Abstract
Purpose To investigate the efficacy and safety of the combination of vinorelbine and intensive temozolomide for recurrent or progressive brain metastases from solid tumors. Methods Patients ≥18 years of age and with Karnofsky performance scale (KPS) ≥ 60, adequate organ function and progressive or recurrent brain metastases were eligible. This was a phase II trial with 28-day cycles using temozolomide (150 mg/m2, days 1–7 and 15–21) and vinorelbine 25 or 30 mg/m2 on days one and eight. The primary endpoint was objective radiographic response. Results Thirty-eight patients (15 men, 23 women) with a median age of 57 years (range, 39–75) and median KPS of 80 were enrolled. The primary tumor sites were lung (n = 20), breast (n = 11), colorectal (n = 2), kidney (n = 2), bladder (n = 1), endometrium (n = 1), head and neck (n = 1). Prior therapies included chemotherapy (97%), whole-brain radiation therapy (79%), brain metastasis resection (53%) and stereotatic radiosurgery (47%). Objective radiographic response rate was 5% (one complete response and one minor response); five patients had stable disease, 29 progressive disease and two patients were not evaluable. Twenty-nine patients (76%) have died and the median follow-up of survivors was six months. Median progression-free and overall survivals were 1.9 and 5 months, respectively. Grade 3/4 toxicities were mainly hematological and two patients discontinued the study due to myelosuppression. Conclusions In this heavily pretreated population of patients with brain metastases, adding vinorelbine and increasing the intensity of temozolomide do not improve response rates compared to previous studies with single-agent temozolomide at standard doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases are a common complication of patients with cancer, occurring in approximately 25% of patients with disseminated disease. The incidence of brain metastases may be increasing as therapies for systemic cancer improve and patients survive longer. Brain metastases have a major impact on quality of life and median survival remains as low as 3–6 months [1]. Surgical resection [2] and stereotactic radiosurgery [3] can benefit a selected group of patients but palliative whole brain radiotherapy (WBRT) remains the standard option for most patients with brain metastases. Patients with recurrent or progressive brain metastases after standard therapies have limited treatment options.
Temozolomide is a well-tolerated oral alkylating agent with excellent central nervous system (CNS) penetration that has demonstrated clinical activity in glioblastoma multiforme [4], recurrent anaplastic gliomas [5], metastatic melanoma [6] and preclinical activity in a variety of solid tumors [7]. Temozolomide is typically administered for five consecutive days every 28 days at a dose of 150–200 mg/m2/d. However, clinical trials utilizing conventional doses of temozolomide have demonstrated only modest efficacy in patients with recurrent or progressive brain metastases [8, 9]. Alternate treatment regimens have been used in an attempt to improve outcome by decreasing the time between dosing intervals and thereby decreasing the regrowth of resistant tumor cells between cycles of chemotherapy [10]. A 1-week on 1-week off schedule of temozolomide at 150 mg/m2/d is feasible and permits a 2.1-fold greater drug exposure than the conventional schedule of 5 days every 28 days [11].
Vinorelbine is a semi-synthetic vinca alkaloid that is active in many tumour types, including breast cancer and non-small cell lung cancer (NSCLC), which account for most cases of brain metastases. Vinorelbine has a favorable safety profile and is a lipophilic agent that potentially crosses the blood-brain barrier [12].
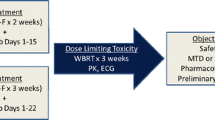
We have previously reported the results of a phase I, dose-finding study demonstrating that temozolomide at 150 mg/m2/d on days 1–7 and 15–21 can be safely combined with vinorelbine on days 1 and 8 of 28-day cycles [13]. The goal of this phase II study was to further investigate the safety and efficacy of this regimen in patients with recurrent brain metastases.
Patients and methods
Patient eligibility
Adult patients (≥18 years of age) with histopathologic confirmation of the diagnosis of a solid tumor and recurrent or refractory brain metastases, with at least one measurable lesion on brain magnetic resonance imaging (MRI), were eligible. Patients were recruited from the Department of Neurology at Memorial Sloan-Kettering Cancer Center (MSKCC) from February 2003 to March 2007. Additional eligibility criteria included Karnofsky performance scale (KPS) ≥ 60, adequate bone marrow function (hemoglobin ≥ 10 g/dl, absolute neutrophil count 1,500/mm3, platelet count ≥ 100,000/mm3), adequate liver function (bilirubin <1.5 times the upper limit of normal, AST and ALT ≤ three times the upper limit of normal, alkaline phosphatase ≤ two times the upper limit of normal), adequate renal function (BUN and creatinine < 1.5 times the upper limit of normal), and life expectancy ≥8 weeks. At least 2 weeks must have elapsed from surgery, 4 weeks from external beam radiotherapy, 8 weeks from stereotactic radiosurgery and patients must have recovered from all acute toxicities of prior chemotherapies before enrolling in this trial. Patients previously treated with stereotactic radiosurgery required evidence of progression at a distant site in the brain or confirmation of tumor progression by biopsy or PET scan. Patients who received prior treatment with temozolomide, dacarbazine or vinorelbine, pregnant or nursing women, patients with serious intercurrent medical illnesses or patients with known HIV or AIDS-related illness were excluded. Additional exclusion criteria included evidence of leptomeningeal or dural metastases.
