Abstract
Inhibition of DNA excision repair can modulate resistance to cisplatin. Cytosine arabinoside (Ara-C) and hydroxyurea (HU), in combination, inhibit the excision-repair system and removal of platinum-DNA adducts. Marked cytotoxic synergy had been demonstrated in vitro at clinically achievable levels. The three-drug regimen was found to be feasible in clinical pilot studies. A Phase II study in patients with relapsed or progressive anaplastic astrocytoma (AA) or glioblastoma multiforme (GBM) was performed in the Southwest Oncology Group. The primary end point was 6 month survival, historically about 42%. A loading dose of HU 1,260 mg/m2 IV over 1 h was followed by Ara-C 1,200 mg/m2 plus HU 5,040 mg/m2 IV over 12 h, followed by cisplatin 100 mg/m2 IV over 1 h. A total of 76 patients were registered. The GBM stratum registered 56 patients in a two-stage accrual. Among 51 eligible GBM patients, the 6-month survival probability was 41% (95% CI 28–55%), and median overall survival was 5 months (95% CI 4–6 months). The 6-month progression-free survival probability was 25% (95% CI 14–37%), and median progression-free survival was 2 months (95% CI 2–4 months). One patient achieved a partial response (2%, 95% CI 0–10%), 13 patients had stable disease (25%, 95% CI 14–39%). Twenty-two patients progressed, and 14 were not assessable for response. The AA stratum was closed early after 20 patients due to slow accrual. Among 19 eligible patients, the 6-month survival probability was 58% (95% CI 36–80%), and median overall survival was 7 months (95% CI 7–14 months). The 6-month progression-free survival probability was 26% (95% CI 6–46%), and median progression-free survival was 3 months (95% CI 2–5 months). No responses were seen. Six patients (32%) had stable disease (95% CI 13–57%), 11 progressed, and 2 were not assessable for response. Of the 70 patients evaluable for toxicity, two died of infection. Twenty-three patients (33%) experienced Grade 4 toxicities, primarily hematological. Cisplatin combined with HU and Ara-C did not improve the 6 month survival rate in patients with relapsed or progressive AA or GBM. Significantly more hematological toxicity was seen than expected from cisplatin alone. Although benefit might be possible in a more platinum-sensitive tumor type, further clinical trials with this regimen for patients with glioblastoma multiforme or anaplastic astrocytoma are not justified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The median survival for adult patients with supratentorial, high grade gliomas after primary surgery and radiotherapy is approximately 1 year, with 80% of patients dead by 24 months [22, 24]. Therapeutic options are particularly limited for patients with relapsed or persistent disease after primary surgery and radiotherapy with or without chemotherapy. For such patients, response rates have been very low and prolonged response duration is the exception [15]. When used together with BCNU, cisplatin provided no additional benefit in a Phase III trial in patients with newly diagnosed glioblastoma multiforme [5]. Stewart et al. tested cisplatin plus Ara-C in adults with malignant gliomas and reported 58% and 23% response rates in untreated and previously treated cohorts, respectively [19].
Modulation of various forms of drug resistance at the cellular level theoretically might improve the therapeutic index of cisplatin, carboplatin and related compounds. The DNA excision repair system is involved in the repair of DNA damage from cisplatin. The ability of a cell to excise UV-induced dimers is inhibited by cytosine arabinoside (Ara-C) [4, 9, 11] and by Hydroxyurea (HU) [3, 6–8, 10, 12, 16, 20, 25, 26]. Cytosine arabinoside (Ara-C) and hydroxyurea (HU), in combination, inhibit the removal of platinum DNA adducts, and marked cytotoxic synergy has been demonstrated in very platinum-resistant HT29 colon carcinoma cells [21]. The HU (1 mM) and Ara-C (1 uM) drug levels required have been achieved in clinical pilot studies of the three-drug regimen [1, 2].
Two pilot studies used a 12-h treatment with HU and Ara-C preceding a 1-h cisplatin infusion. Doses and schedules of the three drugs were chosen from pharmacokinetic data in order to achieve concentrations in vivo similar to those used in the in vitro model. The study accrued 21 patients with prior chemotherapy and 19 patients previously untreated. Partial responses were seen in 9 of 32 patients with measurable disease, and there was significant improvement in 5 of 8 patients with only evaluable disease (one of which was a patient with refractory glioblastoma). Of note, responses were observed in 3 of 8 patients who had previously received cisplatin, suggesting that the HU and Ara-C combination modulated cisplatin resistance. No major acute toxicity was seen. Thrombocytopenia was dose-limiting in patients with a prior history of chemotherapy. Azotemia was treatment limiting in responding and stable patients, suggesting possible synergistic nephrotoxicity. Higher cisplatin-DNA adduct levels in kidney tissue have been associated with a greater incidence of cisplatin induced nephrotoxicity [13, 14].
