Abstract
Eucalyptus globulus Labill and hybrids thereof have low lignin content, favoring cellulose extraction, but are often recalcitrant to clonal propagation. This work analyzed biochemical and morphological changes during adventitious rooting of mini-cuttings of E. globulus × maidenni obtained from mini-stumps cultured in drip fertigated sand bed or intermittent flooding tray commercial propagation systems. Morphological (% rooting, root number and length, mean rooting time) and biochemical parameters (peroxidase activity, total phenolic content and flavonoid content) were monitored to characterize the rooting phases. All of the rooting parameters were equivalent in both systems, indicating comparable efficiency of both methods in clonal propagation. Kinetic profiles of biochemical parameters were also similar, although the activity of peroxidases was an order of magnitude higher and the phenolic content about three times lower in cuttings derived from intermittent flooding-grown mini-stumps than in those derived from sand bed-grown mini-stumps. Taken together, results suggest that rooting phases were similar in both systems: induction before day 5, formation from day 5 to 15, and elongation from day 15 to 45. These data may contribute to the development of rooting phase-specific mineral nutrient solutions to maximize clonal propagation and plant survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eucalyptus has become the most widely planted hardwood genus in the world, with approximately 17.8 million ha of planted area (FAO 2000; Turnbull 1999). In the Americas, eucalypt statistics are dominated by Brazil, where there are estimated three million ha of plantations, the majority of which are used for pulp production (FAO 2000; Turnbull 1999). Brazil is the largest world producer of eucalypt pulp due to clonal forests formed by elite material with high productivity, that can yield values around 45–60 m³ ha−1 year−1 (Mora and Garcia 2000). In southern Brazil, Eucalyptus globulus and its hybrids are of interest for this industry due to their relative high frost resistance and low lignin content, which facilitates cellulose extraction. On the other hand, E. globulus is generally considered recalcitrant for rooting (Serrano et al. 1996; Le Roux and Van Staden 1991), a negative characteristic that is often maintained when interspecific hybrids using this species are produced for commercial purposes.
Mass vegetative propagation has become an important tool for increasing the competitiveness of the forestry based industry. A super-intensive system has been successfully employed for commercial propagation of clonal Eucalyptus; it consists of mini-cuttings kept in indoor mini-hedges and is based on the rooting of axillary shoots from rooted stem-cuttings, also known as mini-stumps (Assis et al. 2004). There are two main systems used by Brazilian companies to keep mini-stumps and to produce mini-cuttings: drip fertigated sand beds and intermittent flooding trays; in the latter, metal lattices holding polyethylene tubes containing mini-stumps have their bases temporarily immersed in a nutritive solution for fertigation (see Assis et al. 2004 for a review). Adventitious root formation is a key step in vegetative propagation (De Klerk et al. 1999) and it is a developmental process consisting of a series of successive and interdependent phases (induction, formation, elongation), each with its own requirements and characteristics (Kevers et al. 1997). Knowledge of biochemical and morphological events associated with root induction and formation may allow improvement in rooting procedures in order to limit losses, particularly towards the final stages of production. Therefore, it would be interesting to identify reliable biochemical marker(s) for rooting in commercial ex vitro systems.
Various studies on adventitious root formation have shown a fundamental role played by peroxidase on the rooting of cuttings (Metaxas et al. 2004; Syros et al. 2004; Hatzilazarou et al. 2006; Husen and Pal 2007). Furthermore, these enzymes have been proposed to be biochemical markers of the successive rooting phases for several species, in which rooting consistently occurred after the cuttings had reached and passed a peak of activity (Rout et al. 2000; Saxena et al. 2000; Caboni et al. 1997; Hand 1993; Fett-Neto et al. 1992; Gaspar et al. 1992). Peroxidase activity has been linked to the oxidation of auxin. Many basic peroxidases have indole-3-acetic acid (IAA) oxidase activity and peroxidases have been shown to be effective in IAA oxidation, at least in vitro (Hand 1993 and references therein). Moreover, phenolic compounds are also known to be involved in rooting (De Klerk et al. 1999). Changes in phenolic compounds might be responsible for the control of peroxidase-IAA-oxidase activity, therefore, affecting the IAA content. However, IAA itself might, by feedback, also control phenolic metabolism (Kevers et al. 1997). Flavonoids are a class of phenolic compounds that are potential biochemical markers of rooting (Hand 1993) and have been shown to be natural inhibitors of basipetal auxin transport in stems (Peer et al. 2004). High concentrations of some flavonoids have been related to an easy- to-root phenotype in Eucalyptus gunnii (Curir et al. 1990).