Study design
The study was approved by the MSKCC Institutional Review Board and written informed consent was obtained from all patients.
Baseline evaluation included brain MRI, physical and neurological examination, and assessment of systemic disease status (usually by body CT scan) within 2 weeks prior to treatment. Patients underwent a weekly complete blood count while on study. Physical and neurological examination, comprehensive metabolic panel, and brain MRI were repeated every two cycles. Re-evaluation of the extent of systemic tumor with body CT was performed after the first two cycles and no less than every four cycles thereafter.
Patients received 28-day cycles with temozolomide (150 mg/m2/d, days 1–7 and 15–21) and intravenous vinorelbine on days one and eight. The phase I maximum tolerated dose (MTD) of vinorelbine in this combination was defined as 30 mg/m2. However, due to frequent myelosuppression observed in the first 14 patients of the study, the vinorelbine dose was decreased to 25 mg/m2. In the absence of disease progression or unacceptable toxicity, patients could continue to receive treatment on study for up to 1 year.
Response and toxicity evaluation
Response to treatment was evaluated by brain MRI. A complete response was defined as total resolution of all measurable radiographic evidence of brain metastases on two assessments separated by at least 4 weeks. The patient could not have any neurological deterioration or be receiving corticosteroids. A partial response required greater than 50% reduction in the size of all measurable brain metastases as defined by the sum of the products of the greatest length and maximum width of the lesions for at least two assessments, separated by at least 4 weeks in a patient with stable or decreasing dose of corticosteroids. Minor response was defined as a 25–50% reduction in the size of all measurable brain metastases as defined by the sum of the products of the greatest length and maximum width of the lesions for at least two assessments separated by at least 4 weeks in a patient with stable or decreasing dose of corticosteroids. Progressive disease was defined as at least 25% increase in tumor size as defined by the sum of the products of the greatest length and maximum width of the lesions or the appearance of any new lesions; stable disease represented all other situations. Toxicity was evaluated using the National Cancer Institute’s Common Toxicity Criteria, version 2.0 throughout the clinical trial until 30 days after removal from the protocol.
Statistics
The primary endpoint was objective radiographic tumor response. Secondary endpoints included progression-free and overall survival and safety. Patients who were treated in the phase I study with vinorelbine doses of 25 or 30 mg/m2 were included in the phase II data analyses. A modified two-stage phase II clinical trial design was used in this study. If zero or only one response were observed in the first 20 patients, it could be concluded that the true response rate was <20% with 93% confidence and no further patients would be enrolled. If two or more responses were observed in the first stage, a total of 35 evaluable patients would be required. If four or more responses were observed, there would be evidence that the regimen used was active. This design was powered to detect a true response rate of 20% with 91% probability and reject a response rate of 5% with 89% probability.
Demographic, safety, laboratory data and treatment response were analyzed using descriptive statistics, while survival analyses were based on Kaplan-Meier estimates. For all patients, progression-free survival was measured from enrollment in the study to disease progression, last contact or death, whichever was earliest. Overall survival was measured from study entry to death or last follow-up. Survival analyses were performed in an intent-to-treat fashion. Follow-up extended thru May 2007.
Results
Patients
A total of 38 patients (15 men, 23 women) with a median age of 57 years were entered in the phase II protocol, including 18 patients who were carried over from the phase I protocol who received a dose of 25 or 30 mg/m2 of vinorelbine. Baseline patient characteristics are presented in Table 1. The most common primary cancer diagnoses were lung (53%) and breast (29%). Most patients (84%) had active systemic disease, with a median of two extracranial sites involved (range, 0–6). Previous treatment for the brain metastases included WBRT (79%), surgical resection (53%) and stereotactic radiosurgery (47%).
Patients completed a median of two cycles of therapy (range, 0–6 cycles), and two patients did not receive the first cycle. One patient had clinical deterioration and another patient had improvement of the brain lesions before starting the first cycle of treatment. Twenty-two patients received vinorelbine at a dose of 25 mg/m2 and 14 patients received 30 mg/m2. Thirty-three patients discontinued the study due to progression of disease or clinical deterioration, two patients discontinued due to toxicity, and one patient withdrew consent.
