Abstract
Forest restoration is carried out with varying objectives in mind, one of which is biodiversity conservation. The present study examines the extent by which tree biodiversity could potentially be maximized in the context of the pan-African Great Green Wall for the Sahara and the Sahel Initiative (GGW). Towards this end, ten indigenous tree species were selected for study in the Ferlo region in Northern Senegal based on previous ethnobotanical studies in the zone. The species included Acacia senegal, Acacia nilotica, Acacia tortilis subsp. raddiana, Acacia seyal, Adansonia digitata, Balanites aegyptiaca, Dalbergia melanoxylon, Sclerocarya birrea, Tamarindus indica and Ziziphus mauritiana. Germination experiments were first performed in the laboratory on seed lots from Senegal, Burkina Faso, Kenya, and South Africa prior to in situ sapling production in the nursery in Northern Senegal situated along the GGW. A split plot field design was employed and the effects of seed provenance (two per species) and the addition of organic fertilizer at the timing of planting were determined. Over the course of the 2 year experimental period, the newly planted trees, in addition to the naturally regenerating woody vegetation and herbaceous grasses were monitored in the fenced-in experimental field plot. Of the ten species, only B. aegyptiaca, A. tortilis subsp. raddiana, and S. birrea exhibited moderate survival rates. The effects of provenance and fertilizer addition were sporadic and species-dependent. Natural regeneration of woody species was abundant albeit characterized by low biodiversity whereas herbaceous grass species showed extensive biodiversity, especially under tree canopies as compared to open areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2009, a groundbreaking Earth system framework emerged with “planetary boundaries” as its core concept (Rockström et al. 2009). Planetary boundaries define an acceptable upper limit or “safe planetary operating space” for each of nine critical environmental Earth System processes that would allow humankind to continue to develop and thrive for generations to come. According to the authors, the planetary boundary of biodiversity loss has already been crossed. Beyond global considerations, biodiversity conservation in the African drylands is particularly crucial as people inhabiting these regions are highly dependent on local biodiversity to meet their daily needs (Davies et al. 2012).
Trees are essential components of the landscape in terms of providing a wide array of direct and indirect benefits for people, so-called ecosystem services (ES) (MEA, Millennium Ecosystem Assessment 2005). Most emphasis is usually placed on the direct benefits, or provisioning ES (food, fuel, fodder, construction, medicine). However, trees also provide regulating (water regulation, erosion control, macro-climate and micro-climate), supporting (nutrient cycling, soil moisture and fertility, biodiversity), and cultural (spiritual, recreation) ES that indirectly benefit environmental and human-well-being. Due to the wide array of direct and indirect benefits they provide, it is now generally accepted that trees play an essential role in enhancing the overall resilience of livelihoods and economies throughout the Africa drylands (De Leeuw et al. 2014).
Since the droughts of the 1970s and 1980s, the countries in the Sahel region have undergone desertification, as defined by the UNCCD to be “land degradation in arid, semi-arid and dry sub-humid areas resulting from various factors including climatic variation and human activity” (UNCCD UNCCD 1994). If the scientific community and natural resource management decision makers do not always agree on the relative importance of human versus climatic causes, it is commonly admitted that the capacity of current Sahelian ecosystems to provide sufficient livelihoods for its inhabitants has been compromised. In what may appear to be in contradiction to the desertification paradigm is the large body of remote-sensing based data pointing to recent re-greening throughout the Sahel (Mbow et al. 2015; Brandt et al. 2015). That said, despite reports showing increases in vegetation cover since the 1990s, tree biodiversity is on the decline at an alarming rate in the Sahel (Herrmann and Tappan 2013). In this particular study from central Senegal, since 1983 a reduction in woody species richness, a loss of large trees, an increasing dominance of shrubs, and a shift towards more arid-tolerant, Sahelian species were reported. In agreement with these field observations, a study based on interviews with local populations at the regional level in Sahelian West Africa (Senegal, Burkina Faso, and Niger) indicated that 79% of the woody species were perceived as decreased in abundance or disappeared completely, most of which were of significant socioeconomic importance (Wezel and Lykke 2006).
In response to the growing social and ecological challenges in the Sahel, in 2007, eleven founding countries adopted the Great Green Wall for the Sahara and the Sahel Initiative (GGW) (Guissé et al. 2013). The GGW is just one of the many examples of the historical momentum of reforestation efforts that humankind is experiencing worldwide (Jacobs et al. 2015). Originally conceived as a planted band of vegetation over 7000 km long and 15 km wide across the African continent along the Sahel, it has now expanded to include more than 20 countries in Africa, with a strategy to harmonize interventions with other on-going, ambitious initiatives in the region (African Union and Pan African GGW Agency 2010). Moreover, the vision has shifted from primarily a reforestation project to more of a multi-sectorial, series of landscape scale interventions designed to improve both social and ecological well-being in the region. Although the GGW is no longer considered as “just a wall of trees”, tree planting is nevertheless still one of the core GGW activities. Given the geographic scope of the GGW, strategies aimed at maximizing its biodiversity could potentially have far reaching implications at the global scale. At the onset of the GGW project, each member country defined a set of indigenous tree species for reforestation based on ecological adaptability and usefulness for local populations. For Senegal, Acacia tortilis subsp. raddiana, Acacia senegal, Balanites aegyptiaca and Ziziphus mauritiana were selected (Verte 2009). As a pre-requisite to enhance tree biodiversity along the GGW path in Senegal, a previous ethnobotanical study coupled together with woody vegetation inventories was performed in the vicinity of the study zone described herein (Niang et al. 2014). As a result, ten indigenous, useful native species were identified for reforestation field trials in the present study; many of them have become infrequent (i.e. Adansonia digitata, Sclerocarya birrea, Z. mauritiana) or have even disappeared (i.e. Dalbergia melanoxylon, Tamarindus indica) from the study zone.
In this follow-up study, we fill a scientific and practical knowledge gap in relation to African reforestation initiatives, and in particular to currently ongoing GGW reforestation practices in the study zone of northern Senegal. Generally speaking for the Sahel, there are very little experimental data pertaining to the selection and field performance of woody species to reliably inform large-scale tree planting initiatives. Moreover, huge expanses of the Sahel are characterized by extensive livestock herding which implies that fencing and livestock exclusion are unavoidable features of reforestation activities in the zone. The objectives of this study are therefore as follows: (1) to explore the feasibility of reintroducing indigenous tree species through large-scale tree planting initiatives with the aim of re-greening the Sahel; (2) to identify tree species that are most well-suited for large-scale tree planting in the study zone; and (3) to determine the impact of livestock exclusion on woody and herbaceous species diversity.
By performing a fencing-in experimental field trial, this study provides a unique opportunity not only to generate scientific data for tree planting of a biodiverse panel of indigenous trees that are essential for local livelihoods, but as importantly, to address the impact of livestock exclusion on natural vegetation dynamics. While this study points to a positive effect of trees on promoting pasture grass biodiversity, it also raises questions concerning the added value of tree planting over passive natural woody species regeneration simply by creating exclosures in the zone. Finally, in keeping with this action research approach, the data generated herein are currently being integrated into large-scale GGW natural resource management strategies in the zone.
