Abstract
Tree growth is regulated by a combination of exogenous and endogenous factors. Such factors also interact with each other, complicating the understanding of causal links. IN particular, resource allocation is sensitive to reproductive investment, especially in masting species, which in turn is regulated by climatic variables. Both resource allocation and seed production patterns are also sensitive to tree age. This study aims to (1) evaluate the effects of tree age and local and regional climate on tree ring width and seed production by Spanish black pine (Pinus nigra Arn. ssp. salzmannii) forest in Cuenca Mountains (Spain), and (2) assess the relationship between seed production and secondary growth of Spanish black pine. Seed fall was estimated using 60 rectangular seed traps (40 × 50 × 15 cm) from 2000 to 2014, randomly distributed across the study area. Standardized tree-ring chronologies were calculated using a random sample of 106 trees stratified into three age classes (> 80 years; 26–80 years, and ≤ 25 years). Local climate data was obtained from a meteorological station, and regional climate data from the CRU-TS 3.1 dataset. Average seed production ranged over time from 2 to 189 seeds m−2 (coefficient of variation = 157%). We identified four masting years (2000, 2003, 2006, and 2014) using a classification based on percentile seed production. Seed production was regulated by climate of the previous 2–3 years, while tree growth responded to precipitation and temperature in the previous and current year. Independent of climate, high seed production had a negative effect on tree ring width and weakened climate growth relationships, indicating resource depletion. Tree age modulated climate sensitivity, increasing correlations between climate and tree-ring index in older trees. P. nigra has been showed to be a climate sensitive species with a bimodal masting behaviour, which should be taking into account for management purposes and silvicultural guidelines under climate change scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global climate observations and predictions for the twenty-first century show the existence of a warming trend, as well as higher frequency of extreme climatic events and longer and more severe droughts, particularly in Mediterranean areas (IPCC 2013). Decreasing precipitation, increasing temperatures and extreme drought events have the potential to reduced forest productivity, increase forest vulnerability to mortality agents, induce regeneration bottlenecks, and shift the distribution of many tree populations in Mediterranean ecosystems (Resco de Dios et al. 2007; Martínez-Vilalta et al., 2008; Allen et al. 2009; Béllard et al. 2014; Candel-Pérez et al. 2012; Vacchiano et al. 2013; Castagneri et al. 2015). Even though warmer temperatures could extend the available growing period (Wullschleger et al. 2002; Boisvenue and Running 2006), a simultaneous increase of extreme drought events may reduce the amount of time the plants are able to keep their stomata open, therefore reducing carbon uptake and shortening the time span for plant growth and development (McDowell et al. 2008).
The ability of plant species to tolerate such changing conditions is influenced by many factors such as phenotypic plasticity, genetic variability within and among populations, and interactions with site factors and disturbances. The future composition of plant communities remains difficult to predict reliably, and some authors have shown apparent contradictions (Lloret et al. 2012). For example, different studies showed an increase in abundance or cover of certain species with rising temperatures or aridity (Benavides et al. 2013), or even argued for the absence of drought sensitivity under warming conditions (Candel-Pérez et al. 2012).
Climate also regulates plant reproduction and establishment, either by a direct effect on the seed production (Ascoli et al. 2017) and on the regeneration micro-environment (Pearson and Dawson 2003; Linares and Tíscar 2010a, b), or indirectly via its effects on disturbance timing and severity (Ascoli et al. 2015). Favourable establishment syndromes may mitigate or compensate the negative effects induced by climate change on the growth and vitality of adult trees. Therefore, assessing how water stress and warming temperatures interact with local site conditions or forest structure to affect simultaneously both tree regeneration and tree and growth is essential to understand the response of many key forest ecosystems to climate change (Vaganov et al. 2006; Vacchiano and Motta 2015) and to gauge the climatic resilience of trees in the face of increasing climatic variability across different life stages at the local and regional level (Matías and Jump 2012; Candel-Pérez et al. 2012).
Flowering, fruiting, and seed rain are key controlling factors of natural recruitment of tree populations, and are tightly coupled with the year-to-year variability in the allocation of resources within the tree (Allen et al. 2010). Tree growth and ring width are also controlled by carbon supply (Fritts et al. 1991). These processes may be near-instantaneous (e.g. photosynthesis controlling carbon supply), or involve carry-over processes from previous years (e.g. remobilisation of carbon reserves). Many tree species display strong inter-annual variation in allocation to reproduction (Schauber et al. 2002), a phenomenon known as masting. The synchronized annual variability displayed by masting is explained by several theories (Kelly 1994; Herrera et al. 1998; Koenig and Knops 2000; Parker et al. 2013), although no consensus exists. Masting years (i.e., heavy seed production events across many individuals and populations) are “cued” by particular climatic conditions in the antecedent years (Vacchiano et al. 2017), and may involve a resource trade-off between growth and reproduction, i.e., a narrower ring is produced in the year of masting (Piovesan and Schirone 2000). However, there is a need to better understand the interactions between climate, growth and reproduction in masting species subject to increasing drought pressure (e.g., Koenig and Knops 2000; Redmond et al. 2012). On top of this, the effect of individual tree age on both climate-growth and climate-reproduction relationships is still poorly understood. In this article we aim to evaluate (1) if and how tree ring width and seed production of Spanish black pine are influenced by climate; (2) which type of climate (regional vs. local) has a stronger influence on masting; (3) if there is any relationship between seed production and secondary growth of Spanish black pine; (4) if tree age modulates climate sensitivity and reproduction-growth tradeoffs.
