Abstract
Tropical peat-swamp forests are one of the largest near-surface reserves of terrestrial carbon. However, many peat-swamp forest tree species have resulted in the reduction due to over-exploitation, forest fires and conversion into agricultural land in Indonesia. The objective of this study was to determine the effects of two arbuscular mycorrhizal (AM) fungi, Glomus clarum and G. aggregatum, on the early growth of two slow-growing peat-swamp forest tree species, Ploiarium alternifolium and Calophyllum hosei, under greenhouse conditions. Cuttings of P. alternifolium and C. hosei were uninoculated or inoculated with G. clarum and G. aggregatum and grown under greenhouse conditions for 6 months. Percentage AM colonization, plant growth, phosphorus (P) concentration and survival rate were measured. The AM colonization of P. alternifolium and C. hosei ranged from 27 to 32% and 18 to 19%, respectively. Colonization by G. clarum and G. aggregatum increased shoot height, stem diameter, leaf number, and shoot and root dry weights. Cutting shoot P content were increased by AM fungal colonization. The survival rates of inoculated plants were higher (100%) than those of control plants (67%). The results suggest that inoculation with AM fungi improves early growth of P. alternifolium and C. hosei in a tropical peat-swamp forest and can therefore contribute to rehabilitation of peat-swamps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical peat-swamp forests in Southeast Asia have the large area of peat and support luxuriant growth of peat-swamp tree species overlying peat deposits up to 20 m thick (Page et al. 2002). In Indonesia, tropical peat-swamp forests are located at low altitudes in the coastal and sub-coastal lowlands of Irian Jaya (4.6 Mha), Sumatra (8.3 Mha) and Kalimantan (6.8 Mha) (Page et al. 1999). In Kalimantan, trees of the family Dipterocarpaceae dominate in terms of abundance, density and biomass (Appanah and Turnbull 1998). Slik et al. (2003) found that the 10 most abundant tree families after Dipterocarpaceae were Euphorbiaceae, Myrtaceae, Sapotaceae, Lauraceae, Myristicaceae, Burseraceae, Anacardiaceae, Ebenaceae, Annonaceae and Guttiferae. Ploiarium and Calophyllum are important genera of the family Guttiferae (Kobuski 1950; Soerianegara and Lemmens 1994). Timbers from Calophyllum are used in the construction of boats and furniture and as plywood, and those from Ploiarium are used to build houses and as firewood and charcoal by local people (Sosef et al. 1998). Several species of Ploiarium and Calophyllum are used in medicine because of their antimicrobial activity and of the presence of other biologically active compounds such as coumarins, xanthones, and terpenoids. Recently, Calophyllum species have received considerable attention from a pharmacological viewpoint because they contain compounds that strongly inhibit HIV (human immunodeficiency virus) reverse transcriptase (Reyes-Chilpa et al. 1997).
Tropical peat-swamp forests have diminished due to forest fires, over-exploitation and conversion into highways, industrial and agricultural lands, and plantation estates (Phillips 1998). Today, degraded peat-swamp forests require extensive rehabilitation and an annual supply of high-quality seedling stocks for reforestation. The major obstacle in the rehabilitation of peat-swamp forests is slow growth and high mortality of seedlings in the nursery. Ploiarium and Calophyllum are slow-growing tree species and appear to have irregular flowering and fruiting habits (Soerianegara and Lemmens 1994; Sosef et al. 1998). Ploiarium seeds germinate insufficiently when sown immediately after collection. Scarification may be required to germinate well. Calophyllum seeds have low germinative ability and are often lost in floods during the rainy season, further limiting reproduction (Nair and Seeni 2003).