The present work characterizes the phases of adventitious rooting in mini-cuttings of E. globulus × maidennii obtained from mini-stumps commercially cultivated in drip fertigated sand bed or intermittent flooding system. The aims of the study were to compare the rooting response of mini-cuttings derived from mini-stumps cultivated in the two propagation systems and to establish a frame for the development of root phase-specific nutrient solutions.
Material and methods
Plant material–E. globulus × maidennii
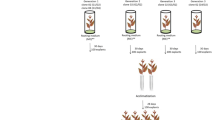
The genetic material used in the experiment was the hybrid clone 19 of E. globulus × maidenii developed by Aracruz Celulose S.A. This hybrid has been on commercial production due to its interesting wood properties, such as low lignin content; however, it shows some recalcitrance to clonal propagation, with relatively high initial mini-cutting losses. Mini-cuttings are young sprouts with 4–6 leaves harvested from mini-stumps (i.e., mother plants with at least 3 and at most 12 months of age) cultivated in indoor clonal hedges (in greenhouse) using intermittent flooding trays or drip fertigated sand bed systems (Fig. 1, see also Assis et al. 2004 for a review). For the experiments herein described approximate age of mini-stumps was 6 months. Mini-cuttings were harvested every 5 days during the winter/spring of 2005 from the onset of the experiment on August 30th (when the mini-cuttings were obtained from mini-stumps) up to 45 days on October 14th (when the rooted mini-cuttings were transferred from the greenhouse to the “hardening house”) at Aracruz Celulose S.A (Barra do Ribeiro, RS, Brazil–latitude 30º17′28″ S, longitude 51º18′04″ W). The mini-cuttings were used to evaluate morphological and biochemical changes associated with the adventitious rooting process.
Vegetative propagation of E. globulus × maidennii. (a). Indoor hydroponics mini-clonal hedge with mini-stumps (mother-plant) maintained in sand bed or intermittent flooding trays. In detail, the production of mini-cuttings (with four leaves to six leaves in this experiment) from sprouts of mini-stumps. (b). Mini-cutting in polyethylene tubes containing substrate (carbonized rice hulls and vermiculite 1:1). (c). Rooting of mini-cuttings. (d). Rooted mini-cutting ready to initiate adaptation to field conditions (hardening)
Experimental conditions
Mini-stumps cultivated in drip fertigated sand bed system received a nutritional solution containing (1,000 g.l−1): calcium nitrate, 1250; Krista K®, 420; Krista® MPK; magnesium sulphate, 505; iron chelate, 41.5; organic boron, 5; manganese sulphate, 4; copper sulphate, 0.4; zinc sulphate, 0.8 (Yara Adubos–Porto Alegre/RS, Brazil). Mineral nutrient quantities (mg l−1): N, 175.4; P-36.4; K, 212.8; Ca, 242.5; Mg, 48; S, 66; B, 0.5; Fe, 5; Cu, 0.1; Mn, 1; Zn, 0.2. The frequency of fertigation was once a day and the volume fertigated was 1.5–2 l m−2 of bed. The mini-stumps were planted at a density of 100 plant m−2 in the sand bed. In order to set the experiment, mini-cuttings within the appropriate size range (4–6 leaves) were randomly harvested from a population of approximately 100 mini-stumps used in the operational routine of the drip fertigated sand bed system.