The second pilot study was designed with modifications dictated by the toxicities in the first trial (nausea, vomiting, thrombocytopenia, and azotemia) in order to determine the optimal Phase II doses in previously untreated and treated cohorts. Allopurinol was given to eliminate the elevation of uric acid observed in the first study which may have contributed to the azotemia. A parenteral preparation of hydoxyurea was substituted for the oral dose, and the 12-h continuous infusion was found to be more reliable and effective in reaching a 12-h steady state. Serum levels could also be monitored for Ara-C and HU, allowing tailoring of drug dose to optimize dose level. This study accrued 45 patients, with a wide range of refractory solid tumors (27 no or limited prior chemotherapy and 18 extensive prior chemotherapy). The substitution of parenteral hydroxyurea for oral HU virtually eliminated the nausea and vomiting seen in the first pilot study. There was significantly less azotemia, only 1 out of 96 cycles resulted in reversible grade 4 azotemia.
The recommended Phase II dose of 100 mg/m2/h achieved steady state Ara-C levels of 2–4 μM, slightly above the target of 1 μM. Target level of 1,000 μM of hydroxyurea was achieved with a loading dose of 1,260 mg/m2 and an hourly dose of 420 mg/m2/h × 12 in the untreated cohort. The pilot studies showed this triple drug combination to be tolerable and to result in some clinical responses in solid tumor patients.
Therapeutic options for patients with relapsed or progressive grade 3 or 4 glioma are limited. This three-drug regimen was particularly attractive to test in this population. The primary objective of the current study was to evaluate 6-month survival rate. Based on Southwest Oncology Group experience with Phase II agents concluded to be inactive in similar patient populations (in both anaplastic astrocytoma and glioblastoma multiforme), the typical 6-month survival rate is 42%
Patients and treatment
Patients with histologically confirmed Grade 3 (anaplastic astrocytoma) or 4 (glioblastoma multiforme) were enrolled into the trial. All patients had to have received adequate radiation therapy and have disease that was recurrent (re-appearance of tumor), persistent (persistent tumor at least 3 months beyond completion of radiation and causing clinical neurologic deterioration), or progressive disease (25% increase in tumor size or greater), as documented by CT scan or MRI after prior surgery and/or radiotherapy. It was required that biopsy slides documenting the histology were available to submit for pathology review to verify diagnosis. Additionally, the patient was required to have bidimensionally measurable disease defined by contrast enhancing margins on CT or MRI scan, with the same technique of scan available and used consistently throughout the study. Up to four cycles of one prior chemotherapy regimen not containing cisplatin was allowed prior to enrollment. Patients who experienced any Grade 3 or higher hematologic toxicity while receiving prior chemotherapy were ineligible because of concerns regarding myelotoxicity from the three drug combination regimen. Stable steroid dose prior to imaging was required. No hormonal or biologic therapies other than G-CSF were allowed. An adequate marrow reserve was required as indicated by WBC > 4,000, ANC > 1,500, platelets greater than the institutional lower limit of normal, and HGB > 10 g%. These tests were performed within 14 days prior to registration. Of note, all patients had a 24 h creatinine clearance > 70 ml/min as well as a serum uric acid <1.2 times the institutional upper limit of normal and SGOT and SGPT < 2 times the institutional upper limit of normal all within 14 days prior to registration. Measurements at baseline of LDH, potassium, and phosphorus as well as a baseline audiogram were required. Patients had to have a SWOG performance status of ≤2.
Patients were stratified by histology: anaplastic astrocytoma versus glioblastoma multiforme. At registration, patients were described by the disease status (recurrent, progressive or persistent), performance status (0–1 vs. 2), prior chemotherapy (yes or no) and prior nitrosureas (yes or no).
The planned accrual was 55 eligible patients in each subgroup with a two-stage design. For each subgroup, a total of 30 eligible patients were to be accrued to the first stage. In the first stage, it was planned that if 11 or fewer patients survived more than 6 months then the study would be closed to that particular subgroup and the regimen would be concluded to be inactive in that patient population. If 12 or more patients survived longer than 6 months within a subgroup, then an additional 25 patients would be accrued in stage two, for a total of 55 in the histologic subgroup.
Treatment
Patients received a loading dose of hydroxyurea 1,260 mg/m2 IV over 1 h followed by Ara-C 1,200 mg/m2 plus hydroxyurea 5,040 mg/m2 IV over 12 h, followed by cisplatin 100 mg/m2 IV over 1 h. Allopurinol. Mannitol, and aggressive hydration were used, as well as dexamethasone to prevent cerebral edema. Standard antiemetic regimens were used.