Numerous studies of tropical rain forest mycorrhizas have indicated the dominance of arbuscular mycorrhiza (AM) (Janos 1980; Béreau and Garbaye 1994). The highest percentage AM colonization was reported in a tropical Mexican wet forest (Guadarrama and Álvarez-Sánchez 1999). AM colonization was approximately 40% in tree species in tropical heath forests and mixed Dipterocarpaceae forest in Brunei (Moyersoen et al. 2001). AM colonization was observed 17 of 22 wild seedlings of peat-swamp tree species in Central Kalimantan, Indonesia (Tawaraya et al. 2003). Dipterocarpaceae is one of most important families in peat swamp forests. It is well known that ectomycorrhiza is formed in the roots of Dipterocarpaceae (Lee and Lim 1989; Turner et al. 1993; Yazid et al. 1994). Little is known about the effects of AM colonization on the growth of tropical peat-swamp tree species. The objective of this study was to determine the effects of two AM fungi, Glomus aggregatum and G. clarum, on the early growth of two slow-growing peat-swamp forest tree species, Ploiarium alternifolium (Vahl) Melchior (synonym = Hypericum alternifolium Vahl) (Kobuski 1950) and Calophyllum hosei Ridley, under greenhouse conditions. The isolate of G. aggregatum originated in Japan and the isolate of G. clarum is native to the peat-swamp forest of Central Kalimantan, Indonesia. These fungi were chosen because G. aggregatum had been shown to be a highly effective symbiont with Acacia crassicarpa and Eucalyptus spp. (Turjaman et al. 2000), and Khaya ivorensis (Santoso et al. 2003) in previous study.
Materials and methods
Cutting propagation method
Cutting materials of P. alternifolium and C. hosei were collected from wildlings at Nyaru Menteng arboretum near Palangka Raya in Central Kalimantan, Indonesia (2°43′ S; 111°38′ E). Since the flowering and fruiting characteristics of those two tree species in peat-swamp forests are not well understood, it was difficult to obtain the seeds. The wildlings were 1–2 years old and their heights were approximately 0.5–1.5 m. The wildlings with orthotropic position were cut to a length of approximately 10 cm with two leaves. The leaf area was reduced to approximately half its original size. All cutting materials from Central Kalimantan were placed in an icebox and brought to greenhouse of Forest and Nature Conservation Research and Development Centre (FNCRDC) in Bogor, West Java (6°36′ S, 106°45′ E). Roots of the two seedling species were stimulated with the ROOTON (Ishihara Sangyo Co., Japan). Rooton contains 0.1% naphthyl acetic acid in powder type. Cut ends were dipped into the rooton. Cutting medium consisting of crushed coconut fiber mixed with rice husks (2:1, v/v) was sterilized in an autoclave at 121°C for 50 min. Subsequently, one cutting was planted in one hole of a 45-hole tray (52 × 29 × 5.6 cm). The volume of one hole was 140 ml. The trays were placed inside a propagator. The propagator is transparent plastic box (66 × 37 × 33 cm) to keep a constant temperature and relative humidity in the trays. Each species were prepared 45 cuttings. A fog evaporative cooling system was installed inside the greenhouse to lower temperature inside the propagator (Sakai et al. 2002).
Arbuscular mycorrhizal fungi
Spores of Glomus clarum Nicholson & Schenk were isolated from peat soil of Kalampangan, Palangka Raya, Central Kalimantan (2°13′ S, 113°56′ E) by trap culture (Brundrett et al. 1996). The pot culture was established as mixed propagule cultures. The mixed propagule consists of spores, external hyphae and roots colonized by G. clarum. Glomus clarum was propagated in pot cultures of Pueraria javanica. Plastic pots were filled with 175 g of sterilized zeolite and 5 g of AM fungal inoculum was placed in the planting hole. Two 6-day-old P. javanica seedlings were transplanted into the pots and grown under natural light greenhouse conditions with no temperature and humidity control. Plastic pots were place 15 cm apart in a greenhouse to avoid contamination. A preliminary experiment showed that AM fungal inoculum was effective to give high AM fungal colonization in the roots of P. javanica without application of microbial filtrate. After 90 days, spores, external hyphae and roots colonized by G. clarum were observed in the zeolite. Spore numbers in the zeolite were 713 spores 10 g−1. AM fungal inoculum of G. aggregatum Schenk & Smith was obtained from Research and Development Division, Osaka Gas Ltd., Japan.