Response to treatment
Two patients (5%; 95% confidence interval (CI), 0.6–19%) had an objective radiographic response, including a complete response in a patient with NSCLC and a minor response in a patient with breast cancer. The duration of the response in these two patients were 5 and 1 months, respectively. Five patients (13%) had stable disease for a median of 2.8 months, 26 patients (69%) had progressive disease confirmed by imaging (23 in the brain, three systemically) and three patients (8%) had clinical progressive disease. The two patients (5%) who did not receive one cycle of chemotherapy were not assessed for response.
Survival
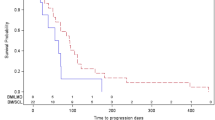
To date, among the 38 patients enrolled, 29 (76%) had died. The median follow-up among survivors was six months. The Kaplan-Meier estimate of progression-free and overall survival for all patients is shown in Fig. 1. Median progression-free survival was 1.9 months (95% CI, 1.8–2.2 months) and median overall survival was five months (95% CI, 3.6–9.8 months).
Safety
Grade 3 and 4 toxicities were mainly hematologic and consisted primarily of leukopenia, lymphopenia, and neutropenia (Table 2). The group who received vinorelbine at a dose of 30 mg/m2 developed more grade 3 and 4 toxicities. Leukopenia and neutropenia were statistically more frequent in the group who received vinorelbine 30 mg/m2 (P < 0.001 and P = 0.005, exact Fisher’s test). Two patients discontinued the protocol due to hematological toxicity. All non-hematologic adverse events were manageable.
Discussion
The results of this study suggest that the combination of a more intense course of temozolomide coupled with vinorelbine has only moderate clinical activity in this heavily pretreated population with brain metastases. Temozolomide alone at conventional doses administered for five consecutive days every 28 days at a dose of 150–200 mg/m2/d has shown modest efficacy in a similar population of brain metastases patients in two different phase II trials [8, 9]. In one study, a partial response was achieved in 1 (4%) and disease stabilization was observed in 4 (17%) of 24 evaluable patients [9]. In an MSKCC study, there were two partial responses (6%) and 15 patients with stable disease (44%) among 34 patients assessed for radiographic response [8]. All three patients with an objective radiographic response had NSCLC, which was the most common primary tumor enrolled in these two clinical trials. Subsequently, two phase II clinical trials with single-agent temozolomide in brain metastases from NSCLC were reported. An European Organization for Research and Treatment of Cancer (EORTC) study was terminated early because none of 12 patients with NSCLC and brain metastases and 13 patients with advanced NSCLC without brain metastases had an objective radiographic response [14]. Another phase II study in heavily pre-treated patients with brain metastases from NSCLC showed an objective response in three patients (10%) and stable disease in three patients (10%) [15]. One limitation of our study was the inclusion of multiple different types of cancer and histology with variable degree of chemosensitivity. However, these patients were heavily pretreated and often did not have any therapeutic options other than supportive care or clinical trials such as this one.
Because single-agent temozolomide had only modest activity in patients with recurrent or refractory brain metastases but was relatively well tolerated, we and other investigators have tried to combine temozolomide with other cytotoxic agents in an attempt to improve efficacy. One study combined temozolomide at standard doses (150–200 mg/m2/d for 5 days every 28 days) with cisplatin 75 mg/m2 on day one. Among 32 patients with mostly breast or lung cancer, there were 10 objective responses (31%) and five with (16%) stable disease. Grade III-IV toxicities with this regimen were mostly hematological and one patient died from septicemia/neutropenic fever [16]. Another study combined temozolomide (200 mg/m2/d for 5 days every 28 days) with pegylated liposomal doxorubicin (35 mg/m2 on day one) and found seven objective responses (37%) among 19 patients [17].
Vinorelbine was chosen based on its in vitro and in vivo broad-spectrum of action in solid tumors (lung, breast, ovarian), side effect profile, and lipophilic properties, which may enhance penetration across the blood-brain barrier [12]. Moreover, vinorelbine in combination with other cytotoxic agents has shown activity in brain metastases from NSCLC, which is the most common cause of metastatic brain disease. Vinorelbine (30 mg/m2 on days one, eight, 15, 22 of 28-day cycles) in combination with cisplatin (100 mg/m2 on day one) and delayed WBRT has shown activity as initial treatment of brain metastases from NSCLC. Among 76 patients, there was a 21% objective response rate after two cycles of chemotherapy alone, but grade 4 neutropenia occurred in 35% of patients and six patients had treatment-related deaths [18]. A phase II study with vinorelbine, gemcitabine and carboplatin as initial treatment for brain metastases from NSCLC showed a response rate of 45% in 20 evaluable patients [19].
