Abstract
Malignant gliomas are the most common and devastating primary tumors of the adult central nervous system. Dexamethasone, a synthetic glucocorticoid, is commonly co-administered to control edema in the management of brain tumors during chemotherapy and radiotherapy. In the present study, the effect of dexamethasone on proliferation and ectonucleotidase activities in rat C6 glioma cell line was investigated. Dexamethasone concentrations ranging from 0.001 to 10 μM induced a time- and concentration-dependent inhibition of C6 rat glioma cell proliferation after 24, 48 and 72-h treatment. The tetrazolium reduction assay (MTT) indicated a reduction of in cell viability (44 ± 7.6%) after 48-h treatment with 1 μM dexamethasone. Pretreatment with 10 μM of RU38486, an antagonist of glucocorticoid receptors, abolished the effect of 1 μM dexamethasone by 78 ± 9.8% after 48 h of treatment, indicating that this action is mediated via the glucocorticoid receptor. Members of the E-NTPDase family and ecto-5′-nucleotidase/CD73 can modulate extracellular ATP degradation and adenosine formation, both of which have been described as proliferation factors. Treatment of C6 glioma cells for 48 h with 1 μM dexamethasone increased in 38 ± 8.09% the AMP hydrolysis and in 3.7-fold the ecto-5′-nucleotidase/CD73 expression, suggesting an increase in adenosine formation and, therefore, a possible modulatory role in the elicitation of cell death responses. In addition, pretreatment with 5 μM GF 109203X, a protein kinase C (PKC) inhibitor, abolished the effect of dexamethasone on cell proliferation and on ecto-5′-NT activity, suggesting that dexamethasone could exert this action via PKC. The alterations in the catabolism of extracellular purines induced by dexamethasone treatment in glioma C6 cells could be related to its pharmacological effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dexamethasone is a synthetic glucocorticoid with a potent anti-inflammatory and immunosupressor action, commonly administered to control edema in the management of brain tumors during chemotherapy and radiotherapy. There is a large body of evidence to demonstrate that steroids, such as dexamethasone, inhibit drug cytotoxicity in human malignant glioma cells during chemotherapy treatment [1, 2]. For instance, dexamethasone has been described to induce partial resistance to methotrexate and cisplatinum in C6 glioma cells [3, 4] thus limiting chemotherapy efficacy. The reduction in the efficacy of various chemotherapy agents caused by dexamethasone can be, at least in part, explained by the induction of cytochrome P450 expression by this drug [5, 6]. Otherwise, dexamethasone itself has been shown to exert antiproliferative effects in human malignant glioma cells [7].
Adenine nucleotides represent an important class of extracellular molecules involved in modulation of signaling pathways that are crucial for normal functioning of the nervous system. ATP is a fast excitatory synaptic transmitter in both central and peripheral nervous system, by binding to either G protein-coupled P2Y or ligant-gated P2X receptors [8, 9]. Besides the well-established physiological effects of ATP, there is evidence showing that extracellular ATP can stimulate mitogenesis and cellular proliferation and cause cytotoxic effects on tumor cells, depending on the cell type and the ATP concentration [10].
The events induced by extracellular adenine nucleotides are controlled by the action of ectonucleotidases, which play a central role in modulating the extracellular levels of these important signaling molecules. NTPDases are enzymes that hydrolyze nucleosides tri- and diphosphates with different preferences for substrate, generating their respective nucleoside monophosphates [11]. Ecto-5′-nucleotidase (ecto-5′-NT/CD73) hydrolyses nucleoside monophosphates to the respective nucleosides and is a key enzyme in the nucleotide degradation pathway [12]. The molecular properties, functional roles and nomenclature of nucleotidases have been reviewed [11, 13].
Adenosine, the final product of ATP hydrolysis, elicits important physiological responses related to neurotransmission modulation, neuroprotection and cell survival/death [14], activating specific receptors. These effects are closely related to extracellular adenosine concentrations, cell surface expression of different adenosine receptors subtypes and signal transduction mechanisms activated following the binding of specific agonists [15].
C6 glioma, a cell line originated from N-nitrosomethylurea treated rats [16], is morphologically similar to glioblastoma multiform (GBM) when injected into rat brain [17]. Besides being used as an in vitro model to investigate several aspects of the biological function of glial cells, rat C6 glioma cell line are also widely used to evaluate the effects of novel therapies for GBM treatment [18].