Materials and methods
Target species
Pinus nigra Arn. is the most widely distributed pine species in mountain areas of Mediterranean Basin. The subspecies salzmannii occurs in central and eastern Spain and southern France. It attains sexual maturity from 15 years of age onwards (Vidakovic 1974). Pollen is usually released from May to June; fertilization takes place 13 months after pollination (Van Haverbeke 1990), cone and seed maturation during the second year, and seed dispersal during late winter of the second year up to the spring of the third year.
European populations of black pine are known to have highly variable fecundity through time (Coutts et al. 2012). Kerr (2000) reported that P. nigra subsp. laricio has the capacity to produce seeds every year, but good seed years occur only every 3–5 years. Large fluctuations in cone and seed production are also reported for Spanish black pine (Ordóñez et al. 2006; Del Cerro et al. 2009). Early reports indicated the occurrence of large seed outputs every 3–4 years, and small crops in between (Ruiz de la Torre 1979). Often, few trees per stand are highly productive, while the remaining ones produce consistently few cones (Tíscar and Linares 2011; Coutts et al. 2012).
The Convention for the Conservation of European Wildlife and Natural Habitats (EC Resolution 4/1996) classified Spanish black pine forests as “habitats of European interest” requiring specific conservation measures, partly due to failing regeneration in stands of this long-lived species (Kerr 2000). Different problems such as irregular masting, seed mass dependence on climatic conditions, seed predation, repeated dry summers, excessive grazing, and uncontrolled ploughing have been suggested as regeneration obstacles (Del Cerro et al. 2009; Tíscar and Linares 2014). In drought-prone black pine forests, warmer temperatures and water deficit were found to reduce both radial growth (Nabuurs et al. 2013) and seed rain (Lucas-Borja et al. 2012).
Study area
This study was conducted from 2000 to 2014 in the Palancares y Agregados forest (1177–1233 m above sea level, 40°01′50″N; 1°59′10″W, Cuenca Mountains, Spain, Fig. 1). Climate in Cuenca Mountains is classified as Mediterranean humid, with a mean annual temperature of 11.9 °C (average minimum temperatures of the coldest month: − 0.5 °C; average maximum of the hottest month: 30.5 °C) and a mean annual precipitation of 595 mm (99 mm in summer) (Allué 1990). Calcareous, sandy soils dominate the study area (Table 1). The studied forest is dominated by Spanish black pine, occurring in a mosaic of even-aged stands with different mean age, due to the legacy of past management. Stands are on average quite dense (955 trees ha−1), with a mean diameter of 22 cm (Table 1) and a canopy cover between 70 and 85%. Understory vegetation includes shade-tolerant species (such as Geranium sylvaticum, Corylus avellana, Crataegus monogyna, Teucrium chamaedrys, Teucrium gnaphalodes) as well as some light-demanding species occurring in open gaps (Centaurea paniculata, Plantago media, Lotus corniculatus, Juniperus oxycedrus, Genista scorpius, Amelanchier ovalis, Acer campestre, Viburnum lantana, Rubus idaeus, Rosa spp., and Prunus spinosa).
Spanish Black pine forests in the Cuenca Mountains have traditionally been managed using the shelterwood system, with a shelter-phase of 20–25 years and a rotation period of 100–125 years (Lucas-Borja et al. 2011). This regeneration method involves a two-phase opening of the canopy and relies on natural regeneration without soil preparation. The first management plan of Palancares y Agregados forest was written in 1895. The forest was then divided into management units up to 50 ha wide, and for each management unit tactical planning considerations, i.e. where and when silvicultural treatments would be applied, were defined. Palancares y Agregados forest plans were valid for a decade and have been revised 10 times. Data from forest surveys carried out at each plan revision allow to reconstruct forest structure at the management unit level for different moments in the past.