Materials and methods
Study site
The study was conducted in Northern Senegal in Widou Thiengoly, a village located in the Commune of Tessékéré, the Département of Linguère, and the Région of Louga. It is within the ZSP (Zone Sylvo-Pastorale) that lies between 14°37 and 16°50 latitude North and 12°56 and 16°26 longitude West (Sy 2003). Widou Thiengoly was selected as the study site because of the ongoing and active GGW reforestation activities in the surrounding areas.
The climate is semi-arid with alternating dry (October to June) and rainy (July to September) seasons (Aubreville 1949). Annual rainfall ranges between 200 and 400 mm and temperatures oscillate with annual averages of 25–30 °C (Le Houérou 1989). The soil is slightly leached ferruginous, tending toward leached (Leprun 1971). Topographic relief is minimal with tropical sub-arid reddish-brown soil made up of sandy material which is poor in clay (Diallo et al. 2013). Soil organic matter is low, ranging from 0.11 to 0.7% for plateaus and 0.53–1.45% for depresssions (Ndiaye et al. 2013). In general, the region is a savannah consisting of open, extensive grasslands and sparsely scattered shrubs and trees. More precisely, Widou Thiengoly and its surroundings is a thorny savannah where B. aegyptiaca, A. tortilis subsp. raddiana and A. senegal dominate the landscape (Niang et al. 2014). The herbaceous stratum is primarily made up of annual grasses including Alysicarpus ovalifolius, Indigofera colutea, Indigofera hirsuta, Brachiaria ramosa, Chloris prieurii, and Cenchrus biflorus (Akpo and Grouzis 1996). Finally, year-round groundwater supply is insured by a network of deep boreholes dug in the 1950s. During the rainy season, rain water-fed, temporary ponds also provide water for herd and human consumption.
Choice of species and seed provenances
The woody species were chosen based on two criteria; they were i) indigenous and ii) highly valued by local populations. The ten candidate species were: Acacia nilotica, A. senegal, Acacia seyal, A. tortilis subsp. raddiana, A. digitata, B. aegyptiaca, D. melanoxylon, S. birrea, T. indica and Z. mauritiana. If some of these species are currently undergoing large-scale GGW plantation (i.e. B. aegyptiaca and A. tortilis subsp. raddiana), others have become rare in the study area and the feasibility of their reintroduction warrants attention (Niang et al. 2014).
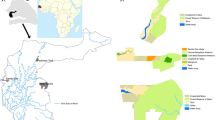
Originally, several provenances per species were obtained for laboratory germination tests. However, many of the seed lots did not germinate or exhibited poor germination rates (data not shown). From here on, only seed lots corresponding to the two provenances/species selected for subsequent field trials will be described (Fig. 1). Seed lots for all ten species were obtained from forest seed resource centres from different countries in Africa: in Senegal from PRONASEF (National Project of Forest Seeds of Senegal) and the ANGMV (the Senegalese National GGW Agency), in Burkina Faso (National Centre of Forest Seeds) and in Kenya (Kenya Forestry Research Institute). Seeds were also obtained from a private supplier in South Africa (Silver Hill Seeds, Cape Town, South Africa). All seed lots were stored at room temperature prior to germination testing.
Localities of seed provenances for the ten indigenous tree species tested in field trials. Seeds came from Senegal, Burkina Faso, Kenya, and South Africa. For each species, there were two provenances (P1 and P2, indicated as 1 and 2 respectively on the figure) and will be referred to as such throughout the article. Localities for A. seyal (Burkina Faso—P1), D. melanoxylon (Burkina Faso—P1) and S. birrea (South Africa—P2) were not provided by the respective seed suppliers. (Color figure online)
Germination tests
A randomized complete block design was used for germination tests. For each species, 100 seeds were divided into four replicates of 25 seeds each. Germination protocols were defined using a practical agroforestry manual (Roussel 1995) and recommendations by the respective seed suppliers. Seed pre-treatments varied according to species and were as follows: A. nilotica, A. senegal, A. seyal, and A. tortilis subsp. raddiana were soaked in room temperature water for 24 h, A. digitata and T. indica were soaked in hot water for 24 h, and for B. aegyptiaca, D. melanoxylon, S. birrea and Z. mauritiana, seed coats were peeled or fractured prior to soaking in room temperature water for 24–48 h. After pre-treatment, the seeds were placed in individual plastic compartments filled with damp hydrophilic cotton. They were then put in a heated chamber at 30 °C in the dark. Germination, defined as radicle breakthrough, was monitored over a two-week period. The cotton was moistened as needed over the study period.
Nursery establishment in Widou Thiengoly
Seeds were planted from May 8–15, 2013 in the communal village nursery of Widou Thiengoly. Plastic sheaths were filled with local soil pre-mixed with animal manure and arranged in rows. In order to be sure to have 120 healthy trees/species (60 trees/provenance), a total of 2000 sheaths were sown for the ten species. The seeds were pre-treated according to the established laboratory protocols described above and then planted directly in the sheaths. For the 3 month nursery period, the saplings were watered twice a day with reduced watering frequency in the last 2 weeks prior to planting.
Experimental field trial set-up
An experimental field plot was established along the GGW, situated 4 km southeast of Widou Thiengoly (Fig. 2). The geographical coordinates of the four corners were as follows: 15°58.549′N/15°17.221′W, 15°58.402′N/015°17.253′W, 15°58.366′N/15°17.149′W and 15°58.507′N/15°17.121′W. It covered an area of 5.2 ha in which the soil and climate conditions are relatively homogenous (flat terrain with sandy substrata). After the first rainfall, furrows measuring 5 m apart were ploughed by a tractor length and width-wise in a chessboard configuration. This was done to maximize rain water infiltration. The existing woody vegetation as well as the herbaceous grass cover outside of the furrows were conserved. Fencing 1.5 m high was erected around the plot to keep livestock from entering. The locations of rectangular sub-plots; each corresponding to one of the ten species, are indicated in Fig. 3a. Iron poles and panels were used to delineate the sub-plot corners and identify the location of each species. For each species sub-plot, 120 holes located at the intersection of furrows (corresponding to the 120 trees of each species to be planted) were dug immediately before planting. In order to monitor daily rainfall in 2013–2015, a rain gauge was installed in the experimental field plot and readings were recorded manually after each rainfall event.
