reflected also by the varying results, in terms of prognosis (median survival), of reported series [20]. Although Ki-67 labeling index is not formally considered as a prognostic marker used for the classification (2000), an index over 4% is generally considered to be predictive of a more aggressive clinical behavior, as previously suggested by several studies [22–24]. Indeed, Neder et al. found a close relationship between MIB-1 labeling index and survival of patients with astrocytomes [23]. Wakimoto et al. also concluded that Ki-67 PI was a significant prognostic factor for gliomas [24]. Moreover, a histological classification made of different classes is by essence dichotomic [25]. Thus, it is unable to represent such a dynamic pathology, which behaves along a clinical, radiological and biological continuum from slow-growing grade 2 gliomas to their high-grade counterparts by anaplastic transformation.
Hence, a more precise assessment of the more or less aggressive potential of a grade 2 glioma, e.g. the knowledge of its evolutive stage at the time of its discovery and then its propensity to undergo malignant transformation), may yield important consequences on the therapeutic management of the patients [26, 27]. It has been demonstrated that prognosis may be greatly different between LGGs with similar features on both pathologic examination and conventional MRI, without contrast enhancement [20]. Indeed, in our study, 7 patients with a Ki-67 ≥ 8% did not show any post contrast enhancement after gadolinium administration. Otherwise, as reported in many studies, when the modifications become visible on standard MRI, e.g. mainly the blood-brain barrier disruption on post-contrast T1-weighted images, anaplastic transformation is already effective [26, 28–30]. The major point here is to establish by non-invasive imaging the progressive transformation from low-grade 2 to “intermediate” and then to grade 3 gliomas of the WHO classification. Pathological, immunohistological and molecular biological criteria, while still controversial, are widely used as they could reflect a more or less aggressive potential and could be predictive of further anaplastic transformation [22, 31]. The presence of prominent nuclear or cellular atypias (anisocaryosis with complex nuclear morphology, anisocytosis, dystrophic cells), as well as elevated Ki-67 indices, are suggestive of a more aggressive behavior of a grade 2 glioma, in the absence of true cytological signs of anaplasia as hypercellularity, increased mitotic count, microvascularisation and necrosis.
Some previous studies have already demonstrated correlations between Cho level and KI-67 levels for gliomas of various grades, but not specifically regarding the degree of aggressivity within a homogeneous group of WHO grade 2 gliomas [32, 33].
Our study shows strong correlations between specific spectral patterns and three ranges of Ki-67 indices, using not only the choline index, but also other metabolites as Naa, lactates and lipids. Mean value of Cho/Cr increased from one group to the next (e.g. from group 1 to group 2 and from group 2 to group 3), with a significantly lower mean value in group 1 (Fig. 1G1). On the other hand, a low cellular density tends to correlate only with spectral patterns of group 1 or 2 (P = 0.089), without reaching statistical significance. This feature is consistent with previous studies suggesting that Choline index correlated with cell(s) density only for tumors without necrotic or cystic areas [7, 8, 33].
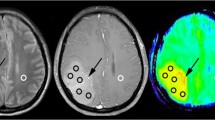
Right temporal lesion (G1a). MRS: slight increased CNI at 1.1, without significant resonance of free lipids or lactates (G1b;c). Ki-67 value is 3. Left frontoparietal mass (G2a). MRS: increased CNI at 2.0, marked resonance of lactates (G2c), without associated free lipids resonance (G2b). Ki-67 value is 5%. Right insular WHO2 glioma (G3a), without post-contrast enhancement on T1-weighted images (G3b). Increased CNI at 2.5, with deeply decreased Naa/Cr (G3d) and clear resonance of free lipids (G3c). No evidence of lactates (G3d)
The rise of lactate resonance (Fig. 1G2) suggests anaerobic metabolism within proliferating cells (tumoral anabolism), with progressive increase of CNI, and precedes the increase of microvascular density [34]. This point is in concordance with the fact that an increase of cellular density induces vascular development, since the normal brain parenchymatous vascular network becomes insufficient, as reported in previous histopathological studies [35–38]. Moreover, the resonance of lactate appears in our study as an independent predictive factor of a Ki-67 between 4 and 8%.