Seed rain, ring width and climate data
Seed fall was estimated using 60 rectangular seed traps (40 × 50 × 15 cm), randomly distributed along the Palancares y Agregados forest. The number of traps is set so as to limit the relative error around the mean (er) to ± 25% assuming that the coefficient of variation (CV) of the measured variable is lower or equal to 1 (N = 2002 CV2 e −2r , after Mace 1964). The only canopy species was Spanish black pine. The minimum distance between seed traps was 200 m, allowing them to be considered as independent data sets in the analyses. The top of the traps was protected with wire netting (1 × 1 cm mesh size) to avoid seed predation. Dispersed and filled seeds were collected in years 2000–2014 on seven dates per year, beginning in early January and continuing until the final collection in late May, i.e., the season when seed fall was observed (Del Cerro et al. 2009). Yearly seed rain intensity was computed by summing seed counts from all traps. Due to the strict bimodal pattern of black pine fructification (Lucas-Borja et al. 2011), seed rain data were converted to a binary series (masting/non-masting) using the 75th percentile as a cutoff (Kelly 1994).
For the quantification of secondary growth, we sampled 106 randomly distributed trees with a diameter at breast height (dbh) larger than 7.5 cm, avoiding those with asymmetrical growth and a non-circular bole. In a circular area (radius = 15 m) centered on each sample tree, we measured percent canopy cover and the diameter and height of all trees with dbh > 7.5 cm, then calculated tree density, total basal area, quadratic mean diameter, and mean tree height. Each tree was measured for dbh and bark thickness; then, two cores per tree were extracted perpendicular to the terrain slope at breast height (Fritts 1976). Sampled trees were located close to the seed traps. Cores were sanded and visually cross-dated, and their ring-width series were counted and measured to the nearest 0.001 mm with the help of a stereomicroscope mounted above a LINTAB™ 5 RINNTECH® device linked to a computer. Cross-dating was checked using COFECHA (Holmes 1983). We averaged tree-ring widths from the same trees and detrended tree chronologies using a cubic spline with a frequency response of 0.50 at a wavelength of (0.67 × series length in years). This ensured that both age-related trends and non-climatic bias due to different competition status were removed, while preserving the climatic signal. The effectiveness of this detrending in removing competition-related bias was showed by the fact that linear regression of mean individual tree-ring index for the period 1994–2014 against basal area in larger trees as a competition index produced a non-significant regression coefficient (p = 0.933); when the same regression was run using raw tree-ring width, a significant and negative slope (p < 0.001) was obtained. Finally, we built a site chronology by averaging the series of annual tree-ring index across all trees, and three age-dependent chronologies by averaging series of only old (breast height age > 80 years), medium (26–80 years) and young (≤ 25 years) trees, respectively.
Local climate data (mean monthly temperature T and total monthly precipitation P) were obtained from a meteorological station located inside the study forest for the period 1997–2014. Regional climate data were obtained at a daily resolution for the same period from the CRU-TS 3.1 dataset (Harris et al. 2014) using the 1 × 1 km cell where the local weather station was located. Daily climate data were summarized by monthly average (temperature) or sum (precipitation). As an additional climate variable potentially associated to masting, we computed the temperature difference between two subsequent years (DT) at a monthly resolution (Kelly et al. 2013).
Data analysis
Tree-ring chronologies and time series of seed and climate (P, T and ·T from each month) were checked for temporal autocorrelation with a lag of 1 year by fitting a linear regression between each year’s value and the value of the previous year. Also, all data series were check for temporal trends by linear regression of each series against time. If a significant (p < 0.05) value was found in a series for the autocorrelation coefficient or the slope of the regression against time, the series were pre-whitened for all subsequent analyses by taking the residuals from an autoregressive model (lag 1) or from a time-dependent linear model, respectively.
Similarity between local and regional monthly climate series was assessed by Pearson’s correlation. Local and regional monthly climate variables (P, T, DT) were tested for significant differences between masting and non-masting years by a two-sample t test. The Was homoscedasticity assumption was satisfied. Due to the specific flowering ecology of black pine, climate in the year of seed production (0) and up to 3 years before (− 3) was tested. The influence of climate on secondary growth was tested by Pearson’s correlations between the mean site chronology and local and regional monthly climate variables between June of year − 1 and September of the current year, by using the function dcc() of the treeclim package (Zang and Biondi 2015) for the R statistical framework (R Core Team 2008).
Trade-offs between seed production and growth were assessed by a two-sample t-test of tree-ring index (in both year 0 and year + 1, i.e., 1 year after seed production) in masting versus non-masting years, under the hypothesis that growth would be lower in or after a masting year. Subsequently, to disentangle the influence of climate and masting on tree growth, we fitted linear regression models between tree-ring index (in both year 0 and year + 1) and selected climate variables (sum of P in April–May of year 0 from regional climate, average T in March–June of year 0 from local climate) separately for masting and non-masting years, under the competing hypotheses that climate-growth relationships in or after masting years would be either significantly stronger (due to resource limitation) or significantly weaker (due to resource depletion) than in or after non-masting years.