Mini-stumps cultivated in the intermittent flooding system received a nutritional solution containing 985 g Calcinit®, 946 g Kristasol®, 140 g Magnitra-L®, 20 ml Boron Organic® and 116 g HydroFerro® (Hidro Fertilizantes–Barueri/SP–Brazil). Mineral nutrient quantities (mg l−1): N, 195.3; NH4, 24; P2O5, 113.5; K2O, 340.5; Ca, 187.1; S, 18.9; B, 2.24; CuEDTA, 0.38; FeEDTA, 7.34; MnEDTA, 0.38; ZnEDTA, 0.24; Mo, 0.38. The frequency of flooding was once to twice a day, the first with nutrient solution and a second with water as required (warmer days). Flooding duration was approximately 30 min, as judged from the visible presence of solution outside the plastic containers with mini-stumps. The mini-stumps were kept in black conical polyethylene tubes (12 cm long × 2 cm diameter; 53 cm3 volume capacity) filled with carbonized rice hulls and vermiculite (1:1 v/v) as substrate, at a density of 250 plants.m-². Mini-cuttings within the appropriate size range (4–6 leaves) were randomly harvested from a population of approximately 50 mini-stumps used in the operational routine of the intermittent flooding system.
Mini-cuttings were cultivated in black conical polyethylene tubes (12 cm long × 2 cm diameter; 53 cm3 volume capacity) filled with carbonized rice hulls and fine vermiculite (1:1 v/v) as substrate with added fertilizers: 2.5 Kg m−3 of PG mix (Yara Adubos–Porto Alegre/RS, Brazil), 1.5 Kg m−3 19-06-01 NPK of Osmocote® Classic (Scotts, Ohio, USA) and 2 g m−3 of Simple super phosphate (20% P2O5). Nutrient contents in Kg m−3: phosphorus pentoxide, 0.4; ammonium sulphate, 1.04; potassium chloride, 0.208; zinc sulphate, 0.014; copper sulphate, 0.014; manganese sulphate, 0.014; boric acid, 0.028).
The greenhouse temperatures during winter were 5–10°C (morning) and 18–28°C (afternoon), whereas during the spring temperatures were 23–29°C (morning) and 26–35°C (afternoon). Photosynthetically active radiation (provided by sunlight) at plant level in the greenhouse ranged from 80 to 200 μmol m−2 s−1; photoperiod varied from 11 to 13 h. Air relative humidity was kept between 84 and 93%.
Morphological analyses
At each sampling time, young sprouts were harvested from the mini-stumps to generate mini-cuttings. For each sampling, 20 mini-cuttings from the intermittent flooding system (a total of 200 were used) and 80 mini-cuttings from the sand bed (a total of 800 were used) were simultaneously evaluated. The difference in number was due to the available operating facilities in each system at the time of the experiment. Final data were taken in the end of the experiment at 45 days. Parameters examined were percent of mortality, percent of rooting (calculated considering only the surviving plants), root number (root density–roots per rooted mini-cutting), root length (considering the length of the longest root) and mean rooting time (Fett-Neto et al. 2001).
Biochemical analyses
Individual samples for biochemical analyses consisted of approximately 2 cm of the basal part of 4 mini-cuttings randomly grouped for each sampling time harvested. Three replicate samples were used for each time point. Flavonoid content was evaluated only in cuttings obtained from the drip fertigated sand bed system. All reagents used in the biochemical assays were analytical grade.
Peroxidase activity
Approximately 100 mg of frozen plant tissues (basal part) were ground in liquid nitrogen. Peroxidase specific activity was quantified according to Fett-Neto et al. (1992), except that guaiacol was used as substrate. Crude protein extracts in 0.2 M pH 7.0 phosphate buffer were used to determine enzyme activity at 420 nm in a Cintra 5 spectrophotometer (GBC, Victoria, Australia). Protein was quantified by the method of Bradford (1976).