We have previously demonstrated that glioma cell lines present low rates of extracellular ATP hydrolysis and high rates of extracellular AMP hydrolysis when compared to astrocytes. We hypothesized that changes in the ectonucleotidase pathway are a characteristic of this kind of tumor and possibly represent an important mechanism associated with malignant transformation of glioma cells [19, 20].
Considering the controversial therapeutical effects of dexamethasone on brain tumors and our previous results showing the possible involvement of the nucleotidases on glioma growth, in the present study, we investigated the effects of dexamethasone on C6 glioma cell line proliferation and ectonucleotidases activities. A possible mechanism involved in the dexamethasone effects was also investigated, suggesting that dexamethasone could exert this action on the ectonucleotidase cascade via protein kinase C (PKC) signaling.
Materials and methods
Chemicals
Dexamethasone, RU38486 (mifepristone), GF 109203X (3-[1-[3-(dimethylamino)propyl]1H-indol-3-yl]-4-(1H-indol-3-yl)1H-pyrrole-2,5-dione) and nucleotides were purchased from Sigma; CellTiter 96®AQueous One Solution Cell Proliferation Assay, M-MLV RT and dNTPs were purchased from Promega. Cell culture medium, penicillin/streptomycin and trypsin/EDTA solution were obtained from Gibco (Gibco BRL, Carlsbad, CA, USA); fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil); Trizol LS reagent (Life Technologies); Taq polymerase (CenBiot-UFRGS) and oligonucleotides were obtained from Invitrogen.
Maintenance of cell line and treatments
The rat C6 glioma cell line was obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were grown (passage 10–20) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% (v/v) FBS containing the antibiotics penicillin/streptomycin (0.5 U/mL). The cultures were maintained in 5% CO2/95% air at 37°C. The cells were seeded at 5 × 103 cells per well in 24-well plates, and allowed to grow to semi-confluence.
Dexamethasone and RU38486 were dissolved in ethanol/water (1:1, v/v) before use and added to the culture medium to give a final concentration of 0.001–10 μM and 10 μM, respectively. The final concentration of ethanol never exceeded 0.1% and had no significant effect in any of the experiments performed. GF 109203X was dissolved in water and used at a final concentration of 5 μM.
After semi-confluence, the medium was changed and the cells were treated with different concentrations of dexamethasone (0.001–10 μM) in DMEM/5% FBS for different times (24, 48 and 72 h). In experiments performed in the presence of the glucocorticoid receptors antagonist (RU38486, 10 μM) or PKC inhibitor (GF 109203X, 5 μM), the drugs were added to culture medium 30 min before the dexamethasone treatment for 48 h. Control cultures were performed in the absence of treatment.
Cell counting
At the end of different treatments above described, the medium was removed, cells were washed with phosphate buffered saline and 200 μL of 0.25% trypsin/EDTA solution was added to detach the cells, which were counted immediately in a hemocytometer.
MTT cell viability assay
This method provides a quantitative measurement of the number of cells with metabolically active mitochondria and is based on the mitochondrial reduction of a tetrazolium bromide salt (MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay). Cells were plated in a 96-well plate at 104 cells/well and treated with: 0.001–10 μM dexamethasone, 10 μM RU38486 or 10 μM RU38486 plus 1.0 μM dexamethasone. After 48-h treatment, 20 μL of the CellTiter 96AQueous One Solution Reagent was added to culture wells and incubated for 2 h. The absorbance was read by an ELISA plate reader at 490 nm. This absorbance was linearly proportional to the number of live cells with active mitochondria.