Geographical location of the experimental field plot along the GGW path in Northern Senegal. Above: at the national scale; below: a close up of the boxed-in area from above. The 5.2 ha plot (red circle) is situated 4 km southeast of the village of Widou Thiengoly in a zone of active, ongoing GGW reforestation (GGW plots indicated by dark green polygons below). The experimental field plot is enlarged for illustration purposes. (Color figure online)
General schema of the experimental field plot. a The locations of the 10 different tree species subplots. b The experimental design. S. birrea from a is given as an example. 120 trees in total per species were planted, corresponding to 30 trees/provenance/treatment. Each boxed-in group of ten trees represents a replicate
Planting trees in the experimental field plot
Nursery-grown trees were planted in the plot during the rainy season from August 23–26, 2013. All trees were planted manually in the pre-dug holes located at the intersection of the furrows described in the previous section. A split-plot design was used for each species (Fig. 3b). The first variable was the seed provenance (P1 and P2), and the second the addition (or not) of organic fertilizer (Tref BIO, Jiffy International AS, Holland) at the time of planting. The fertilizer was comprised of 60% black peat and 40% blond peat. Organic fertilizer treatment was chosen as the second variable as it has a high water-holding capacity (670 ml/l) and provides a source of organic matter for poor soils. To apply the organic fertilizer, 1/3 fertilizer and 2/3 soil were first mixed together. Two spades (roughly equivalent to 3 litres of the fertilizer/soil mix) were added to each hole at the time of planting. For each species, a total of 120 trees per species were planted. This corresponded to 30 trees per provenance and per treatment with three replicates of 10 trees each (Fig. 3b). Finally, trees were not watered in accordance with GGW reforestation protocols.
Vegetation monitoring in the experimental field plot
The trees were monitored in the experimental field plot from 2013 to 2015. Based on visible criteria, they were individually scored as alive or dead at 2, 12 and 24 months after planting. A tree was considered alive when the main stem was still flexible and moist. Although the presence of green leaves was an indicator of a living tree, it was not considered as an absolute criteria as some species lose their leaves during the dry season. To circumvent this ambiguity, trees were scored during the rainy season (for 12 and 24 month readings) as the onset of foliation in response to rainfall was already initiated for all of the species.
Tree height was measured once a month during the 3 months of the nursery phase, and beginning in October 2013, twice a year after planting from 2013 to 2015: once during the rainy season (every August) and once after the rainy season (every October). When measuring tree height, only the flexible, moist part of the stem of living trees was taken into account.
To calculate natural woody species regeneration, individuals with a basal trunk diameter of < 3.5 cm (< 10 cm in circumference) were exhaustively inventoried in the entire 5.2 ha experimental field plot in 2013 and 2015 (Akpo and Grouzis 1996). In addition to determining the total number of individual stems, the relative importance of each species (number of young trees of a given species/total number of young trees) and the natural regeneration capacity (the total number of young trees/total number of young trees + adult trees) were calculated for 2013 and 2015 (Poupon 1980; Akpo and Grouzis 1996).
For the herbaceous stratum, a total of 50 randomly distributed inventories throughout the experimental field plot was performed. They covered the two contrasting biotopes: (1) under adult tree canopy cover and (2) open field, i.e. at a distance from any adult trees. The inventories were carried out in the same locations in October 2013, 2014 and 2015. For both under tree canopy and open field inventories, a circle with a 4 m radius, equivalent to a surface area of approximately 50 m2 was used to delimit the sample zone. This is considered the minimal area to inventory herbaceous vegetation in the Sahel (Akpo and Grouzis 1996). For each under tree canopy inventory, the adult tree was located at the centre of the circle. It is important to note that inventories were not performed under newly planted trees. For each inventory, species presence was recorded in the most exhaustive manner possible. Species samples were collected and identified using “Énumération des Plantes à Fleurs d’Afrique Tropicale” (Lebrun and Stork 1991–2015).
Statistical analysis of data
The data was statistically analyzed using SigmaPlot 11 software. After validation of the normality of distribution using the Shapiro–Wilk test, the T test comparing two Student–Newman–Keuls averages was used to evaluate the effect of seed provenance on germination rates, and tree survival rates at 2, 12 and 24 months after planting, on the one hand between provenances for a given species, and in the absence/presence of organic fertilizer on the other hand. In all cases, the differences were considered significant when the probability for the T-test was less than 0.05 (P ≤ 5%).
Results
Germination rates
The maximal germination rates are shown for all ten species in Fig. 4. Based on germinative capacity, three groups could be distinguished. The first group, with germination rates > 70%, included A. senegal, D. melanoxylon and T. indica for both seed lots and the Bambey, Senegal provenance of B. aegyptiaca (P1). The second group, with germination rates ranging from 40 ≥ 70%, was made up of both seed lots of A. nilotica, A. seyal, and Z. mauritiana, the Senegal provenance of A. digitata (P1) and the Tessékéré, Senegal provenance of B. aegyptiaca (P2). The third group had the lowest germination rates (< 40%) and was comprised of both seed lots of A. tortilis subsp. raddiana, S. birrea, and the Kenyan provenance of A. digitata (P2).
Maximal germination rates (%) of the ten selected tree species. P1 = Provenance 1; P2 = Provenance 2. The countries and localities of seed lots are indicated in Fig. 1. Asterisks indicate statistical significance as follows: *P ≤ 0.05, **P ≤ 0.01
Only three species showed significant differences in germination rates between provenances. For A. digitata, the Senegal provenance (P1 = 51 ± 13.6%) had a higher germination rate than the Kenya provenance (P2 = 13 ± 6.8%). The Burkina Faso provenance of A. tortilis subsp. raddiana (P1 = 34 ± 10.5%) exhibited a higher germination rate than that of Senegal (P2 = 12 ± 3.2%) and finally, seeds of B. aegyptiaca (P1 = 71 ± 8.8%) from Bambey, Senegal provenance germinated better than that of Tessékéré, Senegal (P2 = 47 ± 12.8%). These germination data obtained under laboratory conditions were transposed to the subsequent nursery phase. Finally, it is important to note that a scarification pre-treatment of Acacia spp. seeds led to an overall increase in laboratory germination rates (date not shown); however it was considered technically ill-adapted to the communal nursery setting, especially since seed availability was not limiting for these species.
Daily rainfall measurements
Due to its critical nature, daily rainfall was registered during the entire experimental period (Supplementary Fig. 1). Annual rainfall was low and exhibited substantial inter-annual variation: 249 mm in 2013, 108 mm in 2014 and 294 mm in 2015. These values were below the annual 302 mm average recorded for the study area between 1961 and 2010 (Miehe et al. 2010).
Survival rates of planted trees
For each species, survival rates at 2, 12 and 24 months after planting were determined (Fig. 5). For A. nilotica (Fig. 5a), average survival rates at 2 months were between 56.7 and 90%. Although the Burkina Faso provenance presented a survival rate that tended to be higher than the Senegal provenance in either the absence or presence of organic fertilizer, and fertilizer treatment tended to reduce survival rates within each provenance, no statistically significant differences were observed between provenances or fertilizer treatment 2 months after planting. At 12 months, after the first dry season, survival rates were generally very low for both provenances, with or without fertilizer. For the Senegal provenance, the control (without fertilizer) presented the best survival rate (6.7%) while that of Burkina Faso the lowest (0%). At 24 months, only 3.3% of individuals of Senegal provenance without fertilizer and 3.3% of Burkina Faso provenance with fertilizer were still alive.