Total phenolic content
Approximately 50 mg of frozen plant tissues (basal part) were ground in liquid nitrogen, extracted in 1.5 ml 0.1 N HCl and submitted to sonication in a water bath for 30 min. The extracts were centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was collected and the pellet was re-extracted. The supernatants were pooled and the volume was completed to 5 ml with 0.1 N HCl. For quantification, 1 ml of 20% (w/v) Na2CO3 and 0.5 ml of Folin-Ciocalteu reagent were added, mixed and then incubated at 100°C for 1 min. After cooling, the extract was diluted to 50 ml with water and filtered through Whatman no. 1 filter paper. Reading was at 750 nm. The standard curve was established with 0.1% (w/v) pyrogallol in 0.1 N HCl (Fett-Neto et al. 1992).
Flavonoid content
The flavonoid content was spectrophotometrically determined by the aluminum chloride complexation method described by Zhishen et al. (1999). Approximately 200 mg of frozen plant tissues (basal part) were ground in liquid nitrogen, extracted in 5 ml of 95% (v/v) ethanol and submitted to sonication in a water bath for 30 min in the dark. The extracts were centrifuged at 7,000 × g for 5 min. The supernatant was collected and a 1 ml aliquot was diluted to 5 ml with H2O. For quantification, 0.3 ml of 5% (w/v) NaNO2 was added, followed by mixing and then incubation at 25°C for 5 min. Next, 0.3 ml of 1% (w/v) AlCl3 was added, the resulting solution was mixed and then incubated at 25°C for 6 min. Finally, 2 ml of 1 M NaOH and 2.4 ml of H2O were added followed by agitation. Reading was at 510 nm. The standard curve was established with quercetin (Sigma, USA).
Statistical analyses
Experimental design was totally randomized. Mini-cutting samples were harvested along their position on the tube trays, from left to right. Statistical analyses were done by t-test or percentage confidence intervals, always with a P ≤ 0.05 (Sokal and Rohlf 1981). For biochemical analyses only descriptive statistics was applied (mean and standard error).
Results
The morphological parameters analyzed showed equivalent results for both systems at the end of the rooting process (Table 1). Mini-cuttings from both systems displayed callus formation (around 20% of the plants) after 10 days and the first roots appeared after 15 days in cuttings derived from both systems. At this time, 21% of sand bed-derived mini-cuttings were rooted versus 33% of intermittent flooding-derived ones. Moreover, after 20 days the majority of the mini-cuttings were rooted showing similar condition of the root systems: 70% of sand bed and 89% of intermittent flooding-derived cuttings. On the other hand, intermittent flooding led to a higher mortality rate (20.5%) than sand bed (14%) (Table 1). In both cases, the mortality happened among the non-rooted mini-cuttings.
Mini-cuttings originated from both systems showed a peak in peroxidase activity after 15 days of culture, coincident with the emergence of the first roots. After that, peroxidase activity sharply decreased by day 20, remaining stable afterwards. This pattern was consistent in mini-cuttings derived from both culture systems (Fig. 2). In spite of the similar activity profile displayed by mini-cuttings derived from both mini-stumps operational systems, peroxidase activity of mini-cuttings obtained from mini-stumps under the intermittent flooding system was higher by about one order of magnitude (Fig. 2).
Peroxidase activity in mini-cuttings of E. globulus × maidenni obtained from mini-stumps cultured in drip fertigated sand bed or in intermittent flooding. Inset: peroxidase activity in mini-cuttings obtained from mini-stumps cultured in drip fertigated sand bed shown alone for better clarity of temporal profile. Bars are standard deviations
Changes in content of phenolic compounds were also similar between mini-cuttings derived from both culture methods. For the drip fertigated sand bed treatment, total phenolic content increased up to day 30, whereas for intermittent flooding treatment it increased from day 5 until day 25. Then, phenolic content reached a variable plateau until the end of the experiment for both treatments (Fig. 3). The content of phenolic compounds was 2.5–3 times higher in mini-cuttings derived from mini-stumps grown in the drip fertigated sand bed system compared to those derived from intermittent flooding.