Ectonucleotidase assay
To determine ecto-5′-NT activity, after the different treatments described in Sect. 2.2, the 24 multiwell plates containing glioma cells were washed three times with incubation medium. The reaction was started by the addition of 200 μL of incubation medium containing 2 mM MgCl2, 120 mM NaCl, 5 mM KCl, 10 mM glucose, 20 mM Hepes, pH 7.4 and 2 mM of AMP at 37°C. After 10 min of incubation [19], the reaction was stopped by taking an aliquot of the incubation medium, which was transferred to Eppendorf tubes containing trichloroacetic acid (final concentration 5%, w/v) previously placed on ice. The inorganic phosphate (Pi) released was measured by malachite green method [21], using KH2PO4 as a Pi standard. The non-enzymatic Pi released from nucleotide into the assay medium without cells was subtracted from the total Pi released during the incubation, giving net values for enzymatic activity. To evaluate the ATP and ADP hydrolysis, the incubation medium was the same as the one used for ecto-5′-NT activity, except that 2 mM CaCl2 was used instead of MgCl2 and 1 mM ATP or 1 mM ADP as substrate. The incubation time used in this case was 30 min [19]. The other conditions were the same as used to determine the AMP hydrolysis. All samples were run in triplicate. Specific activity was expressed as nmol Pi released/min/mg of protein.
Protein determination
Cells in the 24-well microplates were solubilized with 100 μL NaOH (1.0 M) and frozen overnight. An aliquot was collected and protein was measured by the Coomassie blue method [22] using bovine serum albumin as standard. The protein determinations were carried out in all experiments.
RT-PCR analysis
Total RNA from C6 glioma cell line culture, with or without 1.0 μM dexamethasone treatment for 48 h, was isolated with Trizol LS reagent in accordance with the manufacturer’s instructions. The cDNA species were synthesized with M-MLV Reverse Transcriptase from 5 μg total RNA in a final volume of 25 μL with a random hexamer primer in accordance to the manufacturer’s instructions. cDNA reactions were performed for 1 h at 37°C and stopped by cooling at 4°C. RT (1.0 μL) reaction mix was used for PCR in a total volume of 20 μL using a concentration of 50 μM of dNTP and 1 U Taq polymerase in the supplied reaction buffer and 0.5 μM of each primer for CD73 or β-actin, as indicated below. The PCR cycling conditions were as follows: 1 min at 95°C, 1 min at 94°C, 1 min at annealing temperature (60°C for both primers), 1 min at 72°C. All PCRs were carried out for 35 cycles and included a final 10-min extension at 72°C. PCR (10 μL) reaction was analyzed on a 1.0% agarose gel. The following sets of primers were used: for ecto-5′-nucleotidase/CD73, 5′CCC GGG GGC CAC TAG CAC CTC A3′ and 5′GCC TGG ACC ACG GGA ACC TT3′ (amplification product 403 bp) and for β-actin, 5′TAT GCC AAC ACA GTG CTG TCT GG3′ and 5′TAC TCC TGC TTC CTG ATC CAC AT3′ (amplification product 210 bp). Negative controls were performed with templates substituted by DNAse, RNAse free distilled water for each PCR reaction. Ecto-5′-nucleotidase mRNA expression was determined as the ratio of the ecto-5′-nucleotidase to β-actin band density.
Statistical analysis
The data obtained are presented as mean ± SEM of at least three independent experiments. Statistical analysis was performed using one-way ANOVA followed by Tukey post-hoc. The differences were considered significant at p < 0.05.
Results
Dexamethasone inhibits cell growth of the C6 rat glioma cell line
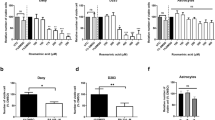
As shown in Fig. 1A, the time course experiments revealed a decrease in the number of C6 cells treated with dexamethasone in the range of 0.01–10 μM. The inhibitory effect with the lowest concentration (0.001 μM) was observed only after 72 h of treatment. These data demonstrate that dexamethasone inhibits C6 cell proliferation in a dose- and time-dependent way. In order to examine whether the effect of dexamethasone was mediated by glucocorticoid receptors, the C6 cells were pretreated for 30 min with 10 μM RU38486, an antagonist of glucocorticoid receptors, and then treated for 48 h with 1.0 μM of dexamethasone, the physiological concentration of glucocorticoids [23]. The pretreatment with RU38486 prevented the inhibitory effect of dexamethasone on cell proliferation (Fig. 1B), suggesting that dexamethasone effect is mediated via glucocorticoid receptors.