Survival rates at 2, 12, and 24 months after planting. SN = Senegal, BF = Burkina Faso, KN = Kenya, SA = South Africa. 1 and 2 indicate the provenance numbers in accordance with Fig. 1. − and + indicate the absence or presence of added organic fertilizer at the time of planting respectively
The 2-month survival rates of A. seyal (Fig. 5b) were between 53.3 and 66.7% without significant differences between provenances and/or fertilizer treatment. At 12 months after planting, only the Burkina Faso provenance control trees were still alive, albeit with a low survival rate of 6.7%. At 24 months, all of the A. seyal trees had died.
The survival rates of A. senegal at 2 months after planting were between 46.7 and 86.7% (Fig. 5c). For both provenances, the trees treated with fertilizer had higher survival rates. For the Senegal provenance (86.7 vs. 46.7%), the difference tended towards significant (P = 0.055). The survival rate at 12 months greatly decreased to 3.3% for the Senegal provenance with or without fertilizer. For the Burkina Faso provenance, the control and the fertilizer-treated trees had survival rates of 6.7 and 0% respectively. Finally, at 24 months, only the controls of both provenances survived at a rate of 3.3%, suggesting that the positive effect of the fertilizer treatment observed in the first 2 months did not have any long-term effect.
For A. tortilis subsp. raddiana (Fig. 5d), the survival rate at 2 months for both provenances and both treatments were quite similar (averages ranged between 63.3 and 73.3%). Compared to the other Acacia species tested, Acacia tortilis var. raddiana exhibited lower mortality rates after the first dry season with survival rates at 12 months after planting between 13.3 and 33.3%. If survival rates tended to be greater among control trees for both provenances compared to fertilizer treatment, the differences were, however, not significant. At 24 months, survival rates were 16.7% for the Senegal provenance with or without fertilizer while for the Burkina Faso provenance, average survival rates were 23.3 and 6.7% respectively for the control and fertilizer treatment respectively. Among all of the Acacia species tested herein, A. tortilis subsp. raddiana was the most drought-resistant, exhibiting the best field performance over the 24-month period.
For B. aegyptiaca (Fig. 5e), average survival rates at 2 months were very high (between 90 and 100%). At 12 months after planting, these rates fell sharply to 33.3–53.3%. No significant differences between provenances or fertilizer treatment were observed. At 24 months, survival rates ranged from 6.7 to 36.7%. The addition of fertilizer tended towards a positive effect for the Senegal/Bambey provenance (P1) (36.7 vs. 6.7% in controls) although it was not statistically significant (P-value of 0.07).
The survival rates at 2 months were high for S. birrea (Fig. 5f), ranging from 86.7 to 100%. At 12 months, averages rates decreased to 6.7–33.3%. For the South Africa provenance, fertilizer treatment (33.3%) presented a significantly higher survival rate than the control (6.7%) (P-value = 0.047). At 24 months, the control trees of both provenances died while the survival rate with fertilizer was 13.3 and 33.3% respectively for the Burkina Faso and South Africa provenances. Interestingly, the survival rates for both provenances in the presence of fertilizer were stable between 12 and 24 months.
Ziziphus mauritiana (Fig. 5g) presented survival rates at 2 months between 30 and 83.3%. In the absence of fertilizer, there were no significant differences between the two provenances; however, in the presence of fertilizer the Burkina Faso provenance survival rates were higher than that of Senegal with 63.3 versus 30% respectively (P = 0.007). Moreover, the presence of fertilizer had a significant negative effect on the 2-month survival rates of both provenances. At 12 months, the negative effect of fertilizer treatment continued as all the fertilizer-treated saplings of both provenances were dead whereas 3.3% of control trees for both provenances were still alive. At 24 months, the survival rates for control trees were identical to those observed at 12 months.
Adansonia digitata (Fig. 5h) exhibited 2-month survival rates that averaged between 46 and 60%. Statistical analysis showed no significant differences between provenances or treatments. At 12 months, only saplings of the Kenya provenance survived with a rate of 10% among control trees, and 6.7% for those receiving fertilizer treatment. At 24 months after planting, 6.7% of control trees of the Kenya provenance were still alive.
At 2 months after planting, the survival rates of D. melanoxylon were between 20 and 60% (Fig. 5i). However, statistical analysis did not show significant differences either between provenances or fertilizer treatments. That said, for the Burkina Faso provenance, the addition of fertilizer tended to reduce the survival rate (P = 0.091) and the Kenya (53.3%) provenance tended to perform better that the Burkina Faso provenance (20%) in the presence of fertilizer (P = 0.083). At 12 months after planting, all the individuals of both provenances had died.
Survival rate averages of T. indica were generally the lowest of all of the species tested (Fig. 5j) and ranged from 16 to 46% at 2 months after planting. Statistical analysis did not show any significant differences either between provenances or treatments. As was the case for D. melanoxylon; after 12 months, all the individuals of both provenances had died.
Growth of planted trees
As expected, for all the species, tree height steadily increased in the nursery phase during which trees were watered daily (Fig. 6). Immediately following planting, tree height was generally lower (month 5—October 2013 compared to month 3—August 2013). This is explained by the fact that trees were measured prior to planting in August 2013 and planted deeper than collar level in the field. Thereafter, the trees followed somewhat similar growth patterns, albeit with some degree of species specificity. Generally speaking, during the dry season the height of the young trees either decreased (due to dieback) or remained relatively unchanged. Tree growth was generally restricted to the rainy seasons. However, for B. aegyptiaca, A. tortilis subsp. raddiana and S. birrea, tree height was relatively stable, especially during the first dry season, followed by less pronounced seasonal fluctuations. Finally, in October 2015 (month 29), none of the trees were taller than 60 cm, indicating little net aerial growth beyond what was recorded in October 2013 (month 5) when the first in situ measurements were taken.
Tree height for the ten species throughout the field experiment. Trees were measured once a month during the nursery phase, and after planting, every October (after the rainy season) and August (during the rainy season) in 2013, 2014, and 2015. The post-planting heights indicated are the averages of all living trees (both provenances and treatments combined) for each time point. N = Nursery phase, DS = Dry season, RS = Rainy season
Natural woody vegetation in the experimental field plot
In 2013, a total of 136 adult trees was recorded and localized in the plot (Fig. 7a). The density was 26.2 trees/hectare with a slightly unequal spatial distribution. Six species were inventoried. B. aegyptiaca greatly dominated the adult population with 108 individuals or a density of 20.7 trees/hectare, followed by S. birrea and Boscia senegalensis (8 individuals or 1.5 trees/hectare), Calotropis procera (6 individuals or 1.2 trees/hectare), A. tortilis subsp. raddiana (5 individuals or 0.9 trees/hectare) and A. senegal (1 individual or 0.2 trees/hectare).