The analysis of flavonoid content in mini-cuttings derived from sand bed displayed a profile similar to that shown by total phenolics with an increase from day 5 until day 25. The highest flavonoid content was observed between days 20 and 30 and a slow, but apparent decrease was observed until day 45 (Fig. 4).
Discussion
Gaspar et al. (1992) described a model for grapevine adventitious rooting that included three phases: (1) an induction phase, characterized by lower peroxidase activity (lack of morphological changes and high auxin content), (2) an initiation (root formation) phase, occurring between the minimum and maximum of peroxidase activity (cell division and decrease in auxin content), and (3) an expression (root elongation) phase, characterized by a gradual decline in peroxidase activity followed by the first visible signs of roots. Various studies have shown that peroxidase activity profiles during adventitious rooting of several species are consistent with this general model (Hatzilazarou et al. 2006; Metaxas et al. 2004; Qaddoury and Amssa 2004; Rout et al. 2000; Saxena et al. 2000; Rival et al. 1997). In the case of Eucalyptus mini-cuttings, it seems that the induction phase occurred in the first days after obtaining the cutting (before 5 days). The formation phase appears to have taken place from day 5 to day 15, during the increase and peak of peroxidase activity. Finally, the elongation phase occurred between days 15 and 20, corresponding to the emergence of the first roots and rooting of the majority of mini-cuttings. At this phase, a decrease in peroxidase activity would be expected due to the stabilization of auxins at lower concentrations after the catabolic action of the enzymes on auxin at the root formation step. The same trends were observed when analyzing total peroxidase activity data (weight basis), indicating that changes were not the result of differences in protein content throughout the rooting process, but of enzyme activities and/or effectors (data not shown).
The much higher peroxidase activity in mini-cuttings derived from the intermittent flooding system could have been induced by the temporary hypoxia and re-oxygenation phases of the mini-stump cultivation system. In plantlets of wheat, it was seen that periods of hypoxia, as well as hypoxia and aeration, where capable of increasing the activity of ascorbate peroxidase in roots (Biemelt et al. 1998). Studies in Lupinus showed that cycles of hypoxia and re-aeration represent sources of oxidative stress, as they increase the concentration of free radicals and induce antioxidant enzymes, such as superoxide dismutase (Garnczarska et al. 2004).
According to Berthon et al. (1993) phenolic compounds are involved in different steps of adventitious root formation. De Klerk et al. (1999) pointed out that phenolic compounds can act as antioxidants, thereby protecting IAA from oxidation and plant tissue from oxidative stress due to wounding. In the present study, it was not possible to identify a peak of total phenolic content during the early part of the experimental period as reported in other studies with rooting of Vigna radiata, Prunus dulcis and Sequoia sempervirens (Nag et al. 2001; Caboni et al. 1997; Fett-Neto et al. 1992); this fact reinforces the possibility that the root induction phase happened during the first 5 days. During the root formation phase, the content of phenolics increased, which may reflect a need to reduce auxin transport from the shoot at this phase. Flavonoids have been shown to inhibit the basipetal transport of auxin in stems (Wasson et al. 2006; Peer et al. 2004). In fact, at least for the sand bed-derived mini-cuttings, the content of total phenolics and flavonoids increased in very similar fashion. In the expression phase, the content of phenolics remained high, but fluctuated, which may be due to differences in root growth between mini-cuttings.
The higher phenolic content in the sand bed system-derived mini-cuttings can also be related to differences in the nutrient solution application method in both culture systems. Studies with Hypericum brasiliense showed an increase in the content of total soluble phenolics in the shoots under hypoxia due to continuous flooding, whereas roots did not present a significant change (De Abreu and Mazzafera 2005). Mini-stumps under intermittent flooding are likely experiencing recurrent but transient oxidative stress and it is possible that phenolics could participate in the mitigation of this phenomenon by acting as antioxidants, leading to reductions in their steady-state content. The opposite magnitude of difference observed for peroxidase activity and content of phenolic compounds between mini-cuttings derived from mini-stumps grown in sand bed and intermittent flooding supports the general view that these biochemical parameters display opposite behaviors in relation to the rooting process (Gaspar et al. 1992).