Soil preparation and seedling inoculation
Ultisol was collected from Haurbentes Experimental Forest, Jasinga,West Java (6°32′–33′ S, 108°26′ E) and stored in a greenhouse. The soil was passed through a 5 mm sieve and mixed with river sand (3:1, v/v) to improve drainage. The pH (H2O) of the mixed soil was 4.8 and available P (Bray-1) was 0.17 mg kg−1. The mixed soil was sterilized in an autoclave at 121°C for 30 min.
Polyethylene pots (15 × 10 cm) were filled with 500 g of the sterilized soil mixture. AM fungal inoculation was accomplished by placing 5 g of inoculum of G. clarum or G. aggregatum 1–3 cm below the cuttings. One 90-day-old P. alternifolium or C. hosei cutting was transplanted into each pot. Control cuttings were not inoculated. The cuttings were watered daily with tap water to field capacity. All pots in each treatment were placed 15 cm apart on benches in a greenhouse to avoid contamination. Weeds and pests were removed manually. The cuttings were grown for 180 days in a greenhouse at the FNCRDC, Bogor,West Java. Daily temperatures varied between 26 and 35°C, relative humidity was 80–90% and photoperiod was approximately 12 h.
Growth measurement
The experiment consisted of three treatments of P. alternifolium and C. hosei cuttings: (1) control (no inoculation); (2) inoculation with G. aggregatum; and (3) inoculation with G. clarum. There were 15 replications per treatment. Shoot height and stem diameter at 1 cm from the soil surface were measured 180 days after transplantation. After 180 days, shoots and roots were separated and oven-dried at 70°C for 72 h before weighing. Shoots were ground and digested with H2SO4 and H2O2 solution (3:1, v/v). P concentration in the digested solution was determined by the vanadomolybdate yellow assay (Olsen and Sommers 1982). Shoot P content was calculated by multiplying the shoot P concentrations and shoot dry weights.
An additional 15 cuttings each of P. alternifolium and C. hosei uninoculated or inoculated with G. aggregatum or G. clarum were grown under the same conditions as those of the cuttings in the above experiment. Numbers of viable cutting were counted 180 days after transplantation. Survival rate was calculated as follows: Survival rate (%) = number of viable cuttings/number of initial cuttings × 100.
Arbuscular mycorrhizal colonization
Roots of P. alternifolium and C. hosei were washed over a 2 mm sieve under running tap water to separate them from soil particles. The roots were cleared in 100 g l−1 KOH for 1 h, acidified with diluted HCl and stained with 500 mg l−1 trypan blue in lactoglycerol (Brundrett et al. 1996). Roots were destained with 50% glycerol and were viewed under a compound microscope at 200× magnification. Percentage AM colonization was examined using the gridline intersect method (Giovannetti and Mosse 1980).
Mycorrhizal dependency (MD) was calculated according to Plenchette et al. (1983): MD (%) = [(dry weight of mycorrhizal plant − dry weight of non mycorrhizal plant)/dry weight of mycorrhizal plant] × 100.
Data were statistically analyzed using analysis of variance with the statistical software StatView 5.0 (Abacus Concepts). Comparison of means was done using the least significant difference method at 5% probability level where the F-value was significant. Data of AM colonization were transformed by arcsine (square root) before statistical analysis.
Results
Arbuscular mycorrhizal colonization
Roots of P. alternifolium and C. hosei were colonized by G. aggregatum or G. clarum, 6 months after transplantation under greenhouse conditions (Table 1). Percentage AM colonization of P. alternifolium and C. hosei by G. aggregatum was not significantly different from that by G. clarum. Control cuttings of P. alternifolium and C. hosei were colonized by indigenous AM fungi as evidence by wind and/or insects carried the AM fungi inoculum.
Shoot phosphorus content
P content was higher in shoot of P. alternifolium inoculated with G. aggregatum and G. clarum than in control cuttings (Table 1). There was no significant difference in shoot P content between P. alternifolium inoculated with the two AM fungi. Inoculation of G. clarum increased shoot P content of C. hosei. However, there was no significant difference in shoot P content between G. aggregatum and control.
