Abstract
Pinus patula and high-elevation (HE) sources of P. tecunumanii exhibit intermediate levels of resistance to pitch canker (Fusarium circinatum), compared to extremely resistant species such as P. oocarpa, and extremely susceptible species such as P. radiata. Seedlings from 20 P. patula provenances and 15 HE P. tecunumanii provenances were artificially inoculated with the pitch canker fungus at 21 and 12 weeks of age, respectively, and assessed for resistance 12–20 weeks later. There was important provenance variation in pitch canker resistance for both species. The 20-week LiveStem percentage ranged from 70.3% to 43.6% among the P. patula provenances and 59.6% to 11.7% among HE P. tecunumanii provenances. There was a geographic pattern to the provenance variation, and in both species, low altitude sources demonstrated more resistance than those from high elevation. Provenance variation in pitch canker resistance could be useful when making selection and breeding decisions with these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pitch canker is a disease of pines, which is caused by the pathogen Fusarium circinatum ex Pinus spp. (Nirenberg and O’Donnell 1998). The disease results in dieback of branch tips, formation of cankers on the main stem, and production of copious amounts of resin. The disease was first described in the southeastern US in the late 1940s (Hepting and Roth 1946), but the pathogen is also widely distributed in Mexico, having been identified on 19 pine species in 14 states (Guerra-Santos 1999). Under the worst-case scenario, an infected tree can eventually lose vigor and die. Pitch canker has never reached epidemic proportion in the southern US, and its severity varies year to year apparently depending on the degree of weather-related and mechanical damage to trees (Dwinell et al. 1985).
In the last two decades, the disease has been reported in natural stands of P. radiata in California where it has reached epidemic proportions (Gordon et al. 2001). It has also spread to Pinus patula (Vilojen et al. 1994) and P. radiata nurseries (J. Mather, personal communication) in South Africa and P. radiata nurseries in Chile (Wingfield et al. 2002). Together, both species account for 4.7 million hectares of exotic plantations world-wide (Balocchi 1997; Birks and Barnes 1991), and therefore control of the disease is of great importance to the wood and forest products industry.
In efforts to find pine species that might be resistant to pitch canker either as pure species or in hybrid combination, the Camcore program at North Carolina State University screened seedlings of 16 pine species in the Oocarpae (Mexican closed pines), Australes (Southern US & Caribbean pines), and Attenuatae (California closed-cone pines) subsections and Pseuduostrobus Group at the USDA US Forest Service Resistance Screening Center (RSC) in Bent Creek, NC (Hodge and Dvorak 2000). The results indicated that the some species like Pinus oocarpa, P. jaliscana, and P. pringlei were very resistant to the disease, while others like P. patula and P. greggii showed extreme susceptibility. Interestingly, the susceptibility of P. tecunumanii in the Oocarpae varied considerably. Seedlings grown from seed samples of P. tecunumanii taken below 1,500 m altitude in Mexico and Central America (low elevation (LE) sources) were very resistant, while those sampled above 1,500 m altitude (high elevation (HE) sources) were only moderately resistant.
Even though there is now some information about the susceptibility of various pine species to pitch canker, little is known about provenance variation in susceptibility to the disease and the potential for developing resistant plantations through provenance selection and breeding. The objectives of this study were to examine provenance variation in pitch canker resistance for both P. patula and P. tecunumanii (HE), and to offer explanations why some populations in Mexico and Central America might be more susceptible than others.
Materials and methods
The 20 P. patula provenances and the 15 P. tecunumanii (HE) provenances included in these studies are presented in Tables 1 and 2. In the original seed collections completed by Camcore, around 20 mother trees per provenance were sampled, with a goal of at least 100 m between selected trees. The bulk provenance mixtures were composed of open-pollinated seed from a minimum of 6 randomly selected families. In addition to these provenances, the P. tecunumanii (HE) experiment included bulk mixes of P. oocarpa, P. maximinoi, P. patula, and LE P. tecunumanii. A P. elliottii pitch-canker-susceptible seedlot (FA2) was also included in both experiments according to the standard protocol at the RSC.
All seedlings were subjected to the pitch canker resistance screening protocols as developed by Oak et al. (1987), in which seedlings are challenged with the pitch canker fungus and their resultant responses are used to gauge relative resistance to infection. The P. patula experiment was conducted in 2003, and the P. tecunumanii experiment was conducted in 2004, with the dates of all activities listed in Table 3. Seed of all taxa were soaked in cold water for 24 h prior to sowing, and seedlings were grown in Ray Leach® containers (115 ml). Single spore isolates (i.e., a single genotype) were used to prepare four bulks of conidia of Fusarium circinatum according to McRae et al. (1987). The isolates originated in four locations in Georgia and Florida in the southeastern US (Table 4). The seedlings were wounded by severing the stem just below the apical meristem, and the excised apical portion was removed. The seedlings were then inoculated by atomizing an aqueous spore suspension onto the fresh wounds, with a concentration of 25,000 spores/ml for the P. patula study and 50,000 spores/ml for the P. tecunumanii study. The atomized spore suspension was sprayed directly onto the wound surface from a distance of around 25 cm, passing 3 times over each tree. Each family was represented by 80 seedlings, with 20 seedlings in each of four replications. Each replication was inoculated with one of the four single-spore isolate mixtures.
All seedlings in the HE P. tecunumanii experiment were inoculated at age 12 weeks, and all seedlings in the Pinus patula experiment were inoculated at age 21 weeks. Previous work at the RSC with P. patula and P. radiata families has shown that better differentiation among families was obtained using older seedlings.