Finally, climate-growth correlations and masting-growth t-tests were run separately for old, medium, and young tree chronologies, to ascertain the effect of age on climate sensitivity and masting-growth trade-offs.
Results
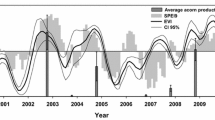
Seed production in the study area was markedly bimodal during the studied period 2000–2014, ranging from 2 to 189 seeds m−2 on average (coefficient of variation = 157%). Classification based on percentile seed production identified four masting years (2000, 2003, 2006, and 2014) (Fig. 2). The series exhibited a weak negative temporal trend and a weak negative temporal autocorrelation at lag 1, but both were non-significant (p = 0.68 and 0.46, respectively).
Cored trees exhibited some age-dependent difference in standardized growth (Fig. 3), with either old or young trees showing higher average tree-ring index in certain years within the period 1997–2014 (Table 2).
Local climate series indicated on average 14.9% more precipitation and 7.6% higher temperatures than CRU in the study period. Correlations between local and regional climate were higher for precipitation (R between 0.48 and 0.94 depending on the month), lower for temperature (0.20–0.90), and lowest for DT (− 0.35–0.46) (Fig. 4). A significant late winter—early spring cooling and drying trend in emerged between 1997 and 2014, i.e., decreasing local precipitation in February, decreasing local temperature in April, and decreasing regional temperature in February and March (all with p < 0.05).
Climate, both local and regional, significantly affected tree-ring index (Fig. 5). The effect was greater for winter-spring precipitation in year 0 (positive correlation), spring–summer temperature in year 0 (negative correlation), and fall-winter temperature in year − 1 (positive correlation). Regional climate data produced higher correlations than their local analogue for precipitation, and lower for temperature. In younger trees, growth was less sensitive to the previous summer and current spring P and T.
In the study period, climate had a limited influence on fructification. Three years before fructification, DT in June (local climate) and temperature in December (regional) had a positive effect on seed production. Two years before fructification, we could detect a negative effect of April temperature (local) and June (regional) or August (local) precipitation, and finally a positive effect of current year’s temperature (in January using local climate, and May using regional climate) (Fig. 6).
All chronologies (old, medium, young, and all trees pooled) showed a lower tree-ring index in the year of masting versus non-masting years, and a higher tree-ring index in the year following masting, although such differences were never larger than the 95% significance threshold. Young trees exhibited the smallest differences in growth between masting and non-masting years (t-statistic: + 0.43 and − 0.53 in year 0 and year + 1, respectively), old trees the highest (t-statistic: + 1.68 and − 1.24).
Finally, masting had a weakening effect on sensitivity of tree growth to climate. In fact, the slope of climate-growth regressions was always flatter in masting relative to non-masting years, both at the time of seed production and in the following year (Fig. 7), lending support to the resource depletion hypothesis.
Discussion
Masting and climate
Our findings on correlations between climate and seed production can be interpreted in the light of the specific reproductive ecology of black pine and its interactions with the proximate causes of masting, i.e., the processes and resources (water, carbon, nitrogen) involved in the 2- to 3-year long seed development. The positive influence of DT in June-3 and T in December-2, as well as the negative effect of T in April-2, can be related to resource accumulation and faster mineralization rate of needle litter by soil organisms (this involves especially nitrogen, Allen et al. 2017). Cool summers 2 years before flowering induce resource accumulation in many other species, e.g. Fagus and Picea (Vacchiano et al. 2017). The negative effect of P in June and August − 2 may be related to lower pollination efficiency in presence of rain; no temperature effects were detected in summer − 2 to justify an effect on flower induction. The absence of effects in year − 1 could have to do with the fact that fecundation (13 months after pollination) is not influenced by climate, as it happens inside the cone scales. Finally, the positive effect of current January and May T can have to do with seed release mechanisms. In the genus Pinus, seeds are often released as cones dry out (cones are xerochastic; Greene et al. 2008), and several cases have documented that dry and windy conditions, i.e., when long distance dispersal is facilitated, promote seed abscission in conifer species (Dawson et al. 1997).
In the study area, seed production between 2000 and 2014 was markedly bimodal, and tree rings were significantly narrower in mast years (two-sample t-test), which is consistent with findings by Linares and Tíscar (2010a). According to the classification by Kelly (1994), “normal” masting species are characterised by (1) a marked bimodal seed output throughout the years, and (2) the presence of switching, i.e., in years of large crops, resources are diverted from vegetative growth or reserves. As other Pinus species (Koenig and Knops 2000), black pine conforms to this description. The coefficient of variation for seed output found in this study (1.57), which is extremely similar to that previously found by Tíscar and Linares (2011) in a 4-year study, indicates the likely occurrence of resource switching (if CV > 1.6 according to Kelly 1994). Moreover, high production years were always followed by scarce or null seed production, consistent with findings in both Spain (Mackay 1926 in Sierra de Cazorla) and elsewhere (Coutts et al. 2012 in New Zealand invasive populations). This is a tell-tale sign of switching and depletion of resources during masting.