Plant growth
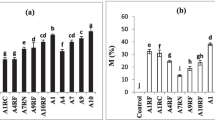
AM colonization increased shoot height, stem diameter and leaf number of P. alternifolium 6 months after transplantation (Fig. 1). The inoculation of G. aggregatum and G. clarum increased shoot dry weight and root dry weight (Table 1). There was no significant difference in stem diameter, leaf number, and shoot and root dry weights between P. alternifolium inoculated with the two fungi.
AM colonization by G. aggregatum increased shoot height and stem diameter but not leaf number of C. hosei (Fig. 2). By contrast, G. clarum colonization increased shoot height, stem diameter and leaf number of C. hosei. Inoculation of the two AM fungi increased shoot dry weight and root dry weight of C. hosei compared with control (Table 1). However, there was no significant difference in shoot dry weight and root dry weight between C. hosei inoculated with the two fungi.
Mycorrhizal dependency
For a given fungus, MD values varied depending on the host species. MD values obtained in this study with both cutting species exceeded 50%. The MD of P. alternifolium and C. hosei ranged from 55–58% and 57–63%, respectively.
Survival rate
The survival rates of P. alternifolium cuttings inoculated with G. aggregatum and G. clarum were higher than that of control cuttings 6 months after transplantation under greenhouse conditions (Fig. 3). The survival rates of C. hosei inoculated with the two fungi were also higher than that of control cuttings. No difference in survival rate was observed between cuttings inoculated with G. aggregatum and G. clarum for both host species.
Discussion
In this study, we showed the importance of AM fungi for early growth and shoot P nutrition of tropical peat-swamp forest tree species. We demonstrated for the first time the positive effects of two AM fungi, G. aggregatum and G. clarum, on P. alternifolium and C. hosei, and confirmed the previously reported AM colonization of a native tree genus of Calophyllum in a lowland tropical rain forest of Singapore (Burslem et al. 1995), and two native tree species of Calophyllum in a tropical peat-swamp forest in Central Kalimantan, Indonesia (Tawaraya et al. 2003). To the best of our knowledge, this is the first demonstration of AM colonization in P. alternifolium and C. hosei.
The extent of AM colonization in the roots of P. alternifolium and C. hosei was comparable to that reported previously. Tawaraya et al. (2003) reported that natural AM colonization of C. sclerophyllum and C. soulattri growing in peat-swamp forest was between 18 and 60%. Natural AM colonization was observed in control P. alternifolium cuttings, but the extent was lower (3%) than that observed in cuttings colonized by G. aggregatum and G. clarum. Béreau et al. (2000) observed that AM colonization of Dicorynia guianensis control seedlings was very low (<10%). It is very likely that AM colonization of control seedlings originated from the soil underneath the pots since the pots were placed directly above the ceramic surface without any border. The direct contact between the bottom of pots and the greenhouse benches may be a reason for the natural AM fungal colonization. We confirmed that the AM fungi that colonized the control cuttings were not G. aggregatum or G. clarum by morphological features.
AM colonization by G. aggregatum and G. clarum increased the P contents of P. alternifolium and C. hosei by as much as 164–171% and 39–67%, respectively. There are some nutrient constrains in degraded peat-swamp forest and P is most limiting factor for early growth of both tree species. P. alternifolium and C. hosei are important trees in the tropical peat-swamp forests of Southeast Asia because they have medicinal properties and are used as timber. Shoot height, stem diameter and leaf number were also increased by inoculating with those two AM fungi. Increases of these parameters may lead to increased yield from the medicinal plant and timber of both species. Furthermore, AM fungal inoculation induces vigorous growth of seedlings, which would be advantageous to reforestation activities. This experiment was carried out in the greenhouse conditions, where there was no competition with other fungi. The field experiment is underway in degraded peat-swamp forest.