Effect of dexamethasone on cell proliferation. (A) Semi-confluent cultures of C6 cells were treated with 0.001–10 μM of dexamethasone for 24, 48 or 72 h or (B) pretreated with RU38486 (10 μM) for 30 min, and then treated with dexamethasone (1 μM) for 48 h. Cells were then detached with 0.25% trypsin/EDTA and counted in a hemocytometer. Data are the means ± SEM of three independent experiments. The control was considered as 100%. The absolute cell numbers for the controls were: (A) 359,300 ± 5,000 for 24 h; 365,000 ± 4,500 for 48 h; 380,000 ± 3,200; (B) 400,000 ± 4,850. Statistical analysis was performed by ANOVA followed by Tukey test. In (A), *p < 0.05, **p < 0.01 and ***p < 0.001 significantly different from the control groups. In (B), asignificantly different from the control group (p < 0.05); bsignificantly different from the dexamethasone group (p < 0.05)
Dexamethasone decreases cell viability in rat glioma cells
To investigate whether dexamethasone could modulate cell viability, in a second set of experiments, we performed a MTT assay, which measures the mitochondrial activity and, indirectly, cell viability, even of the spontaneously detached cells in the culture medium. Analysis of the MTT assay showed that treatment with dexamethasone for 48 h, as described in Sect. 2.4, significantly decreases cell viability at a concentration of 0.01–10 μM. This effect was reverted by the 10 μM of the antagonist, RU38486 (Fig. 2). Interestingly, RU38486 was able to increase the mitochondrial viability per se.
Cell viability dose–response curve of dexamethasone, as measured by MTT accumulation in the C6 glioma cell line. Semi-confluent cells were pretreated with RU38486 (10 μM) for 30 min, and then treated with dexamethasone (1 μM) for 48 h. Cell viability was then evaluated by MTT assay, as described in Sect. 2. Values are means ± SEM. The effect was statistically different in comparison with control at *p < 0.05, **p < 0.01 and ***p < 0.001, as determined by ANOVA followed by Tukey test
Dexamethasone stimulates ecto-5′-nucleotidase activity in C6 glioma cells
To evaluate whether dexamethasone could affect the ectonucleotidase activities in C6, initially we tested 1 μM of this drug, the physiological glucocorticoid concentration [23], on ATP, ADP and AMP hydrolysis. This treatment with dexamethasone for 48 h did not affect the ATP and ADP hydrolysis, while it caused an increase in AMP hydrolysis (data not shown). The effect of different concentrations of dexamethasone for different times on AMP hydrolysis was investigated. Figure 3A shows a dose- and time-dependent increase in ecto-5′-NT activity. To evaluate whether the glucocorticoid receptor antagonist, RU38486, could revert the ecto-5′-NT activation induced by dexamethasone, cells were pretreated with RU38486 (10 μM), before dexamethasone treatment, and the AMP hydrolysis was measured, as described in Sect. 2. As shown in Fig. 3B, the pretreatment with RU38486 before dexamethasone addition abolished significantly the glucocorticoid stimulatory effect on ecto-5′-NT. This result indicates that the increase in ecto-5′-NT/CD73 activity by dexamethasone involves the glucocorticoid receptors activation.
Dose–response curve for activation of ecto-5′-nucleotidase by dexamethasone and reversion of activation induced by RU38486. After reaching semi-confluence, (A) C6 cells were treated with increasing concentrations of dexamethasone (0.001–10 μM) for 24, 48 or 72 h or (B) pretreated with RU38486 (10 μM) for 30 min and then treated with dexamethasone (1 μM) for 48 h. Cells were then incubated with AMP for 10 min. Specific activity values are expressed as nmol Pi/min/mg protein. The values represent means ± SEM from three independent experiments. The effect was statistically different in relation to the control at *p < 0.05, **p < 0.01 and ***p < 0.001 as determined by ANOVA followed by Tukey test
Dexamethasone increases ecto-5′-nucleotidase/CD73 mRNA expression in C6 glioma cells
Since dexamethasone promoted a significant increase in AMP hydrolysis, to verify whether this increase was a result of an enhancement in ecto-5′-NT/CD73 expression, we performed RT-PCR from control and dexamethasone treated cells. mRNA from control and treated C6 cells revealed a specific signal (403 bp fragment), corresponding to mRNA for ecto-5′-NT/CD73 (Fig. 4A). In cells treated with 1.0 μM of dexamethasone, the ecto-5′-NT/CD73 mRNA expression was 3.7-fold higher than that of the control cultures (Fig. 4B). These results support the hypothesis that the increase in ecto-5′-NT/CD73 activity is related to an overexpression of the CD73 mRNA levels and probably to an increase in protein synthesis.