This study does not directly inquire the ultimate causes of masting in black pine. However, existing observations may support both the pollen coupling and the predator satiation hypotheses for masting (Pearse et al. 2016). The pollen coupling hypothesis states that wind-pollinated plants obtain reproductive benefits by synchronizing flowering efforts over large areas, because this increases the probability of cross-pollination (Smith et al. 1990). It has been observed that black pine produces higher percentages of empty seeds (unpollinated) in low flowering years (Tíscar, 2007). Similarly, the predator satiation hypothesis states that large seed crops are likely to satiate seed predators, which thus destroy a lower percentage of the crop (Kelly 1994). Recent studies showed that black pine seeds are highly predated by rodents and birds in low seed years, while a higher percentage survives predation in high seed years (Tíscar 2007). In a similar study, predation rates were found to be influenced by the seed crop size, as predators consumed more than 75% of seeds in years with lower production and less than 15% in a mast year (Lucas-Borja et al. 2012).
Secondary growth and climate
There is abundant literature (also in the Mediterranean forest) that relates growth response to species’ resilience to climate change and potential vitality declines (Linares et al. 2009; de Luis et al. 2013). However, the relationship is far from linear, as climate-growth responses may depend on many tree- and stand-level factors, and individuals or populations of sensitive species are also capable of showing remarkable resilience (i.e., growth release) when adverse climate stresses relax (Hacket-Pain et al. 2016).
In Spain, black pine has been found to be a drought-sensitive species (Candel-Pérez et al. 2012). In this study, winter-spring precipitation and fall-winter temperatures of the previous year showed a positive and significant correlation with secondary growth, while the correlation with spring–summer temperatures was negative. This is consistent with Martín-Benito et al. (2008, 2012), who showed that radial growth increased after a cool, wet autumn and spring, and/or mild winter, and decreased in years with a warm summer. In fact, warmer temperatures in late winter or early spring may cause early cambium activation and promote secondary growth, while summer water stress would allow less carbon to be invested in growth (Chaves et al. 2003; Martín-Benito et al. 2008).
Regional versus local climate
Understanding how climate change affects tree ecosystems is important for anticipating its impacts on terrestrial ecosystems (Shestakova et al. 2016). Our results demonstrated that correlations between local and regional climate were on average higher for precipitation, lower for temperature, and lowest for DT. However, climate-growth relationships were stronger when using regional precipitation and local temperature data. Thus, no clear trend was found, and it is difficult to evaluate which type of climate (regional vs. local) has a stronger influence on secondary growth and masting.
Masting-growth tradeoffs
Climate in year − 1 does not seem to influence masting as it does growth. However, T in year 0 has self-reinforcing effects (positive effect on seed release and output, and negative on growth). A reason for this study was also to warn dendroclimatologist about the potential effects of masting on tree ring width and the risk of confounding climatically negative years with high production years. Growth reductions indeed happen in the masting year regardless of the climate due to resource depletion effects, so this is useful to report.
Age effects
As has been showed in other black pine forests (Candel-Pérez et al. 2012), climate–growth relationships are modulated by forest age. Our results showed that tree-ring index in older and medium-aged trees presented higher correlation with climate than in younger trees for the period 1997–2014. However, trees sampled for this study are still relatively young relative to the life span of black pine, which is one of the longest-lived European tree species with individuals more than 1000 years old (Creus 1998).
Due to the study design (i.e., traps collecting seeds from trees of various ages), we could not directly test the effect of age on reproductive output. From the literature, the reproductive age of black pine starts at 15–40 years, while maximum production is reached between 100 and 120 years of age (Tíscar 2002). Therefore, it makes sense to pool the reproductive output of all three sexually mature tree age classes, even if in reality stands will regenerate after the seed cut stage of the shelterwood system (80–100 years). The relationship between fertility and tree age has been studied in the neighbouring Cazorla–Segura mountain range, where trees > 200 years old showed significantly reduced fertility (i.e., the capacity to produce sound seeds able to germinate) compared to those < 120 years old (Tíscar 2002). Further research partially with this finding, and trees up to 600 years old were observed producing abundant crops in masting years (Tíscar and Linares 2011).