Studies on these tree species and other tropical tree species have indicated that AM fungal inoculation reduces the amount of chemical fertilizers required for seedling production (Habte et al. 1993; Siqueira et al. 1998; Muthukumar and Udaiyan 2006). The production of seedlings of fast growing species such as Acacia, Eucalyptus and Gmelina in commercial nursery scales requires application of large amounts of chemical fertilizers, which entails high cost. Without fertilizer application, intensively managed tree plantations generally have low harvest. Moreover, fertilization cost is highest for degraded forest lands (Mackensen and Föster 2000). However, heavy fertilization often reduces the formation of some AM (Johnson 1993; Titus and Leps 2000). If the production cost using AM inoculum is lower than that using fertilizers, AM fungal inoculation can replace or reduce the amount of chemical fertilizers used in nurseries and in the field, without loss of efficiency of AM fungal colonization.
AM colonization in cuttings of P. alternifolium and C. hosei species differed between both AM fungi. P. alternifolium showed better response to AM colonization than C. hosei because percentage AM colonization and root dry weight of P. alternifolium were higher than those of C. hosei. For example, G. aggregatum colonized P. alternifolium quite well, but colonized C. hosei only poorly. Nevertheless, both AM fungi stimulated the root system of both cuttings, suggesting that there is no clear relationship between the degree to which a cutting is colonized by AM fungi and the potential for the cutting to benefit from this. MD (Plenchette et al. 1983) was 55 and 58% in P. alternifolium inoculated with G. aggregatum and G. clarum, and 63 and 57% in C. hosei, respectively. Ploiarium alternifolium and C. hosei were considered to be highly dependent according to the MD categories defined by Habte and Manajunath (1991). These values for AM dependency in both cutting species are also similar when compared with Sesbania padulosa, Acacia radiana, and Rhynchosia minima inoculated by G. aggregatum (Duponnois et al. 2001).
The survival rate of seedling stocks in the field is vital to reforestation. The survival rates of both AM-inoculated cuttings were 100% after 6 months (Fig. 3), and were higher than the survival rates of two tropical tree species from Panama inoculated with AM fungi, Ochroma pyramidale (97%) and Luehea seemannii (52%) (Kiers et al. 2000). Generally, 120% seedling stock is necessary for reforestation; however, our study shows that much more seedlings are needed when the seedlings are not inoculated with AM fungi, because field conditions are much more extreme than nursery conditions. The use of AM-inoculated seedlings might solve this problem: it would reduce significantly the cost of seedlings required for reforesting vast areas such as peat-swamp forests. However, it was difficult to get both species seedlings from the field to produce seedling stocks for planting activity. Among methods of vegetative propagation of grafting, air layering, tissue culture and cutting propagation, the latter is the most commonly used technique (Weinland 1998). Development of hedge orchard is a strategy to sustain the resource of cuttings and this technique has been used also to produce cuttings some species of dipterocarps. The hedge orchard can serve as a continuous supply for large-scale nursery seedling stocks.
We succeeded in making rootings of two peat-swamp tree cuttings, P. alternifolium and C. hosei, with the propagation cutting system, utilizing the greenhouse-use fogging system developed by Sakai et al. (2002). This technique has been used to produce seedlings of Shorea leprosula, S. selanica and S. platyclados (Dipterocarpaceae). To accelerate reforestation programs, Nair and Seeni (2003) proposed that Calophyllum seedlings be multiplied by tissue culture technology, but the Calophyllum roots grew slowly.
In conclusion, the inoculation of G. aggregatum and G. clarum improved plant growth and increased shoot P contents and survival rates of P. alternifolium and C. hosei cuttings 6 months after transplantation under greenhouse conditions. There was no significant difference in plant growth between the cuttings inoculated with the two AM fungi. Although G. aggregatum is an exotic species, it performed well in both tree species. When native species are unavailable, the use of exotic AM fungi should be considered provided that the inoculated cuttings show improved growth. Glomus clarum is beneficial if those two tree species are selected for reforestation activity. Further studies of field conditions are required to confirm this preliminary finding. It may also be possible to increase the reliability of inoculation by using mixed inoculum. Alexander and Lee (2005) suggested that it is best to use mixed AM fungal species inoculum rather than single AM fungal species as different species may have different functional roles. Taken together, our results suggest that the integration of the propagation cutting system with AM fungal inoculation technology can accelerate the establishment of cutting stocks on a large scale in nurseries, to rehabilitate tropical peat-swamp forests.