Ecto-5′-nucleotidase expression in C6 glioma cultures, evaluated by semi-quantitative RT-PCR. After treatment with dexamethasone (1 μM) for 48 h, total RNA was extracted and processed for analysis of ecto-5′-nucleotidase expression (A). The PCR products were separated on a 1% agarose gel and the expression was evaluated by determining the mRNA ratio of CD73 to β-actin (B)
PKC inhibitor prevents the dexamethasone effects on C6 cell proliferation and the ecto-5′-nucleotidase activation
Pretreatment with 5 μM GF 109203X, a PKC inhibitor, significantly prevented the inhibitory effect of 1.0 μM dexamethasone on cell proliferation (Fig. 5A) and was able to revert the ecto-5′-NT activation induced by dexamethasone after 48-h treatment (Fig. 5B). These results suggest that the PKC signal transduction pathway could be involved in modulating the effects of dexamethasone in C6 glioma cells.
GF 109203X prevents the inhibition of cell proliferation and the increase of ecto-5′-nucleotidase activity induced by dexamethasone. Cells were pretreated with GF 109203X (5 μM) for 30 min and then treated with dexamethasone (1 μM) for 48 h. (A) Cells were then detached with trypsin/EDTA and counted in a hemocytometer or (B) were incubated with 2 mM of AMP for 10 min. In (A), the control was considered as 100%. The absolute cell number for the control was 325,000 ± 3,400. Specific activity values were expressed as nmol Pi/min/mg protein (B). The values are represented as means ± SEM of three independent experiments. Statistical analysis was performed by ANOVA followed by Tukey test. aSignificantly different from the control group (p < 0.05); bsignificantly different from the dexamethasone group (p < 0.05)
Discussion
In the present study we demonstrate that dexamethasone induce a time- and concentration-dependent inhibition of C6 rat glioma cell proliferation, probably mediated via glucocorticoid receptors. Dexamethasone increased AMP hydrolysis and ecto-5′-nucleotidase/CD73 expression. In addition, a PKC inhibitor abolished the effects of dexamethasone on C6 cells, suggesting that this drug could exert its action via PKC.
Although dexamethasone is one of the most frequently used glucocorticoids in cancer therapy, the beneficial and deleterious effects of its use remain unclear. Some studies have suggested that dexamethasone attenuates cytotoxicity and growth inhibition of human malignant glioma cells induced by exposure to several chemotherapeutics [2]. According to Gorman et al. [24], dexamethasone can potentially reduce the tendency of a cell to undergo apoptosis induced by many chemotherapeutical agents, by increasing the mitochondrial membrane potential. On the other hand, studies indicate that this glucocorticoid is associated with tumor death induction. Recent investigations have also demonstrated that murine macrophage RAW264.7 cells, which have knocked down glucocorticoid receptors expression, present a proliferative advantage when compared to control cells, indicating that glucocorticoids are involved in antiproliferative events and that this effect depends on the presence of glucocorticoid receptors [25].
The C6 transformed rat glial cell line was used in this study as a model of glioma cells to investigate the effect of dexamethasone on cell proliferation and ectonucleotidase activities. Although the use of C6 glioma cells are limited, this model should give accurate data about the further efficacy of novel possible drugs for therapies of gliomas in subsequent studies using an in vivo rat glioma model [20].
In accordance with data from the literature, we observed that dexamethasone significantly reduced C6 glioma cell proliferation and cell viability, an effect also observed in T98G and U87-MG human malignant glioma cells [1]. Our results show that the effect of dexamethasone on glioma cell proliferation probably occurs through the activation of the glucocorticoid receptors, since the effect was prevented by a selective receptor antagonist. Interestingly, RU38486 alone increase the mitochondrial viability. This result is in accordance with previous reports demonstrating an increase in membrane mitochondrial potential, an indication that this drug could interact directly with mitochondria [26].