In addition to combining temozolomide with vinorelbine, we used a more intensive dosing regimen of temozolomide to enhance efficacy. This regimen increases the intensity of temozolomide exposure and could minimize tumor cell regrowth between cycles. Moreover, this schedule might also be more effective in depleting the DNA repair enzyme O6-alkylguanyl transferase, which is a major mechanism of temozolomide resistance [20]. This same temozolomide regimen has been used successfully in the treatment of recurrent glioblastomas with only moderate toxicity [11].
In this heavily pretreated population of patients with brain metastases, adding vinorelbine to a protracted course of temozolomide does not improve response rates compared to previous studies with single-agent temozolomide at standard doses. Moreover, the treatment of recurrent or progressive brain metastases is only palliative, so the high incidence of toxicity found in this study is unacceptable for this patient population.
References
Omuro AM, Abrey LE (2004) Brain metastases. Curr Neurol Neurosci Rep 4:205–210
Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS et al (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Agarwala SS, Kirkwood JM, Gore M, Dreno B, Thatcher N, Czarnetski B et al (2004) Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol 22:2101–2107
Raymond E, Izbicka E, Soda H, Gerson SL, Dugan M, Von Hoff DD (1997) Activity of temozolomide against human tumor colony-forming units. Clin Cancer Res 3:1769–1774
Abrey LE, Olson JD, Raizer JJ, Mack M, Rodavitch A, Boutros DY et al (2001) A phase II trial of temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 53:259–265
Christodoulou C, Bafaloukos D, Kosmidis P, Samantas E, Bamias A, Papakostas P et al (2001) Phase II study of temozolomide in heavily pretreated cancer patients with brain metastases. Ann Oncol 12:249–254
Fornier M, Norton L (2005) Dose-dense adjuvant chemotherapy for primary breast cancer. Breast Cancer Res 7:64–69
Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M (2004) One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology 62:2113–2115
Gregory RK, Smith IE (2000) Vinorelbine–a clinical review. Br J Cancer 82:1907–1913
Omuro AM, Raizer JJ, Demopoulos A, Malkin MG, Abrey LE (2006) Vinorelbine combined with a protracted course of temozolomide for recurrent brain metastases: a phase I trial. J Neurooncol 78:277–280
Dziadziuszko R, Ardizzoni A, Postmus PE, Smit EF, Price A, Debruyne C et al (2003) Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases. a phase II study of the EORTC Lung Cancer Group (08965). Eur J Cancer 39:1271–1276
Giorgio CG, Giuffrida D, Pappalardo A, Russo A, Santini D, Salice P et al (2005) Oral temozolomide in heavily pre-treated brain metastases from non-small cell lung cancer: phase II study. Lung Cancer 50:247–254
Christodoulou C, Bafaloukos D, Linardou H, Aravantinos G, Bamias A, Carina M et al (2005) Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: a Hellenic Cooperative Oncology Group (HeCOG) phase II study. J Neurooncol 71:61–65
Caraglia M, Addeo R, Costanzo R, Montella L, Faiola V, Marra M et al (2006) Phase II study of temozolomide plus pegylated liposomal doxorubicin in the treatment of brain metastases from solid tumours. Cancer Chemother Pharmacol 57:34–39
Robinet G, Thomas P, Breton JL, Lena H, Gouva S, Dabouis G et al (2001) Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancerologie (GFPC) protocol 95–1. Ann Oncol 12:59–67
Bernardo G, Cuzzoni Q, Strada MR, Bernardo A, Brunetti G, Jedrychowska I et al (2002) First-line chemotherapy with vinorelbine, gemcitabine, and carboplatin in the treatment of brain metastases from non-small-cell lung cancer: a phase II study. Cancer Invest 20:293–302
Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A et al (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011
Acknowledgements
LE Abrey has received honoraria and grant support from Schering Plough, Inc. AM Omuro and JJ Raizer have received honoraria from Schering Plough, Inc. This study was supported in part by an unrestricted educational grant from Integrated Therapy Group, Inc. (ITGI), a subsidiary of Schering-Plough Corporation. We thank Judy Lampron for her expert editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwamoto, F.M., Omuro, A.M., Raizer, J.J. et al. A phase II trial of vinorelbine and intensive temozolomide for patients with recurrent or progressive brain metastases. J Neurooncol 87, 85–90 (2008). https://doi.org/10.1007/s11060-007-9491-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9491-3