References
Alexander I, Lee SS (2005) Mycorrhizas and ecosystems processes in tropical rain forest: implications for diversity. In: Burslem DFRP, Pinard MA, Hartley SE (eds) Biotic interactions in the tropics: their role in the maintenance of species diversity. Cambridge University Press, pp 165–203
Appanah S, Turnbull JM (1998) A review of dipterocarps: taxonomy, ecology and silviculture. Center for International Forestry Research (CIFOR), Bogor, Indonesia, 223 pp
Béreau M, Garbaye J (1994) First observations on the root morphology and symbioses of 21 major tree species in the primary tropical rain forest of French Guyana. Ann Sci For 51:407–416
Béreau M, Barigah TS, Louisanna E, Garbaye J (2000) Effects of endomycorrhizal development and light regimes on the growth of Dicorynia guianensis Ammshoff seedlings. Ann For Sci 57:725–733
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. Monograph 32, ACIAR, Canberra, pp 374
Burslem DFRP, Grubb PJ, Turner IM (1995) Response to nutrient addition among shade-tolerant tree seedlings of lowland tropical forest in Singapore. J Ecol 83:113–122
Duponnois R, Plenchette, Bâ AM (2001) Growth stimulation of seventeen fallow leguminous plants inoculated with Glomus aggregatum in Senegal. Eur J Soil Biol 37:181–186
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Guadarrama P, Álvarez-Sánchez FJ (1999) Abundance of arbuscular mycorrhizal fungi spores in different environments in a tropical rain forest, Veracruz, Mexico. Mycorrhiza 8:267–270
Habte M, Muruleedhara BN, Ikawa H (1993) Response of neem (Azadirachta indica) to soil P concentration and mycorrhizal colonization. Arid Soil Res Rehab 7:327–333
Habte M, Manajunath A (1991) Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza 1:3–12
Janos DP (1980) Vesicular-arbuscular mycorrhizae affect lowland tropical rain forest plant growth. Ecology 61:151–162
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Kiers ET, Lovelock CE, Krueger EL, Herre EA (2000) Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: implications for tropical forest diversity. Ecol Lett 3:106–113
Kobuski CE (1950) Studies in the Theaceae, XIX, the genera Archytaea and Ploiarium. J Arnold Arbor 31:196–207
Lee SS, Lim KL (1989) Mycorrhizal infection and foliar phosphorus content of seedlings of three dipterocarps species growing in a selectively logged forest and a forest plantation. Plant Soil 117:237–241
Mackensen J, Föster H (2000) Cost-analysis for a sustainable nutrient management of fast growing-tree plantations in East-Kalimantan, Indonesia. For Ecol Manage 131:239–253
Moyersoen B, Becker P, Alexander IJ (2001) Are ectomycorrhizas more abundant than arbuscular mycorrhizas in tropical heath forests? New Phytol 150:591–599
Muthukumar T, Udaiyan K (2006) Growth of nursery-grown bamboo inoculated with arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria in two tropical soil types with and without fertilizer application. New Forests 31:469–485
Nair LG, Seeni S (2003) In vitro multiplication of Calophyllum apetalum (Clusiaceae), an endemic medicinal tree of the Western Ghats. Plant Cell Tissue Organ Cult 75:169–174
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL (eds) Methods of soil analysis Part 2.Chemical and microbiological properties. American Society of Agronomy Madison, pp 403–430
Page SE, Rieley JO, Shotyk ØW, Weiss D (1999) Interdependence of peat and vegetation in a tropical peat swamp. Philos Trans R Soc Lond 354:1885–1897
Page SE, Siegert F, Rieley JO, Boehm HV, Jaya A, Limin S (2002) The amount of carbon released from peat and forest fires in Indonesia during 1997. Nature 420:61–65