At a further stage, corresponding to the third Ki-67 range, membrane breakdown may happen within actively growing tumors, without cell necrosis [36, 39] as reflected by the appearance of a resonance of free lipids (Fig. 1G3), and before any visible modification on conventional MR images [30, 40]. Indeed, resonance of free lipids appears in this study as an independent predictive factor of a Ki-67 index of more than 8% (which is agreed to be an abnormally high value for “benign” lesions). Last, the correlation between free lipids resonance and cellular atypia further supports this assumption, and is consistent with the study of Nafe et al. [41]. These authors found a correlation between lipid peak and Ki-67 indices, in a population of 46 heterogeneous grades (WHO grades 2–4). At this last stage, the absence of correlation with lactates resonance may be explained by their washing out, resulting from increased blood flow into the tumoral site, as demonstrated by Law et al. [42], owing to the increase of the microvascular density, thus leading to their inconstant detection at the time of examination (while they are present), as suggested by some studies [34, 42]. Indeed, in our series, 7 patients belonging to the third group of KI-67 did not demonstrate lactate resonance. Then, from a spectro-MR point of view (on spectral grounds) a “critical point”, that is a change in the glioma aggressivity reflected by a higher proliferative capacity, could be located between the first and the second Ki-67 ranges.
By pointing out correlations between immunohistological and spectral data, as well as independent spectroscopic predictive factors of immunohistological results, our study suggests that 1HMRS may be a reliable tool for predicting the proliferative activity of WHO grade 2 gliomas in reference to the Ki-67 index, and also to the presence of histological atypias. 1HMRS may then be useful in the non-invasive monitoring of low-grade gliomas as suggested by previous studies [43], in order to identify sooner, or more subtle modifications, than conventional MRI (e.g. an heterogeneous structure and/or post-contrast enhancement of the tumor). Furthermore, longitudinal monitoring using 1HMRS, may provide kinetic parameters based on successive spectroscopic profiles, thus complementing the information obtained by biopsy or surgery, as they can be obtained repeatedly. Iterative spectroscopic informations can implement other dynamic evaluations (such as the glioma growth curves [12] and the perfusion study of Law [42]), and thus lead to an improvement of the therapeutical strategies, using surgery [44], chemotherapy [45, 46] and radiotherapy [47]. These considerations and hypothesis must be supported by other prospective studies, as they are currently in progress in our institution.
References
Alger JR et al (1990) Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxyglucose PET. Radiology 177(3):633–641
Ott D, Hennig J, Ernst T (1993) Human brain tumors: assessment with in vivo proton MR spectroscopy. Radiology 186(3):745–752
Michaelis T et al (1993) Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology 187(1):219–227
Miller BL (1991) A review of chemical issues in 1H NMR spectroscopy: N-acetyl-l-aspartate, creatine and choline. NMR Biomed 4(2):47–52
Urenjak J et al (1993) Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 13(3):981–989
Kemp GJ (2000) Non-invasive methods for studying brain energy metabolism: what they show and what it means. Dev Neurosci 22(5–6):418–428
Croteau D et al (2001) Correlation between magnetic resonance spectroscopy imaging and image-guided biopsies: semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery 49(4):823–829
Dowling C et al (2001) Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. Am J Neuroradiol 22(4):604–612
Vuori K et al (2004) Low-grade gliomas and focal cortical developmental malformations: differentiation with proton MR spectroscopy. Radiology 230(3):703–708
Herminghaus S et al (2003) Determination of histopathological tumor grade in neuroepithelial brain tumors by using spectral pattern analysis of in vivo spectroscopic data. J Neurosurg 98(1):74–81
Yang D et al (2002) Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology 44(8):656–666
Mandonnet E et al (2003) Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol 53(4):524–528
Hoyt JW et al (1995) Analysis of proliferative grade in glial neoplasms using antibodies to the Ki-67 defined antigen and PCNA in formalin fixed, deparaffinized tissues. J Neurooncol 24(2):163–169
Steck K, el-Naggar AK (1994) Comparative flow cytometric analysis of Ki-67 and proliferating cell nuclear antigen (PCNA) in solid neoplasms. Cytometry 17(3):258–265