In conclusion, the rooting results obtained in both systems were similar. The choice for a system can be made based on operational convenience and production costs. Furthermore, biochemical parameters showed a consistent profile for both culture systems, indicating that it is possible to use them as biochemical markers, especially peroxidase activity. Changes in time course profiles and relative contributions of peroxidase activity and content of phenolics appear more relevant for the final morphogenic rooting response than specific values of these per se. Although further studies with different clones and seasons will be necessary to confirm the use of the rooting markers herein evaluated, the identification of rooting phases may pave the way for a better modulation of the adventitious rooting process of Eucalyptus in the commercial scale set up employing the mini-hedge strategy. The development of rooting phase-specific nutrient solutions, for example, as has been succesfully done for in vitro rooting in E. globulus (Schwambach et al. 2005), may result in significant gains in efficiency of the propagation process.
References
Assis TF, Fett-Neto AG, Alfenas AC (2004) Current techniques and prospects for the clonal propagation of hardwood with emphasis on Eucalyptus. In: Walter C, Carson M (eds) Plantation forest biotechnology for the 21th century, 1st edn. Research Sign Post, New Delhi
Berthon JY, Battraw MJ, Gaspar T, Boyer N (1993) Early test using phenolic compounds and peroxidase activity to improve in vitro rooting of Sequoiadendron giganteum (Lindl.). Buchholz Saussurea 27:7
Biemelt S, Keetman U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to the activation of the antioxidant defense system in roots of wheat seedlings. Plant Physiol 116:651–658. doi:10.1104/pp.116.2.651
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principles of protein dye binding. Anal Biochem 72:245–248. doi:10.1016/0003-2697(76)90527-3
Caboni E, Tonelli MG, Lauri P, Iacovacci P, Kevers C, Damiano C et al (1997) Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol Plant 39(1):91–97. doi:10.1023/A:1000365224324
Curir P, VanSumere CF, Termini A, Barthe P, Marchesini A, Dolci M (1990) Flavonoid accumulation is correlated with adventitious roots formation in Eucalyptus gunni Hook micropropagated through axillary bud stimulation. Plant Physiol 92:1148–1153
De Abreu IN, Mazzafera P (2005) Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem 43:241–248. doi:10.1016/j.plaphy.2005.01.020
De Klerk GJ, Van Der Krieken W, De Jong JC (1999) The formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant 35(3):189–199. doi:10.1007/s11627-999-0076-z
FAO (2000) Global forest resources assesment 2000–Main report. FAO Forestry paper. (www.fao.org/forestry/fo/fra/main/index.jsp)
Fett-Neto AG, Teixeira SL, Da Silva EAM, Sant’Anna R (1992) Biochemical and morphological changes during in vitro rhizogenesis in cuttings of Sequoia sempervirens (D. Don) EndI. J Plant Physiol 140:720–728
Fett-Neto AG, Fett JP, Goulart LWV, Pasquali G, Termignoni RR, Ferreira AG (2001) Distinct effects of auxin and light on adventitious root development in Eucalyptus saligna and Eucalyptus globulus. Tree Physiol 21:457–464
Garnczarska M, Bednarski W, Morkunas I (2004) Re-aeration-induced oxidative stress and antioxidative defenses in hypoxically pretreated lupine roots. J Plant Physiol 161:415–422. doi:10.1078/0176-1617-01073
Gaspar T, Kevers C, Hausman JF, Berthon JY, Ripetti V (1992) Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomie 12:757–765. doi:10.1051/agro:19921003
Hand P (1993) Biochemical and molecular markers of cellular competence for adventitious rooting. In: Davis TD, Haissig BE (eds) Biology of adventitious root formation. Basic life sciences, v. 62. Plenum Press, New York