Results
The study closed on February 15, 2001 with a total of seventy-six patients registered. The anaplastic astrocytoma stratum closed early on June 1, 1998 due to slow accrual (after twenty patients registered). The glioblastoma multiforme stratum was temporarily closed after thirty patients had been accrued. The results of the interim analysis indicated that the second stage of accrual should proceed. The GBM stratum met the accrual goal, with a total of 56 patients.
Five of the GBM patients enrolled in the trial were ineligible: two patients had an inadequate WBC count, two patients failed pathology review (gliosarcoma and one other patient with oligodendroglioma with neuroglial neoplasm), and one patient not having measurable disease.
Overall survival, progression-free survival, and response
Glioblastoma multiforme
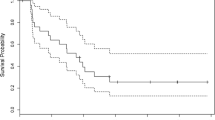
Of the 51 eligible patients, all have died, with a median overall survival of 5 months (95% confidence interval of 4–6 months) (Fig. 1). The 6-month survival probability was 41% (95% confidence interval of 28–55%). All GBM patients have either progressed or died with a median progression-free survival of 2 months (95% confidence interval of 2–4 months) (Fig. 2). The 6-month progression-free survival probability is 25% (95% confidence interval of 14–37%).
Of the 51 eligible GBM patients, one patient achieved a partial response (2%, 95% confidence interval of 0–10%). Thirteen patients (25%, 95% confidence interval of 14–39%) had stable disease. Twenty-two patients had increasing disease, and 14 had disease that was not assessable under the protocol definition.
Anaplastic astrocytoma
Of the 19 eligible patients, 18 have died, with a median overall survival of 7 months (95% confidence interval of 6–14 months) (Fig. 1). The 6-month survival probability was 58% (95% confidence interval of 36–80%). No responses were seen. All AA patients have progressed or died, with a median progression-free survival of 3 months (95% confidence interval of 2–5 months 95%) (Fig. 2). The 6-month progression-free survival probability was 26% (95% confidence interval of 7–46%). Of the 19 eligible patients, 18 have died, with a median overall survival of 7 months (95% confidence interval of 6–14 months) (Fig. 1).
Of the 19 eligible patients, no responses were seen (0%; 95% CI of 0%–18%). Six patients (32%, 95% confidence interval of 13–57%) had stable disease. Eleven patients had increasing disease, and two had disease that could not be assessed.
Toxicity
Hematologic and renal toxicity were expected on the basis of prior clinical experience with this regimen. Worsening of cerebral edema due to vigorous hydration was a theoretical concern in this particular patient population.
Among the 70 eligible patients assessed for toxicity, two died of sepsis. One ineligible glioblastoma multiforme patient also died of sepsis. Twenty patients (16 GBM, 4 AA) experienced grade four hematologic toxicities. A total of 6 patients (5 GBM, 1 AA) experienced grade four non-hematologic toxicities. There was one major protocol deviation due to failure to dose reduce for grade 4 thrombocytopenia. Grade four creatinine increase after inadequate hydration was seen in one patient, a protocol deviation resulting in removal from the study.
In the glioblastoma multiforme cohort, ten patients were removed from protocol treatment because of toxicity; twelve patients were removed from treatment for reasons not specified in the protocol, primarily deteriorating clinical condition or progressive disease not meeting protocol definition of progression (11 patients), and improper collection of creatinine clearance (one patient). Of the nineteen eligible anaplastic astrocytoma patients, four were removed from protocol for reasons not specified in the protocol: two patients had progression or deteriorating condition not meeting protocol definition of progressive disease, one patient was removed for additional surgery after four cycles, and one patient had a deteriorating condition after remaining on protocol treatment following documented progression. Four patients were removed due to toxicity related to treatment: decreased creatinine clearance (1 patient), decision to not restart treatment after dose delay for pneumonia (1 patient), grade 4 convulsions due to patient non-compliance with anticonvulsant medications (1 patient), and renal insufficiency (1 patient). Two patients refused to complete protocol treatment.
Despite initial concerns regarding renal function and cerebral edema, those problems were not encountered when the specified precautions were observed. There were no grade 3 or grade 4 renal toxicities when using prehydration, allopurinol and when avoiding contrast agents prior to platinum therapy. Toxicities ≥ grade 3 are listed in Table 1. Fatigue and anorexia were the most significant non-hematologic toxicities.