The main finding of the present investigation was the stimulatory effect of dexamethasone on ecto-5′-NT/CD73 activity (Fig. 3). The treatment with increased concentrations of dexamethasone resulted in a dose- and time-dependent increase of ecto-5′-NT/CD73 activity that was abolished by the RU38486 antagonist. This may indicate that the increase of ecto-5′-NT/CD73 activity was also mediated via glucocorticoid receptors. Since dexamethasone can potentially induce gene transcription, we investigated whether the increase in AMP hydrolysis was consequence of a positive modulation of ecto-5′-NT/CD73 mRNA levels. In fact, dexamethasone exposure increased ecto-5′-NT/CD73 expression by 3.7-fold (Fig. 4B). Although this enzyme has been extensively characterized, few data are available concerning a possible hormonal regulation of enzymatic activity. A possible explanation for the effect of dexamethasone on ecto-5′-NT/CD73 expression is that this glucocorticoid induces modifications in the conformation of its receptor, which migrates into the nucleus and can activate or repress transcription by binding to specific DNA sequences on target genes. Thus, glucocorticoids can influence many fundamental biological processes, from development and homeostasis to proliferation, differentiation, apoptosis and protein expression [27].
Finally, we found that the observed effects of dexamethasone on cell proliferation and on ecto-5′-NT activity may occur via PKC signaling (Fig. 5). This is in accordance with studies that have shown that glucocorticoids may modulate pathways through the activation of PKC [28, 29]. In addition, PKC signaling may activate the transcription of specific genes, including the ecto-5′-NT/CD73 [30].
The activity of ecto-5′-NT/CD73 is variable in malignant cells. Elevated activity of ecto-5′-NT/CD73 was found in breast carcinoma, gastric cancer, melanoma and glioblastoma [31]. Although it is highly expressed in many tumor cells, its specific function during tumorigenesis is unclear. This enzyme is the major protein responsible for extracellular adenosine generation. By controlling the P1 receptor agonist concentration, it may modulate distinct signaling pathways in glioma cells [14]. The increase in enzymatic activity and expression of ecto-5′-nucleotidase/CD73 showed here, may result in a higher adenosine formation in the extracellular medium. An important characteristic of adenosine is to differentially modulate normal and transformed cell growth, depending on its extracellular concentration, expression of adenosine receptors on the cell surface and the physiological state of the target cell [32]. Inosine, formed as a product of adenosine deamination, may also be released into the extracellular space in conditions of cellular stress, when metabolism of adenosine is high and can be considered a natural trigger of adenosine receptors [33]. Taken together, these results suggest that the increase in extracellular adenosine levels may justify the antiproliferative effects of dexamethasone on C6 glioma cells. However, additional investigations should be performed to confirm this hypothesis, since adenosine presents the dual characteristics of both cell protection and cell death, depending on the activation of distinct receptor subtypes, as well as specific pathophysiological conditions [14].
Although the alterations in the catabolism of extracellular purines induced by dexamethasone showed here could be related to the pharmacological effects previously described for this drug, further investigations, using other gliomas cell line and an in vivo glioma model, should be necessary to better understand the dexamethasone action during chemotherapy.
References
Weller M, Schmidt C, Roth W, Dichgans J (1997) Chemotherapy of human malignant glioma: prevention of efficacy by dexamethasone? Neurology 48:1704–1709
Naumann U, Durka S, Weller M (1998) Dexamethasone-mediated protection from drug cytotoxicity: association with p21WAF1/CIP1 protein accumulation? Oncogene 17:1567–1575
Wolff JE, Jurgens H (1994) Dexamethasone induced partial resistance to methotrexate in C6-glioma cells. Anticancer Res 14:1585–1588
Wolff JE, Denecke J, Jurgens H (1996) Dexamethasone induces partial resistance to cisplatinum in C6 glioma cells. Anticancer Res 16:805–809
Cui X, Thomas A, Han Y, Palamanda J, Montgomery D, White RE, Lorrison RA, Cheng KC (2005) Quantitative PCR assay for cytochromes P450 2B and 3A induction in rat precision-cut liver slices: correlation study with induction in vivo. J Pharmacol Toxicol Methods 52(2):234–243