Phillips VD (1998) Peat swamp ecology and sustainable development in Borneo. Biodivers Conserv 7:651–671
Plenchette C, Fortin JA, Furlan V (1983) Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. I. Mycorrhizal dependency under field conditions. Plant Soil 70:199–209
Reyes-Chilpa R Jimenez-Estrada M, Estrada-Muñiz E (1997) Antifungal xanthones from Calophyllum brasiliensis heartwood. J Chem Ecol 23:1901–1911
Sakai C, Subiakto A, Nuroinah HS, Kamata N, Nakamura K (2002) Mass propagation method from the cutting of three dipterocarp species. J For Res 7:73–80
Santoso E, Irianto RSB, Turjaman M, Widyati E, Yulianti, Sitepu IR (2003) Application of vesicular Arbuscular mycorrhizal to promote Khaya ivorensis seedling growth. Bull Penelitian Hutan 636:27–31
Siqueira JO, Saggin-Júnior OJ, Flores-Ayles WW, Guimarães PTG (1998) Arbuscular mycorrhizal inoculation and superphosphate application influence plant development and yield of coffee in Brazil. Mycorrhiza 7:293–300
Slik JWF, Poulsen AD, Ashton PS, Cannon CH, Eichhorn KAO, Kartawinata K, Lanniari I, Nagamasu H, Nakagawa M, van Nieuwstadt MGL, Payne J, Purwaningsih, Saridan A, Sidiyasa K, Verburg RW, Webb CO, Wilkie P (2003) A floristic analysis of the lowland dipterocarp forests of Borneo. J Biogeogr 30:1517–1531
Soerianegara I, Lemmens RHMJ (1994) Timber trees: major commercial timbers. Plant Resources of South-East Asia No. 5 (1) Prosea Bogor Indonesia
Sosef MSM, Hong LT, Prawirohatmodjo S (1998) Timber trees: lesser-known timbers. Plant Resources of South-East Asia No. 5 (3) Prosea Bogor Indonesia
Tawaraya K, Takaya Y, Turjaman M, Tuah SJ, Limin SH, Tamai Y, Cha JY, Wagatsuma T, Osaki M (2003) Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For Ecol Manage 182:381–386
Titus JH, Leps J (2000) The response of arbuscular mycorrhizae to fertilization, mowing, and removal of dominant species in a diverse oligotrophic wet meadow. Am J Bot 87:392–401
Turjaman M, Sofyan C, Sitepu IR, Santoso E (2000) Field testing of exotic vesicular arbuscular mycorrhizae Glomus aggregatum on Acacia crassicarpa and Eucalyptus spp. at Rumpin, West Java. JSPS-NRCT/DOST/LIPI/VCC. Paper presented at the international workshop on biotechnology for sustainable utilization of biological resources in the tropics, November 22–24, 1999, Penang, Malaysia, 18 pp
Turner IM, Brown ND, Newton AC (1993) The effect of fertilizer application on dipterocarp seedling growth and mycorrhizal infection. For Ecol Manage 57:329–337
Weinland G (1998) Plantations. In: Appanah S, Turnbull JM (eds) A review of dipterocarps: taxonomy, ecology and silviculture. Center for International Forestry Research (CIFOR), Bogor, Indonesia, pp 151–185
Yazid SM, Lee SS, Lapeyrie F (1994) Growth stimulation of Hopea spp. (Dipterocarpaceae) seedlings following ectomycorrhizal inoculation with an exotic strain of Pisolithus tinctorius. For Ecol Manage 67:339–343
Acknowledgments
This research was supported in part by the RONPAKU program, the Core University Program of Japan Society for the Promotion of Science (JSPS), a Grant-in-Aid for Scientific Research from the Ministry of Education, Sports and Culture, Japan (No. 192555016), and the Sumitomo Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turjaman, M., Tamai, Y., Sitepu, I.R. et al. Improvement of early growth of two tropical peat-swamp forest tree species Ploiarium alternifolium and Calophyllum hosei by two arbuscular mycorrhizal fungi under greenhouse conditions. New Forests 36, 1–12 (2008). https://doi.org/10.1007/s11056-008-9084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-008-9084-9