Discussion
Therapeutic options for patients with relapsed or progressive grade 3 or 4 glioma are limited. This three-drug regimen was considered to be attractive to test in this population for a number of reasons. Ara-C, hydroxyurea and cisplatin may each have single agent activity. High concentrations of cisplatin are found in brain tumors after intravenous administration [17], with activity of cisplatin noted in refractory disease [17, 18, 23]. Furthermore, one patient with GBM who had failed surgery, radiotherapy and high dose AZQ with auto BM rescue sustained a response to the 3-drug regimen in the first pilot study [1]. The 3-drug regimen was a logical choice given prior results with these drugs used separately, and in view of the possibility of re-creating in vivo the marked platinum sensitization achieved in vitro. Patients with extensive prior treatment and history of significant hematological toxicities were not included due to concerns over excessive myelosuppression from more-than-additive drug effects.
Cisplatin, when combined with hydroxyurea and cytosine arabinoside as DNA excision-repair inhibitors, did not improve the 6-month survival rate in patients with relapsed or progressive anaplastic astrocytoma or glioblastoma multiforme. Significantly more hematological toxicity was seen than expected from cisplatin alone. The use of hydroxyurea and Ara-C as DNA excision-repair inhibitors to potentiate the cytotoxicity of cisplatin was based on extensive pre-clinical in-vitro modeling. Pharmacokinetic determinations had been made in initial pilot studies to ensure that adequate serum levels of each drug could be achieved to replicate the in vitro conditions in vivo. It is worth noting that this regimen was modeled in vitro and applied directly to human clinical trials. Although drug interactions were evident as enhanced toxicity, no corresponding clinical improvement was detected, in patients with relapsed or persistent anaplastic astrocytoma or glioblastoma multiforme. The additional cytotoxicity therefore did not improve the therapeutic index of cisplatin. These tumor types have long been recognized as being very resistant to a wide range of cytotoxic agents. Studying the regimen in patients with relapsed or refractory disease, while clinically appropriate, further selected for tumor resistance. All patients would have had prior radiation, some both radiation and chemotherapy. Future uses of this approach could perhaps be envisaged for tumor types that are usually sensitive to platinum compounds to the point of complete remission, but where a proportion of patients relapse, presumably due to the persistence of a relatively drug-resistant subpopulation. Although it is possible that clinical benefit might be achieved with this drug combination when used for a more platinum-sensitive tumor type, further clinical trials with this regimen in patients with glioblastoma multiforme or anaplastic astrocytoma are not justified.
References
Albain KS, Swinnen LJ, Erickson LC, Stiff PJ, Fisher RI (1990) Cisplatin preceded by concurrent cytarabine and hydroxyurea: a pilot study based on an in vitro model. Cancer Chemother Pharmacol 27(1):33–40
Albain KS, Swinnen LJ, Erickson LC, Stiff PJ, Fisher SG, Fisher RI (1992) Cytotoxic synergy of cisplatin with concurrent hydroxyurea and cytarabine: summary of an in vitro model and initial clinical pilot experience. Semin Oncol 19(3 Suppl 9):102–109
Beck DJ, Brubaker RR (1973) Effect of cis-platinum(II)diamminodichloride on wild type and deoxyribonucleic acid repair deficient mutants of Escherichia coli. J Bacteriol 116(3):1247–1252
Dunn WC, Regan JD (1979) Inhibition of DNA excision repair in human cells by arabinofuranosyl cytosine: effect on normal and xeroderma pigmentosum cells. Mol Pharmacol 15(2):367–374
Grossman SA, O’Neill A, Grunnet M, Mehta M, Pearlman JL, Wagner H, Gilbert M, Newton HB, Hellman R, Eastern Cooperative Oncology Group (2003) Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol: Official J Am Soc Clin Oncol 21(8):1485–1491
Fram RJ, Cusick PS, Marinus MG (1986) Studies on mutagenesis and repair induced by platinum analogs. Mutat Res 173(1):13–18
Fram RJ, Kufe DW (1985) Effect of 1-beta-D-arabinofuranosyl cytosine and hydroxyurea on the repair of X-ray-induced DNA single-strand breaks in human leukemic blasts. Biochem Pharmacol 34(14):2557–2560