a Adult trees inventoried in the experimental field plot in August 2013. Colored circles indicate the location of the different species. The names of the planted species subplots are also indicated. b, c Natural regeneration of woody species recorded in 2013 and 2015 respectively. The relative proportions (%) of the different woody species in the plot are indicated. (Color figure online)
The natural regeneration of woody species (young trees with a basal stem diameter < 3.5 cm) was inventoried in 2013 and 2015 (Fig. 7b, c respectively and Supplementary Table 1). In 2013, 1095 individual stems were recorded in the 5.2 ha plot, corresponding to 210.5 individual stems/ha. The regeneration rate (number of young trees/number of young + adult trees) was 89%. Nine species were recorded (Fig. 7b). The species in descending order of relative importance were as follows: B. aegyptiaca, C. procera and S. birrea, which accounted for 80.5, 9.4 and 4.5% respectively of the population. These were followed by Leptadenia hastata (3%), A. tortilis subsp. raddiana (1.7%), B. senegalensis (0.5%), Leptadenia pyrotechnica (0.2%), A. nilotica (0.1%) and A. senegal (0.1%). In 2015, the number of young trees was greater than in 2013 with a total of 1672 (321.5 individual stems/ha). This represented a regeneration rate of 92%. Eight species were recorded. B. aegyptiaca still dominated the regenerating population, accounting for 76% of the total number of young trees. B. senegalensis (10.1%) and L. hastata (9.9%) increased in relative proportion compared to 2013. In contrast, S. birrea, C. procera, A. tortilis subsp. raddiana, and A. senegal declined with a relative contribution to the total population of 1.2, 1.7, 0.7 and 0.06% respectively. Grewia bicolor (0.2%) was the only species newly encountered in 2015; in contrast, A. nilotica and L. pyrotechnica were no longer present in 2015.
Herbaceous flora in the experimental field plot
A total of 56 herbaceous species divided into 22 families and 40 genera were identified in the experimental plot from the three inventories carried out in 2013, 2014, and 2015 (Supplementary Table 2). The Poaceae family was mostly highly represented with 15 species (26.7%), followed by Fabaceae with 9 species (16.1%), Malvaceae with 4 species (7.1%), Amaranthaceae, Convolvulaceae and Cucurbitaceae, each with 3 species (5.3%), Cyperaceae, Phyllanthaceae and Rubiaceae presented 2 species each (3.5%). The remaining 13 families were less represented with only 1 species each (1.7%). The most represented genus was Indigofera (4 species/10%). It was followed by Ipomoea, Aristida, Eragrostis and Corchorus (3 species/7.5%); then Achyrantes, Momordica, Cyperus, Senna, and Phyllanthus (2 species/5%) and finally, all of the others genera with only 1 species each (2.5%).
Specific richness evolved over time. A total of 42 species was recorded in 2013; 38 in 2014; and 45 in 2015 for both biotopes taken together. Some species were identified every year (i.e. Ipomoea coptica, Cucumis melo var. agrestis, A. ovalifolius, I. colutea, I. hirsuta, Enteropogon prieurii) whilst others were found specifically in 2013 (Pancratium trianthum, Ipomoea triloba, Indigofera diphylla, Corchorus olitarius), 2014 (Tephrosia purpurea) or 2015 (Amaranthus hybridus, Cleome viscosa, Commelina forsskaolii, Corchorus aestuans, Hibiscus sidiformis, Aristida adscensionis, Chloris barbata, Pennisetum violaceum, and Tripogon minimus).
In terms of biotope distribution, three categories of species were distinguished: sciaphilous species found uniquely under tree canopy cover (i.e. Peristrophe bicalyculata, Achyrantes aspera, Achyranthes argentea, Spermacoce ruelliae), heliophilous species found uniquely in the open field biotope (i.e. Heliotropium bacciferum, C. forsskaolii, I. diphylla, and P. violaceum) and species common to both biotopes (i.e. C. melo var. agrestis, A. ovalifolius, I. hirsuta, I. colutea, E. prieurii, Dactylocteniuman aegyptium, C. biflorus, Aristida mutabilis, Phyllanthus niruri, Gisekia pharnaceoides, and Zornia glochidiata). Interestingly, the number of species specifically found under canopy cover was systematically higher compared to in the open field: 10 versus 8 species in 2013, 8 versus 2 species in 2014, and 12 versus 1 species in 2015.
Discussion
The research presented herein is perfectly aligned with The Sustainable Development Goal 15 of the 2030 Agenda for Sustainable Development which is devoted to “protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss” (ICSU, ISSC 2015). The question remains as to the best ways to achieve these goals in the Sahel, one of the most vulnerable regions on Earth. Since the major droughts of the 1970s and 1980s, the Sahel has received enormous international attention from the development community but seldom are these efforts demonstrated to have long-lasting effects. Natural resource decision makers and scientists are becoming increasingly convinced that there is no one-size-fits-all solution to restore or transform the diverse Sahel ecosystems. Success will likely come from contiguous, smaller, landscape-scale interventions that individually take into account both the social and ecological dimensions of these complex social-ecological systems and the application of resilience principles specifically adapted to these vulnerable landscapes (Barrett and Constas 2014; Davies et al. 2015). The GGW is a window of opportunity to test this.
In any reforestation effort, it is important to determine the main objective(s): biodiversity conservation, biomass production, climate adaptation and/or mitigation (Ciccarese et al. 2012). In the context of the GGW, restoration of biodiversity is clearly a priority as local populations are highly dependent on biodiversity for their subsistence needs (De Leeuw et al. 2014). Although the link between biodiversity conservation and poverty reduction is still unclear, biodiversity conservation can contribute to poverty alleviation, at least, under some circumstances (Roe 2010).
In Sahelian landscapes, trees constitute a major source of ES. This is the case for trees growing in all portions of the landscape whether it be in agricultural fields, forests, shrublands, fallow, depressions, or homesteads (Sinare et al. 2016). That said, the feasibility and added value of tree planting in the Senegalese Sahel is a topic that is still open to debate. In the present study, we examined the reintroduction of a biodiverse panel of ten indigenous, useful woody species in a two-year field trial located in the Ferlo in northern Senegal, a region located along the GGW path and characterized by an extremely low degree of natural woody species diversity (Niang et al. 2014). In this previous study, twenty woody species had been identified, only ten of which were found in the immediate vicinity of Widou Thiengoly. Interestingly the majority of the species tested herein had already been identified as endangered species at the West African regional scale (Wezel and Lykke 2006). Herein, B. aegyptiaca exhibited the best overall field performance in terms of survival rates over the two-year experimental period, followed by A. tortilis subsp. raddiana, and Scerlocarya birrea. The extraordinary adaptive capacity of B. aegyptiaca in arid and semi-arid environments has been attributed to a wide array of morphological and physiological features including small, thick leaves with a waxy cuticle (Radwan 2007). It also has an efficient root system comprised of a deep tap root and an extensive lateral root system capable of efficiently capturing surface rains (Breman and Kessler 1995). Despite the fact that B. aegyptiaca demonstrated the best survival rate, little aerial growth of surviving trees over the two-year period was recorded. On the contrary, the root system grew extensively within the first months after planting, thereby favoring water update in young trees. Indeed, high root/shoot ratios (> 1 g/g) at the time of planting and root collar diameter have been reported as potential markers of field performance for two valuable Sudanian savanna species, Acacia macrostachya and Pterocarpus erinaceus, in field trials in Burkina Faso (Zida et al. 2008). The relative success of B. aegyptiaca, A. tortilis subsp. raddiana, and S. birrea in the field trials reported herein is not surprising as they dominate current landscapes in the zone (Ndiaye et al. 2013; Niang et al. 2014). These findings are also in keeping with their low annual precipitation requirements with B. aegyptiaca growing in areas with ≥ 250 mm/year, A. tortilis subsp. raddiana ≥ 100 mm/year, and S. birrea ≥ 200 mm/year (Orwa et al. 2009). Average annual precipitation in our study zone is 302 mm/year with 249, 108, and 294 mm registered in 2013, 2014, and 2015 respectively in the experimental field plot. Although overall survival rates were low herein, the negligible scientific data available for reforestation in the Sahel pointed to equally low rates (i.e. 20% in Niger in the 1970’s). This has understandably resulted in skepticism from the scientific community towards tree planting in the Sahel (Sendzimir et al. 2011). High mortality was principally due to low rainfall, but additional factors including planting shock and termites also had deleterious effects on tree fitness.