Kleihues P, Sobin LH (2000) World Health Organization classification of tumors. Cancer 88(12):2887
Czernicki Z et al (2000) Malignancy of brain tumors evaluated by proton magnetic resonance spectroscopy (1H-MRS) in vitro. Acta Neurochir Suppl 76:17–20
Isobe T et al (2002) Quantification of cerebral metabolites in glioma patients with proton MR spectroscopy using T2 relaxation time correction. Magn Reson Imaging 20(4):343–349
Tedeschi G et al (1997) Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 87(4):516–524
Piepmeier JM (2001) Cerebral gliomas: how tumor biology affects management. Clin Neurosurg 48:3–9
Pallud J et al (2006) Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol 60(3):380–383
Piepmeier J et al (1996) Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery 38(5):872–878 (discussion 878–879)
Louis DN et al (1991) Proliferating cell nuclear antigen and Ki-67 immunohistochemistry in brain tumors: a comparative study. Acta Neuropathol (Berl) 81(6):675–679
Neder L et al (2004) MIB-1 labeling index in astrocytic tumors–a clinicopathologic study. Clin Neuropathol 23(6):262–270
Wakimoto H et al (1996) Prognostic significance of Ki-67 labeling indices obtained using MIB-1 monoclonal antibody in patients with supratentorial astrocytomas. Cancer 77(2):373–380
Daumas-Duport C et al (1988) Grading of astrocytomas. A simple and reproducible method. Cancer 62(10):2152–2165
Kondziolka D, Lunsford LD, Martinez AJ (1993) Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg 79(4):533–536
Cao Y et al (2006) Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol 24(8):1228–1235
Julia-Sape M et al (2006) Comparison between neuroimaging classifications and histopathological diagnoses using an international multicenter brain tumor magnetic resonance imaging database. J Neurosurg 105(1):6–14
Lev MH et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. Am J Neuroradiol 25(2):214–221
Rees J (2003) Advances in magnetic resonance imaging of brain tumours. Curr Opin Neurol 16(6):643–650
Onda K et al (1994) Correlation between the bromodeoxyuridine labeling index and the MIB-1 and Ki-67 proliferating cell indices in cerebral gliomas. Cancer 74(7):1921–1926
Matsumura A et al (2005) Correlation between choline and MIB-1 index in human gliomas. A quantitative in proton MR spectroscopy study. J Clin Neurosci 12(4):416–420
Shimizu H et al (2000) Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. Am J Neuroradiol 21(4):659–665
Law M et al (2006) Perfusion magnetic resonance imaging predicts patient outcome as an adjunct to histopathology: a second reference standard in the surgical and nonsurgical treatment of low-grade gliomas. Neurosurgery 58(6):1099–1107 (discussion 1099–1107)
Cha S et al (2003) Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med 49(5):848–855
Shin JH et al (2002) Using relative cerebral blood flow and volume to evaluate the histopathologic grade of cerebral gliomas: preliminary results. Am J Roentgenol 179(3):783–789
Sugahara T et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. Am J Roentgenol 171(6):1479–1486
Castillo M, Kwock L, Mukherji SK (1996) Clinical applications of proton MR spectroscopy. Am J Neuroradiol 17(1):1–15
Danielsen ER, Ross B (1999) Magnetic resonance spectroscopy diagnosis of neurological diseases (Book). Marcel Dekker, INC. Editor, New York, Basel
Leclerc X, Huisman TA, Sorensen AG (2002) The potential of proton magnetic resonance spectroscopy ((1)H-MRS) in the diagnosis and management of patients with brain tumors. Curr Opin Oncol 14(3):292–298
Nafe R et al (2003) Preoperative proton-MR spectroscopy of gliomas—correlation with quantitative nuclear morphology in surgical specimen. J Neurooncol 63(3):233–245
Law M et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. Am J Neuroradiol 24(10):1989–1998
Reijneveld JC et al (2005) Proton MRS imaging in the follow-up of patients with suspected low-grade gliomas. Neuroradiology 47(12):887–891
Duffau H et al (2005) Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76(6):845–851
Hoang-Xuan K et al (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22(15):3133–3138
Van den Bent MJ et al (2003) Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol 21(13):2525–2528
Van den Bent MJ (2003) Can chemotherapy replace radiotherapy in low-grade gliomas? Time for randomized studies. Semin Oncol 30(6 suppl 19):39–44
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guillevin, R., Menuel, C., Duffau, H. et al. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J Neurooncol 87, 181–187 (2008). https://doi.org/10.1007/s11060-007-9508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9508-y