Fraval HN, Rawlings CJ, Roberts JJ (1978) Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from Micrococcus luteus. Mutat Res 51(1):121–132
Hiss EA, Preston RJ (1977) The effect of cytosine arabinoside on the frequency of single-strand breaks in DNA of mammalian cells following irradiation or chemical treatment. Biochimica et Biophysica Acta 478(1):1–8
Konishi H, Usui T, Sawada H, Uchino H, Kidani Y (1981) Effects of anticancer platinum compounds on Escherichia coli strains with normal and defective DNA repair capacity. Gann = Gan 72(4):627–630
Kufe DW, Weichselbaum R, Egan EM, Dahlberg W, Fram RJ (1984) Lethal effects of 1-beta-D-arabinofuranosylcytosine incorporation into deoxyribonucleic acid during ultraviolet repair. Mol Pharmacol 25(2):322–326
Plooy AC, van Dijk M, Berends F, Lohman PH (1985) Formation and repair of DNA interstrand cross-links in relation to cytotoxicity and unscheduled DNA synthesis induced in control and mutant human cells treated with cis-diamminedichloroplatinum(II). Cancer Res 45(9):4178–4184
Poirier MC (1989) DNA adduct formation in tissues of human cancer patients. Proc Am Assoc Cancer Res 30(1):660–661
Reed E, Burt-Gupta S, Katz D, Poirier MC (1989) Platinum-DNA adducts measured at autposy in multiple human tissues. Proc Am Assoc Cancer Res 30:276
Shapiro WR (1986) Therapy of adult malignant brain tumors: what have the clinical trials taught us? Semin Oncol 13(1):38–45
Snyder RD (1984) The role of deoxynucleoside triphosphate pools in the inhibition of DNA-excision repair and replication in human cells by hydroxyurea. Mutat Res 131(3–4):163–172
Stewart DJ, Leavens M, Maor M, Feun L, Luna M, Bonura J, Caprioli R, Loo TL, Benjamin RS (1982) Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res 42(6):2474–2479
Stewart DJ, O’Bryan RM, Al-Sarraf M, Costanzi JJ, Oishi N (1983) Phase II study of cisplatin in recurrent astrocytomas in adults: a Southwest Oncology Group Study. J Neuro-Oncol 1(2):145–147
Stewart DJ, Richard MT, Benoit B, Hugenholtz H, Russell N, Dennery J, Peterson E, Grahovac Z, Belanger G, Aitkens S (1984) Cisplatin plus cytosine arabinoside in adults with malignant gliomas. J Neuro-Oncol 2(1):29–34
Streifel JA, Howell SB (1981) Synergistic interaction between 1-beta-D-arabinofuranosylcytosine, thymidine and hydroxyurea against human B cells and leukemic blasts in vitro. Proc Natl Acad Sci USA 78(8):5132–5136
Swinnen LJ, Barnes DM, Fisher SG, Albain KS, Fisher RI, Erickson LC (1989) 1-Beta-D-arabinofuranosylcytosine and hydroxyurea production of cytotoxic synergy with cis-diamminedichloroplatinum(II) and modification of platinum-induced DNA interstrand cross-linking. Cancer Res 49(6):1383–1389
Walker MD, Alexander E Jr, Hunt WE, MacCarty CS, Mahaley MS Jr, Mealey J Jr, Norrell HA, Owens G, Ransohoff J, Wilson CB, Gehan EA, Strike TA (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 49(3):333–343
Walker RW, Allen JC (1988) Cisplatin in the treatment of recurrent childhood primary brain tumors. J Clin Oncol: Official J Am Soc Clin Oncol 6(1):62–66
Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, Mahaley MS Jr, Mealey J Jr, Owens G, Ransohoff J 2nd, Robertson JT, Shapiro WR, Smith KR Jr, Wilson CB, Strike TA (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Eng J Med 303(23):1323–1329
Walsh CT, Craig RW, Agarwal RP (1980) Increased activation of 1-beta-D-arabinofuranosylcytosine by hydroxyurea in L1210 cells. Cancer Res 40(9):3286–3292
Warner HR, Demple BF, Deutsch WA, Kane CM, Linn S (1980) Apurinic/apyrimidinic endonucleases in repair of pyrimidine dimers and other lesions in DNA. Proc Natl Acad Sci USA 77(8):4602–4606
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA22433, CA04919, CA20319, CA42777, CA58658, CA04920, CA45807, CA35261, CA46441, CA27057, CA46113, CA45560, CA58882, CA46282, CA58861, CA37981, CA63845, CA16385, CA52654, CA76429, CA63844, CA58416, CA12644, CA46136, CA52772, CA67575, CA58686, CA63850, CA74647, CA28862, CA46368.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swinnen, L.J., Rankin, C., Carraway, H. et al. A Phase II study of cisplatin preceded by a 12-h continuous infusion of concurrent hydroxyurea and cytosine arabinoside (Ara-C) for adult patients with malignant gliomas (Southwest Oncology Group S9149). J Neurooncol 86, 353–358 (2008). https://doi.org/10.1007/s11060-007-9483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9483-3