Hatzilazarou SP, Syros TD, Yupsanis TA, Bosabalidis AM, Economou AS (2006) Peroxidases, lignin and anatomy during in vitro and ex vitro rooting of gardenia (Gardenia jasminoides Ellis) microshoots. J Plant Physiol 163:827–836. doi:10.1016/j.jplph.2005.06.018
Husen A, Pal M (2007) Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New For 33:309–323. doi:10.1007/s11056-006-9030-7
Kevers C, Hausman JF, Faivre-Rampant O, Evers D, Gaspar T (1997) Hormonal control of adventitious rooting: progress and questions. J Appl Bot–Ang Bot 71(3–4):71–79
Le Roux JJ, Van Staden J (1991) Micropropagation and tissue culture of Eucalyptus–a review. Tree Physiol 9:435–477
Metaxas DJ, Syros TD, Yupsanis T, Economou AS (2004) Peroxidases during adventitious rooting in cuttings of Arbutus unedo and Taxus baccata as affected by plant genotype and growth regulator treatment. Plant Growth Regul 44:257–266. doi:10.1007/s10725-004-5931-7
Mora AL, Garcia CH (2000) A cultura do eucalipto no Brasil. Sociedade Brasileira de Silvicultura, São Paulo, Brazil
Nag S, Saha K, Choudhuri MA (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul 20:182–194. doi:10.1007/s003440010016
Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH et al (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16:1898–1911. doi:10.1105/tpc.021501
Qaddoury A, Amssa M (2004) Effect of exogenous indole butyric acid on root formation and peroxidase and indole-3-acetic acid oxidase activities and phenolic contents in date palm offshoots. Bot Bull Acad Sin 45:127–131
Rival A, Bernard F, Mathieu Y (1997) Changes in peroxidase activity during in vitro rooting of oil palm (Elaeis guineensis Jacq). Sci Hortic (Amsterdam) 71:103–112. doi:10.1016/S0304-4238(97)00079-4
Rout GR, Samantaray S, Das P (2000) In vitro rooting of Psoralea corylifolia Linn: peroxidase activity as a marker. Plant Growth Regul 30:215–219. doi:10.1023/A:1006336819887
Saxena C, Samantaray S, Rout GR, Das P (2000) Effect of auxins on in vitro rooting of Plumbago zeylanica: peroxidase activity as a marker for root induction. Biol Plant 43(1):121–124. doi:10.1023/A:1026519417080
Schwambach J, Fadanelli C, Fett-Neto AG (2005) Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus. Tree Physiol 25:487–494
Serrano L, Rochange F, Semblant JP, Marque C, Teulières C, Boudet AM (1996) Genetic transformation of Eucalyptus globulus through biolistics: complementary development of procedures for organogenesis from zygotic embryos and stable transformation of corresponding proliferating tissue. J Exp Bot 45:285–290. doi:10.1093/jxb/47.2.285
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman, San Francisco, 859 p
Syros T, Yupsanis T, Zafiriadis H, Economou A (2004) Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J Plant Physiol 161:69–77. doi:10.1078/0176-1617-00938
Turnbull JW (1999) Eucalypt plantations. New For 17:37–52. doi:10.1023/A:1006524911242
Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18:1617–1629. doi:10.1105/tpc.105.038232
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. doi:10.1016/S0308-8146(98)00102-2
Acknowledgments
The authors thank Aracruz Celulose S.A. for the supply of plant material and for the grant-in-aid of research to carry out this investigation. The Brazilian government agencies CAPES (Federal Agency for Graduate Education) and CNPq (National Council for Scientific and Technological Development) provided graduate, undergraduate and research scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwambach, J., Ruedell, C.M., de Almeida, M.R. et al. Adventitious rooting of Eucalyptus globulus × maidennii mini-cuttings derived from mini-stumps grown in sand bed and intermittent flooding trays: a comparative study. New Forests 36, 261–271 (2008). https://doi.org/10.1007/s11056-008-9099-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-008-9099-2