Baldwin SJ, Bramhall JL, Ashby CA, Yue L, Murdock PR, Hood SR, Ayrton AD, Clarke SE (2006) Cytochrome P450 gene induction in rats ex vivo assessed by quantitative real-time reverse transcriptase-polymerase chain reaction (TaqMan). Drug Metab Dispos 34(6):1063–1069
Kaup B, Schindler I, Knupfer H, Schlenzka A, Preiss R, Knupfer MM (2001) Time-dependent inhibition of glioblastoma cell proliferation by dexamethasone. J Neurooncol 51:105–110
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Burnstock G (2006) Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 27:166–176
White N, Burnstock G (2006) P2 receptors and cancer. Trends Pharmacol Sci 27(4):211–217
Zimmermann H (2001) Ectonucleotidases: some developments and a note on nomenclature. Drug Dev Res 52:44–56
Zimmermann H (1992) 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285(Pt 2):345–365
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure functions relationships and pathophysiological significance. Purinergic Signal 2:409–430
Jacobson KA, Hoffmann C, Cattabeni F, Abbracchio MP (1999) Adenosine-induced cell death: evidence for receptor-mediated signalling. Apoptosis 4:197–211
Merighi S, Mirandola P, Varani K, Gessi S, Leung E, Baraldi PG, Tabrizi MA, Borea PA (2003) A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Ther 100:31–48
Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161:370–371
Grobben B, De Deyn PP, Slegers H (2002) Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res 310:257–270
Jungmann RA, Wang XS, Milkowski DM, Short ML (1992) Glucocorticoid induction of CRE-binding protein isoform mRNAs in rat C6 glioma cells. Nucleic Acids Res 20:825–829
Wink MR, Lenz G, Braganhol E, Tamajusuku ASK, Schwartsmann G, Sarkis JJ, Battastini AMO (2003) Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett 198:211–218
Morrone FB, Oliveira DL, Gamermann P, Stella J, Wofchuk S, Wink MR, Meurer L, Edelweiss MI, Lenz G, Battastini AM (2006) In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer 6:226
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+ -stimulated ATPase activity. Anal Biochem 157:375–380
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Barreto-Chaves ML, Aneas I, Krieger JE (2001) Glucocorticoid regulation of angiotensin-converting enzyme in primary culture of adult cardiac fibroblasts. Am J Physiol Regul Integr Comp Physiol 280:R25–R32
Gorman AM, Hirt UA, Orrenius S, Ceccatelli S (2000) Dexamethasone pre-treatment interferes with apoptotic death in glioma cells. Neuroscience 96:417–425
Zhu XY, Liu YJ, Lu J, Xu RB (2004) Knockdown of glucocorticoid receptor expression by RNA interference promotes cell proliferation in murine macrophage RAW264.7 cells. J Steroid Biochem Mol Biol 92:375–382
Makino A, Ozaki Y, Matsubara H, Sato T, Ikuta K, Nishizawa Y, Suzumori K (2005) Role of apoptosis controlled by cytochrome c released from mitochondria for luteal function in human granulosa cells. Am J Reprod Immunol 53:144–152
Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G (1995) Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621
Yamamoto S, Jiang H, Nishikawa K, Ishihara M, Wang JC, Kato R (1992) Protein kinase C-dependent and -independent actions of a potent protein kinase C inhibitor, staurosporine. Eur J Pharmacol 227:113–122
Maddali KK, Korzick DH, Turk JR, Bowles DK (2005) Isoform-specific modulation of coronary artery PKC by glucocorticoids. Vascul Pharmacol 42:153–162
Node K, Kitakaze M, Minamino T, Tada M, Inoue M, Hori M, Kamada T (1997) Activation of ecto-5′-nucleotidase by protein kinase C and its role in ischaemic tolerance in the canine heart. Br J Pharmacol 120:273–281
Sadej R, Spychala J, Skladanowski AC (2006) Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res 16:213–222
Ohana G, Bar-Yehuda S, Barer F, Fishman P (2001) Differential effect of adenosine on tumor and normal cell growth: focus on the A3 adenosine receptor. J Cell Physiol 186:19–23
Gomez G, Sitkovsky MV (2003) Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood 102:4472–4478
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Luci Bavaresco was recipient of PIBIC-CNPq fellowship. We thank Dr Maria Luisa Barreto-Chaves for experimental suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bavaresco, L., Bernardi, A., Braganhol, E. et al. Dexamethasone inhibits proliferation and stimulates ecto-5′-nucleotidase/CD73 activity in C6 rat glioma cell line. J Neurooncol 84, 1–8 (2007). https://doi.org/10.1007/s11060-007-9342-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-007-9342-2