For some species, a small percentage of individuals were still living 2 years after planting. These species were A. digitata, A. senegal, A. nilotica and Z. mauritiana. Whether any of these species should be conceivably included in large scale reforestation along the GGW should be subjected to in-depth socio-economic and environmental cost–benefit analyses. For the emblematic A. digitata (baobab), a few very large adult individuals still exist in the study zone with unfortunately no observable regeneration. In a study carried out in South Africa the failure of baobab recruitment was not due to viable seed production, but more to the lack of consistent intra-annual rainfall. These authors underlined the need for active planting, protection, and monitoring of saplings to conserve this socio-economically vital species (Venter and Witkowski 2013). Finally, all of the D. melanoxylon and T. indica saplings were dead within the first 12 months after planting. Woody species biodiversity in the zone has become severely impoverished and these species are among those that are no longer present in the zone (Barral et al. 1983; Brandt et al. 2015). These results suggest little hope for their large-scale reintroduction in the zone.
As for the effects of seed provenance on species performance, no consistent effect over the full two-year period for a given species was observed. However, there were some statistically significant, sporadic differences in survival rates between provenances for some species. Although still a matter of debate, it might be reasonable to assume that local provenances would outperform more distant ones as they would theoretically be more well-adapted to local conditions (Sackville Hamilton 2011; Boshier et al. 2015). That said, climatic factors and the eco-geographic region of the seed provenances themselves are also likely to be as critical in affecting tree performance. For logistical reasons, we were only able to test two provenances per species, thereby making it impossible to conclude as to the importance of provenance on tree survival rates. However, one interest species worth noting is A. digitata. Of the 120 trees planted, there were only two trees living after 2 years, both of which were from Kenya. Finally, the addition of organic fertilizer at planting led to some opposing effects according to the different species. At 24 months post-planting, fertilizer positively affected both provenances of S. birrea and one of the Senegal provenances (Téssékéré) of B. aegyptiaca, whereas it negatively affected survival rates of both provenances of Z. mauritiana, A. digitata and A. senegal.
The advantage of a fenced-in field trial is that it allows for the controlled monitoring of natural regeneration processes. Woody species regeneration data were collected at two time points, one at the beginning (October 2013) and the other at the end (October 2015) of the field trial. The biodiversity of natural regeneration was generally low with nine and eight species respectively in 2013 and 2015. This was not unexpected when considering the poor biodiversity of adult populations within the plot (six species) and in the surrounding areas (Niang et al. 2014). In terms of species composition, the regenerating populations mirrored adult populations in the zone. For example, in both 2013 and 2015, the dominant species in the plot was, by far, Balanties aegyptiaca. Some changes in species composition between 2013 and 2015 are worth noting. For example, the number and relative proportion of C. procera, considered as an indicator of land degradation (Arbonnier 2002; Ozer et al. 2010), decreased between 2013 and 2015, indicating a potential improvement of biophysical conditions in the plot. On the contrary, the number and proportion of B. senegalensis dramatically increased between 2013 and 2015. Boscia senegalensis is a multipurpose tree that is highly prized for its edible fruit and wide array of medicinal attributes (Niang et al. 2014). Finally, three G. bicolor saplings were inventoried in 2015, a species that has become extreme rare in the study zone. This could be accounted for by a convergence of required factors: a persistent soil seed bank, sufficient rainfall in 2015 (294 mm annual precipitation) and grazing exclusion over the two-year period.
From a quantitative standpoint, the natural regeneration capacity in the experimental field plot was high (88% in 2013 and 92% in 2015) when compared to regeneration capacities reported in open, unprotected areas surrounding Widou Thiengoly (50.5 and 46.6% in 2010 and 2011 respectively) (Ndiaye et al. 2013). Moreover the number of young trees inventoried in 2015 was higher than in 2013, presumably due to the absence of grazing over the two-year period and adequate rainfall in 2015. These results are of particular interest from the landscape management perspective. Natural regeneration is a viable, cost-effective alternative to tree planting if increasing the biomass production/area is the desired outcome. Regenerating individuals were not only abundant in the plot, they also appeared more vigorous with a far greater overall contribution to (aerial) biomass production than the planted trees. For areas with low biodiversity like the Senegalese Ferlo, natural regeneration might be of limited added value in terms of providing novel provisioning ES, although long-term positive effects on regulating (climate regulation) and supporting (soil formation, nutrient cycling, habitat provision) ES would be expected.
Natural regeneration can also be wisely managed and this has already been proven to be successful in several areas throughout the Sahel. Farmer-managed natural regeneration (FMNR) has been used over the last 25 years to restore over five million hectares of land in the Nigerien Sahel (Reij et al. 2009; Sendzimir et al. 2011) and recently there has been strong advocacy for scaling-up (Reij and Winterbottom 2015). FMNR provides a wide range of environmental, social and economic benefits (Weston et al. 2015). In this extensive study it was shown that asset creation, increased consumption of wild resources, health improvements, and psycho-social benefits were of even greater value in FMNR-adopting households than income and agricultural yields.
The main occupation of local Fulani populations in the Senegalese Ferlo is livestock herding, meaning that any GGW intervention in the zone must be monitored in terms of its impact on pasturelands. In the Ferlo, exclosures are a necessary part of any intervention in order to avoid grazing and trampling of saplings, at least for the first several years. Herein, herbaceous species biodiversity was characterized inside of the exclosures. A total of 56 species were identified in the experimental field plot. These data are comparable to those obtained by Ndiaye et al. (2013) in which 52 species in the vicinity of Widou Thiengoly were reported. The number of species inventoried each year varied according to annual rainfall (2014 < 2013 < 2015).