While it’s unclear if seed output is dependent on tree age in the analyzed stands, resource allocation tradeoffs were sensitive to age, with older trees exhibiting the largest differences in standardized tree-ring width when masting versus non-masting years were compared, both in the year of seed production and in the following one. This can be explained by the increased size and structural complexity of older trees, which raises maintenance respiration costs and lowers the efficiency of the hydraulic pathway (Candel-Pérez et al. 2012).
The production of seeds can also be driven by factors other than age. During the mast year of 2006, higher seedfall was observed at lower elevation and in higher density stands (Lucas-Borja et al. 2012). However, the effect of tree density is contradictory, as Ordóñez et al. (2005) reported that both cone mass and the frequency of years with successful cone production decreased in very dense stands—a fact that can be linked to the lower photosynthetic surface and more limited access to resources for fruit development due to increased intra-specific competition (Arista and Talavera 1996).
Management significance
Seed production and tree growth are both important indicators of tree resilience to climate change, especially in drought-sensitive species such as black pine. However, the existing evidence suggest that seed availability should not be limiting the establishment of natural regeneration, at least in masting years. This is relevant for forest managers, who may for example synchronize regeneration cuts with masting years in order to ensure natural regeneration. Periodic fructification and subsequent dissemination, together with its extended longevity, should ensure black pine persistence. Thus, the main bottleneck in the recruitment dynamics of black pine may in fact be related to microclimatic conditions and occurrence of suitable sites for initial seedling recruitment (Tíscar and Linares 2011).
Conclusion
In the Cuenca Mountains of Spain, tree ring width and seed production were both influenced by climate. Due to resource depletion and switching, masting years produced reduced woody growth and weaken climate-growth relationships. Moreover, tree age modulated climate sensitivity, increasing correlations between climate and tree-ring index in older trees. Black pine has been showed to be sensitive to drought, and to display a bimodal masting behaviour, both of which should be taken into account for management purposes and silvicultural guidelines.
References
Allen CD (2009) Climate-induced forest dieback: an escalating global phenomenon? Unasylva 231/232(60):43–49
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Allen RB, Millard P, Richardson SJ (2017) A resource centric view of climate and mast seeding in trees. Progress in Botany. Springer, Berlin
Allué JL (1990) Atlas Fitoclimático de España. Taxonomías. MAPA. INIA. Colección Monografías INIA, no. 69, Madrid
Arista M, Talavera S (1996) Density effect on the fruit-set, seed crop viability and seedling vigour of Abies pinsapo. Ann Bot 77:187–192
Ascoli D, Vacchiano G, Maringer J, Bovio G, Conedera M (2015) The synchronicity of masting and intermediate severity fire effects favours beech recruitment. For Ecol Manag 353:126–135
Ascoli D, Vacchiano G, Turco M, Conedera M, Drobyshev I, Maringer J, Motta R, Hacket-Pain A (2017) Inter-annual and decadal changes in teleconnections drive continental-scale synchronization of tree reproduction. Nat Commun 8:2205
Béllard C, Leclerc C, Leroy B, Bakkenes M, Veloz S, Thuiller W, Courchamp F (2014) Vulnerability of biodiversity hotspots to global change. Glob Ecol Biogeogr 23:1376–1386
Benavides R, Rabasa SG, Granda E, Escudero A, Hódar JA, Martínez-Vilalta J, Rincón A, Zamora R, Valladares F (2013) Direct and indirect effects of climate on demography and early growth of Pinus sylvestris at the rear edge: changing roles of biotic and abiotic factors. PLoS ONE 8(3):e59824
Boisvenue C, Running SW (2006) Impacts of climate change on natural forest productivity—evidence since the middle of the 20th century. Glob Chang Biol 12(5):862–882
Candel-Pérez D, Linares JC, Viñegla B, Lucas-Borja ME (2012) Assessing climate-growth relationships under contrasting stands of co-occurring Iberian pines along an altitudinal gradient. For Ecol Manag 274:48–57
Castagneri D, Petit G, Carrer M (2015) Divergent climate response on hydraulic-related xylem anatomical traits of Picea abies along a 900-m altitudinal gradient. Tree Physiol 35:1378–1387
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Coutts S, Cousins C, Yvonne Buckley L (2012) Reproductive ecology of Pinus nigra in an invasive population: individual- and population-level variation in seed production and timing of seed release. Ann For Sci 69(4):467–476