In arid rangeland exclosure studies carried out in Tunisia (Tbib and Chaieb 2004) and Algeria (Kherief et al. 2013), herbaceous species biodiversity was systematically higher inside the fenced areas as compared to outside, suggesting ecological benefits to exclosures. However, in zones where extensive livestock herding is the main source of revenues, exclosures must be used with caution as they are often poorly regarded by local populations for socio-economic and cultural reasons. Furthermore, long term experiments will be needed to monitor long term range environmental impacts and potential trade-offs of exclosures. Finally, when comparing herbaceous biodiversity under tree canopies versus open areas within the plot, the biodiversity was systematically greater under the tree canopy compared to open areas. The decreased temperatures, increased humidity, and increase in organic matter/litter production under trees provide a favorable microclimate for herbaceous species growth (Akpo and Grouzis 1996). This underlines the indirect benefits of increasing woody vegetation cover via tree planting and/or natural regeneration for improving pasture grass biodiversity.
Conclusion
This work is particularly timely as active GGW reforestation is underway in the study area in northern Senegal. Herein, we provide critical data from the first GGW experimental field trials to assess the extent by which woody species biodiversity may be reintroduced on a large scale along the GGW. Ten indigenous woody species known to be important for local populations, each for multiple uses including, food, medicine, construction were tested herein. Several of the same species that performed best in reforestation trials were those that also demonstrated high natural regeneration capacity. This suggests that large-scale tree-planting may be of limited added value in terms of actually increasing woody species biodiversity in the study area. It is important to note that these results are particularly applicable to reforestation activities in the surrounding areas of Widou Thiengoly. They could also serve as a starting point for GGW decisions as to which tree species to plant in eco-geographically similar regions. Similar small-scale field trials are currently being scaled-out to different eco-geographic regions along the GGW in Senegal. Together, this series of small-scale field trials along the GGW path will be extremely valuable in that it will (1) contribute to the currently poor understanding of the true benefits of tree planting in the Sahel and (2) provide sound, quantitative data to GGW practitioners as to which species are most well-adapted to the different intervention areas.
Large-scale tree planting is only one of the many natural resource management options along the GGW, and in any given area, multiple landscape-scale solutions must be considered in keeping with the needs of local stakeholders and their landscape use. Along the Senegalese GGW, water is clearly the limiting natural resource. Given this constraint, enhancing woody species biodiversity may only be realistically envisaged at a smaller scale for some selected, high-value species of socio-economic importance as this would imply water use, expensive exclosures and protective measures for the first years in order to ensure successful tree establishment.
References
African Union and Pan African GGW Agency (2010) Harmonised regional strategy for implementation of the “Great Green Wall Initiative of the Sahara and the Sahel”. http://www.greatgreenwallinitiative.org/sites/default/files/publications/harmonized_strategy_GGWSSI-EN_.pdf. Accessed 22 Dec 2016
Akpo LE, Grouzis M (1996) Influence du couvert ligneux sur la régénération de quelques espèces ligneuses sahéliennes (Nord-Sénégal, Afrique occidentale). Webbia 50:247–263
Arbonnier M (2002) Arbres, arbustes et lianes des zones sèches de l’Afrique de l’Ouest. CIRAD – MNHN. Pony-sur-Yonne, France
Aubreville A (1949) Climats, forêts et désertification de l’Afrique tropicale. Société d’éditions géographiques, maritimes et coloniales, Paris, France
Barral H, Bénéfice K, Boudet G et al (1983) Les systèmes de production d’élevage au Sénégal dans la région du Ferlo: synthèse de fin d’études d’une équipe de recherches pluridisciplinaire. ORSTOM, Paris
Barrett CB, Constas MA (2014) Toward a theory of resilience for international development applications. Proc Natl Acad Sci USA 111:14625–14630. https://doi.org/10.1073/pnas.1320880111
Boshier D, Broadhurst L, Cornelius J, Gallo L, Koskela J, Loo J, Petrokofsky G, St Clair B (2015) Is local best? Examining the evidence for local adaptation in trees and its scale. Environ Evid 4:20. https://doi.org/10.1186/s13750-015-0046-3
Brandt M, Mbow C, Diouf AA, Verger A, Samimi C, Fensholt R (2015) Ground- and satellite-based evidence of the biophysical mechanisms behind the greening Sahel. Glob Chang Biol 21:1610–1620. https://doi.org/10.1111/gcb.12807
Breman H, Kessler JJ (1995) Woody plants in agro-ecosystems of semi-arid regions with an emphasis on Sahelian countries. Springer, Berlin
Ciccarese L, Mattsson A, Pettenella D (2012) Ecosystem services from forest restoration: thinking ahead. New Forest 43:543–560. https://doi.org/10.1007/s11056-012-9350-8
Davies J, Poulsen L, Schulte-Herbrüggen B, Mackinnon K, Crawhall N, Henwood WD, Dudley N, Smith J, Gudka M (2012) Conserving dryland biodiversity. International Union for Conservation of Nature, Gland
Davies J, Robinson LW, Ericksen PJ (2015) Development process resilience and sustainable development: insights from the drylands of Eastern Africa. Soc Nat Resour 28:328–343. https://doi.org/10.1080/08941920.2014.970734
De Leeuw J, Njenga M, Wagner B, Iiyama M (2014) Treesilience: an assessment of the resilience provided by trees in the drylands of Eastern Africa. ICRAF, Nairobi
Diallo A, Agbangba EC, Ndiaye O, Guissé A (2013) Ecological structure and prediction equations for estimating tree age, and dendometric parameters of Acacia senegal in the Senegalese semi-arid zone-Ferlo. Am J Plant Sci 4:1046–1053
Guissé A, Boëtsch G, Ducourneau A, Goffner D, Gueye L (2013) L’observatoire homme–milieux international Tessékére (Ohmi): un outil de recherche pour étudier la complexité des écosystèmes arides du Sahel. C R Biol 336:273–277
Herrmann SM, Tappan GG (2013) Vegetation impoverishment despite greening: a case study from central Senegal. J Arid Environ 90:55–66. https://doi.org/10.1016/j.jaridenv.2012.10.020
ICSU, ISSC (2015) Review of the sustainable development goals: the science perspective. International Council for Science (ICSU), Paris
Jacobs DF, Oliet JA, Aronson J, Bolte A, Bullock JM, Donoso PJ, Landhäusser SM, Madsen P, Peng S, Rey-Benayas JM, Weber JC (2015) Restoring forests: what constitutes success in the twenty-first century? New Forest 46:601–614
Kherief SN, Nouasria D, Salemkour N, Benchouk K, Delhamra M (2013) La mise en repos: une technique de gestion des parcours steppiques. Journal Algérien des Régions Arides, Numéro Spécial 12:115–123
Le Houérou HN (1989) La variabilité de la pluviosité annuelle dans quelques régions arides du monde; ses conséquences écologiques. In: Bret B (ed) Les hommes face aux sécheresses IHEAL editions. IHEAL, Paris, pp 127–137
Lebrun JP, Stork AL (1991–2015) Enumération des plantes à fleurs d’Afrique tropicale et tropical african flowering plants: ecology and distribution, vol. 1–7. Conservatoire et jardin botaniques de la ville de Genève, Geneva, Switzerland
Leprun JC (1971) Nouvelles observations sur les formations dunaires sableuses fixées du Ferlo nord occidental (Sénégal). ASEQUA 31:69–78
Mbow C, Brandt M, Ouedraogo I, De Leeuw J, Marshall M (2015) What four decades of earth observation tell us about land degradation in the Sahel? Remote Sens 7:4048–4067. https://doi.org/10.3390/rs70404048
MEA, Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: desertification synthesis. World Resources Institute, Washington DC
Miehe S, Kluge J, Wehrden H, Retzer V (2010) Long-term degradation of Sahelian rangeland detected by 27 years of field study in Senegal. J Appl Ecol 47:692–700
Ndiaye O, Diallo A, Sagna MB, Guissé A (2013) Diversité floristique des peuplements ligneux du Ferlo, Sénégal. VertigO, vol 13, no 3
Niang K, Sagna MB, Ndiaye O, Thiaw A, Diallo A, Akpo LE, Saleh MM, Diome N, Diatta S, Faye MN, Gueye M, Guissé A, Goffner D (2014) Revisiting tree species availability and usage in the Ferlo region of Senegal: a rationale for indigenous tree planting strategies in the context of the Great Green Wall of the Sahara and Sahel Initiative. J Exp Biol Agric Sci 2:529–537
Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S (2009) Agroforestree database: a tree reference and selection guide version 4.0. World Agroforestry Centre, Kenya
Ozer P, Hountondji Y-C, Niang AJ, Karimoune S, Manzo OL, Salmon M (2010) Désertification au Sahel: historique et perspectives. Bulletin de la Société Géographique de Liège 54:69–84
Poupon H (1980) Structure et dynamique de la strate ligneuse d’une steppe sahélienne au nord du Sénégal. ORSTOM, Paris
Radwan UAA (2007) Photosynthetic and leaf anatomical characteristics of the drought-resistant Balanites aegyptiaca (L.) Del. seedlings. Am Eurasian J Agric Environ Sci 2:680–688
Reij C, Winterbottom R (2015) Scaling up regreening: six steps to success. A practical approach to forest and landscape restoration. World Resources Institute technical report. http://www.wri.org/sites/default/files/scaling-regreening-six-steps-success.pdf. Accessed 11 Nov 2016
Reij C, Tappan G, Smale M (2009) Agroenvironmental transformation in the Sahel: another kind of “Green Revolution.” IFPRI Discussion Paper. International Food Policy Research Institute, Washington, D.C.
Rockström J, Steffen WL, Noone K et al (2009) A safe operating space for humanity. Nature 461:472–475. https://doi.org/10.1038/461472a
Roe D (2010) Linking biodiversity conservation and poverty alleviation: a state of knowledge review. CBD Technical Series no 55. https://www.cbd.int/doc/publications/cbd-ts-55-en.pdf. Accessed 23 Jan 2017
Roussel J (1995) Pépinières et plantations forestières en Afrique tropicale sèche: manuel à l’usage des ingénieurs et techniciens du reboisement. ISRA, Dakar
Sackville Hamilton NR (2011) Is local provenance important in habitat creation? A reply. J Appl Ecol 38:1374–1376
Sendzimir J, Reij CP, Magnuszewski P (2011) Rebuilding resilience in the Sahel: regreening in the Maradi and Zinder, regions of Niger. Ecol Soc 16:1. https://doi.org/10.5751/ES-04198-160301
Sinare H, Gordon LJ, Enfors Kautsky E (2016) Assessment of ecosystem services and benefits in village landscapes—a case study from Burkina Faso. Ecosyst Serv 21:141–152
Sy O (2003) Dynamique des ressources en eau et évolution de la mobilité pastorale en zone sylvopastorale. Dissertation, Université Cheikh Anta Diop, Dakar, Senegal
Tbib A, Chaieb M (2004) La mise en défens des parcours en zones arides: avantages écologiques et obstacles socio-économiques. Cahiers Options Méditerranéennes 62:473–476
UNCCD (1994) United Nations Convention to Combat Desertification in those countries experiencing serious drought and/or desertification, Particularly in Africa. UNEP-London. http://www2.unccd.int. Accessed 12 Nov 2016
Venter SM, Witkowski ETF (2013) Where are the young baobabs? Factors affecting regeneration of Adansonia digitata L. in a communally managed region of southern Africa. J Arid Environ 92:1–13. https://doi.org/10.1016/j.jaridenv.2012.12.010
Verte GM (2009) Choix des Espèces végétales et des Systèmes de Mise en valeur et de Suivi. http://www.doc-developpement-durable.org/file/ArbresFruitiers/FICHES_ARBRES/jujubier/ChoixEspecesPourGrandeMurailleVerte.pdf. Accessed 21 Feb 2017
Weston P, Hong R, Kaboré C, Kull CA (2015) Farmer-managed natural regeneration enhances rural livelihoods in dryland West Africa. Environ Manag 55:1402–1417. https://doi.org/10.1007/s00267-015-0469-1
Wezel A, Lykke AM (2006) Woody vegetation change in Sahelian West Africa: evidence from local knowledge. Environ Dev Sustain 8:553–567. https://doi.org/10.1007/s10668-006-9055-2
Zida D, Tigabu M, Sawadogo L, Oden PC (2008) Initial seedling morphological characteristics and field performance of two Sudanian savanna species in relation to nursery production period and watering regimes. Forest Ecol Manag 255:2151–2162
Acknowledgements
The authors sincerely thank the Senegalese National Great Green Wall Agency for technical and logistical assistance. A warm thanks goes to Mbacké Fall and villagers for invaluable help in the nursery at Widou Thiengoly. We also thank Arnaud Ouedraogo who helped us to localize some of the “out-of-the-way” places in Burkina Faso from which our seeds came from. We thank Seyni Sané for help in statistical analyses. We thank Karine Ginoux and Sophie Drame who always insure that our field missions runs as smoothly as possible from an administrative standpoint. The authors acknowledge Institut Klorane and Fondation Véolia Environnement for financial support and a Ph.D. fellowship for T. I. Wade and to Labex DRIIHM (ANR-11-LABX-0010) (Laboratoire d’Excellence Dispositif de Recherche Interdisciplinaire sur les Interactions Hommes-Milieux) for M. Mauclaire’s PhD fellowship. We thank the CNRS Observatoire Hommes-Milieux Tessékéré (OHMi) and the Agence Nationale de la Recherche (French National Research Agency) (project Future Sahel – ANR-15-CE03-0001) for funding this research. Finally, we acknowledge the anonymous reviewers for their comments that have allowed us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wade, T.I., Ndiaye, O., Mauclaire, M. et al. Biodiversity field trials to inform reforestation and natural resource management strategies along the African Great Green Wall in Senegal. New Forests 49, 341–362 (2018). https://doi.org/10.1007/s11056-017-9623-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-017-9623-3