Creus J (1998) A propósito de los árboles más viejos de la España peninsular: los Pinus nigra Arn. ssp. salzmanii (Dunal) Franco de Puertollano-Cabañas sierra de Cazorla, Jaén. Montes 54:68–76
Dawson TP, Curran PJ, Plummer SE (1997) The potential for understanding the biochemical signal in the forest canopies using a coupled leaf and canopy model. In: Guyot A, Phulpin T (eds) Physical measurements and signatures in remote sensing (A). Balkema, Rotterdam, pp 463–470
de Luis M, Cufar K, Di Filippo A, Novak K, Papadopoulos A, Piovesan G, Rathgeber CBK, Raventos J, Saz MA, Smith KT (2013) Plasticity in dendroclimatic response across the distribution range of Aleppo Pine (Pinus halepensis). PLoS ONE 8:1–13
Del Cerro A, Lucas-Borja ME, Martínez García E, López-Serrano FR, Andrés-Abellán M, García-Morote FA, Navarro-López R (2009) Influence of stand density and soil treatment on the Spanish black pine (Pinus nigra Arn. ssp. salzmannii) regeneration in Spain. Invest Agric Sist Rec For 18(2):167–180
Development Core Team R (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Fritts HC (1976) Tree rings and climate London. Academic Press, London
Fritts HC, Vaganov EA, Sviderskaya IV, Shashkin AV (1991) Climatic variation and treering structure in conifers: a statistical simulative model of tree-ring width, number of cells, cell wall-thickness and wood density. Clim Res 1(6):37–54
Greene DF, Quesada M, Calogeropoulos C (2008) Dispersal of seeds by the tropical sea breeze. Ecology 89:118–125
Hacket-Pain AJ, Cavin L, Friend AD, Jump AS (2016) Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur J For Res 135:897. https://doi.org/10.1007/s10342-016-0982-7
Harris I, Jones PD, Osborn TJ, Lister DH (2014) Updated high-resolution grids of monthly climatic observations—the CRU TS3.10 Dataset. Int J Clim 34:623–642
Herrera CM, Jordano P, Guitián J, Traveset A (1998) Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. Am Nat 152:576–594
Holmes RL (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Kelly D (1994) The evolutionary ecology of mast seeding. Trends Ecol Evol 9:465–470
Kelly D, Geldenhuis JA, James A, Holland EP, Plant MJ, Brockie RE (2013) Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecol Lett 16:90–98
Kerr G (2000) Natural regeneration of Corsican pine (Pinus nigra subsp. laricio) in Great Britain. Forestry 73:479–488
Koenig WD, Knops JMH (2000) Patterns of annual seed production by northern hemisphere trees: a global perspective. Am Nat 155:59–69
Linares JC, Tíscar PA (2010a) Climate change impacts and vulnerability of the southern populations of Pinus nigra ssp. salzmannii. Tree Physiol 30:795–806
Linares JC, Tíscar P (2010b) Climate change impacts and vulnerability of the southern populations of Pinus nigra subsp. salzmannii. Tree Physiol 30:795–806
Linares JC, Camarero JJ, Carreira JA (2009) Interacting effects of changes in climate and forest cover on mortality and growth of the southernmost European fir forests. Glob Ecol Biogeogr 18:485–497
Lloret F, Escudero A, Iriondo JM, Martínez-Vilalta J, Valladares F (2012) Extreme climatic events and vegetation: the role of stabilizing processes. Glob Change Biol 18:797–805
Lucas-Borja ME, Fonseca T, Parresol B, Silva-Santos P, García-Morote FA, Tíscar-Oliver PA (2011) Modelling Spanish black pine seedling emergence: establishing management strategies for endangered forest areas. For Ecol Manag 262:195–202
Lucas-Borja ME, Fonseca Fidalgo T, Linares JC, García-Morote FA, López-Serrano FR (2012) Does the recruitment pattern of Spanish black pine (Pinus nigra Arn. ssp. salzmannii) change the regeneration niche over the early life cycle of individuals? For Ecol Manag 284:93–99
Mace AE (1964) Sample size determination. Reinhold, New York
Mackay E (1926) El Pinus laricio Poir. y su aplicación a las repoblaciones forestales de la región mediterránea. I Congresso di Selvicoltura, Roma
Martín-Benito D, Cherubini P, del Rio M, Cañellas I (2008) Growth response to climate and drought in Pinus nigra Arn. trees of different crown classes. Trees 22:363–373
Martín-Benito D, Beeckman H, Cañellas I (2012) Influence of drought on tree rings and tracheid features of Pinus nigra and Pinus sylvestris in a mesic Mediterranean forest. Eur J For Res 132:33–45
Martínez-Vilalta J, López BC, Adell N, Badiella L, Ninyerola M (2008) Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Glob Change Biol 14:2868–2881
Matías L, Jump AS (2012) Interactions between growth, demography and biotic interactions in determining species range limits in a warming world: the case of Pinus sylvestris. For Ecol Manag 282:10–22
McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, Yepez EA (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739
Nabuurs GJ, Lindner M, Verkerk PJ, Gunia K, Deda P, Michalak R, Grassi G (2013) First signs of carbon sink saturation in European forest biomass. Nat Clim Change 3:792–796
Ordóñez J, Retana J, Espelta J (2005) Effects of tree size, crown damage, and tree location on post-fire survival and cone production of Pinus nigra trees. For Ecol Manag 206:109–111
Ordóñez JL, Molowny-Horas R, Retana J (2006) A model of the recruitment of Pinus nigra from unburned edges after large wildfires. Ecol Model 197(3–4):405–417
Parker WC, Noland TL, Morneault AE (2013) Comparative mast seed production in unmanaged and shelterwood white pine (Pinus strobus L.) stands in central Ontario. New For 44(4):613–628
Pearse IS, Koenig WD, Kelly D (2016) Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytol 212(3):546–562
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr 12:361–371
Piovesan G, Schirone B (2000) Winter North Atlantic oscillation effects on the tree rings of the Italian beech (Fagus sylvatica L.). Int J Biometeorol 44(3):121–127
Redmond MD, Forcella F, Barger NN (2012) Declines in pinyon pine cone production associated with regional warming. Ecosphere 3(12):1–14
Resco de Dios V, Fischer C, Colinas C (2007) Climate change effects on Mediterranean forests and preventive measures. New For 33(1):29–40
Ruiz de la Torre (1979) Árboles y arbustos de la España peninsular. Escuela Técnica Superior de Ingenieros de Montes, Sección de Publicaciones, Madrid
Schauber EM, Kelly D, Turchin P, Simon C, Lee WG, Allen RB, Payton IJ, Wilson PR, Cowan PE, Brockie RE (2002) Synchronous and asynchronous masting by 18 New Zealand plant species: the role of temperature cues and implications for climate change. Ecology 83:1214–1225
Shestakova TA, Gutiérrez E, Kirdyanov AV, Camarero JJ, Génova M, Knorre A, Voltas J (2016) Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proc Natl Acad Sci USA 113(3):662–667
Smith CC, Hamrick JL, Kramer CL (1990) The advantage of mast years for wind pollination. Am Nat 136:154–166
Tíscar PA (2002) Capacidad reproductiva de Pinus nigra subsp. salzmannii en relación con la edad de la planta madre. Invest Agrar: Sist Recur For 11:357–371
Tíscar PA (2007) Dinámica de regeneración de Pinus nigra subsp. salzmannii al sur de su área de distribución: etapas, procesos y factores implicados. Inv Agr: Sis Recur For 16:124–135
Tíscar P, Linares JC (2011) Structure and regeneration patterns of Pinus nigra subsp. salzmannii natural forests: a basic knowledge for adaptive management in a changing climate. Forests 2:1013–1030
Tíscar PA, Linares JC (2014) Large-scale regeneration patterns of Pinus nigra subsp. salzmannii: poor evidence of increasing facilitation across a drought Gradient. Forests 5:1–20
Vacchiano G, Motta R (2015) An improved species distribution model for Scots pine and downy oak under future climate change in the NW Italian Alps. Ann For Sci 72(3):321–334
Vacchiano G, Lonati M, Berretti R, Motta R (2013) Drivers of Pinus sylvestris L regeneration following small, high-severity fire in a dry, inner-alpine valley. Plant Biosyst 149:354–363
Vacchiano G, Hacket-Pain A, Turco M, Motta R, Maringer J, Conedera M, Drobyshev I, Ascoli D (2017) Spatial patterns and broad-scale weather cues of beech mast seeding in Europe. New Phytol 215(2):595–608
Vaganov EA, Hughes MK, Shashkin EA (2006) Growth dynamics of conifer tree rings: images of past and future environments. Springer, Berlin
Van Haverbeke DF (1990) Pinus nigra Arnold, European black pine. In: Burns RM, Honkala BH (eds) Silvics of North America, vol 1. Conifers. Agric. Handb. 654. U.S. Department of Agriculture, Forest Service, Washington, pp 395–404
Vidakovic M (1974) Genetics of European Black Pine (Pinus nigra Arn.). Ann For (Anali za Šumarstvo) 6:57–86
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2—implications for water-limited environments. Plant, Cell Environ 25:319–331
Zang C, Biondi F (2015) treeclim: an R package for the numerical calibration of proxy-climate relationships. Ecography 38:431–436
Acknowledgements
This work was supported by Junta de Comunidades de Castilla-La Mancha (JCCM) [POII10-0112-7316]. Consejería de Agricultura (JCCM) provided the necessary support to carry out the field work. Authors also thanks PROFOUND COST-Action (FP1304).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucas-Borja, M.E., Vacchiano, G. Interactions between climate, growth and seed production in Spanish black pine (Pinus nigra Arn. ssp. salzmannii) forests in Cuenca Mountains (Spain). New Forests 49, 399–414 (2018). https://doi.org/10.1007/s11056-018-9626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-018-9626-8