Following inoculation the seedlings were returned to the greenhouse where they were maintained for 20 weeks during which time pathogen colonization was allowed to occur. Two measurements of damage were taken in both experiments: 12 weeks and 20 weeks in the P. patula study, and 16 weeks and 20 weeks in the P. tecunumanii study. In both studies, the total length of the stem from hypocotyl to wound and total length of remaining live stem was measured at the first measurement date. At the second measurement date, the percent of live stem remaining was visually estimated to the nearest 10%. Two criteria were used to estimate damage at each measurement date:
Statistical methods
For each of the two damage traits in both experiments, ANOVA was conducted using SAS Proc GLM (SAS Institute 1989) using a linear model including seedling height as a covariate, isolate-rep, provenance, and provenance × isolate-rep interaction. Since the primary objective of this study was to examine provenance variation, it was convenient to treat each 20-tree replication with a single isolate, thus isolates and replication were confounded. Previous experiments have demonstrated that replication effects are generally small, and therefore all parameters associated with isolate-rep effects were interpreted as being due mostly to isolate effects.
Variance components were estimated and Best Linear Unbiased predictions of provenance effects were calculated using SAS Proc Mixed. The proportion of provenance variance was estimated with the parameter p 2 estimated as
where σ2 p = provenance variance, σ2 p×t = provenance × isolate-rep interaction variance, and σ2 e = error variance. The Type B correlation of provenance effects across isolate-rep treatments was estimated as
This parameter is analogous to the Type B genetic correlation of Burdon (1977), and will approach 1 as the provenance × isolate-rep interaction variance approaches zero.
The repeatability of provenance means (R pm) was estimated as
These parameter estimates were used to evaluate which trait gives the most precise discrimination among provenances.
Results
Height
There were statistically significant effects of seedling height on nearly all damage traits in both experiments (Tables 5 and 6). This effect was also observed in an experiment with 23 pine species/varieties (Hodge and Dvorak 1999). Presumably seedling height is a proxy for some type of physiological change, perhaps degree of lignification that impacts how quickly the pathogen can colonize the stem. All statistical analyses included height as a covariate.
Isolate effects
There were highly significant differences among isolate-reps for all damage traits at both measurements in the P. patula experiment (Table 7). Mean stem height was around 258 mm at the time of inoculation, and mean height in the isolate-reps ranged from 238 mm to 291 mm (Table 4). Isolate-reps were grown near one another, but in different areas of the greenhouse. Although height was used as a covariate, the height differences suggest the possibility of other physiological effects arising from the environmental differences among replications leading to moderate differences in pitch canker damage. Mean Dieback at 12 weeks post-inoculation ranged from 20.5 mm for isolate S402 to 61.4 mm for isolate S298 (Table 5). These values correspond to about 90.7% and 74.5% LiveStem, respectively. The differences persisted until the 20-week measurement, with mean Dieback at that time ranging from 63.1 mm for isolate S402 to 129.6 mm for isolate S298 (LiveStem of 71.7% and 47.2%, respectively).
In contrast to the P. patula experiment, in the P. tecunumanii experiment there were no significant differences among isolate-reps for any trait (Table 8).
Provenance × isolate-rep interaction
In the P. patula experiment, there were statistically significant provenance × isolate-rep interactions for all damage traits. The Type B provenance correlations ranged from 0.41 to 0.84 for the Dieback and LiveStem traits (Table 9), indicating a moderate amount of interaction across isolate-reps. Conversely, there was no significant provenance × isolate-rep interaction for any of the damage traits at either measurement for P. tecunumanii (Table 6). The Type B provenance correlations were uniformly high, ranging from 0.92 to 1.00 for all traits (Table 10), indicating no biologically important interaction with isolates.
Species and provenance differences
The species differences that were observed in an earlier study (Hodge and Dvorak 2000) are completely consistent with those seen in this study (Table 12). In terms of resistance, P. oocarpa > LE P. tecunumanii > P. maximinoi > HE P. tecunumanii > P. patula. In the P. tecunumanii experiment, seedlings of Pinus oocarpa inoculated at 12 weeks had 92.6% LiveStem 20 weeks after inoculation, while LE P. tecunumanii was nearly as resistant, with 90.2% mean LiveStem. On average, HE P. tecunumanii averaged 33.5% LiveStem, while P. patula was almost completely dead, with mean LiveStem only 2.3%. It is difficult to make precise comparisons among the two experiments due to the difference in age of inoculation in the two experiments (21 weeks for the P. patula study, and 12 weeks for the P. tecunumanii study), however the following statements seem reasonable:
-
the most resistant P. patula provenances might approach the mean resistance of HE P. tecunumanii,
-
the most resistant HE P. tecunumanii provenances might approach the mean resistance of P. maximinoi, but would still be less resistant than typical LE P. tecunumanii.
The most useful trait to discriminate among provenances in the P. patula experiment was 20-week LiveStem, with R pm = 0.85 (repeatability of provenance means, Table 9). In the P. tecunumanii experiment, there was little difference among traits for R pm, with values ranging from 0.88 to 0.91 (Table 10). As one might expect, there were no important differences in how HE P. tecunumanii provenances ranked according to the different damage traits.
There were significant and important differences among provenances for all damage traits in both the P. patula and the P. tecunumanii experiments (Table 5 and 6). Using 20-week LiveStem to rank the P. patula provenances, the most resistant provenance (El Cielo) had 70.3% LiveStem, while the most susceptible provenance (Corralitla) had only 43.6% LiveStem (Table 11). In the P. tecunumanii experiment, 20-week LiveStem ranged from 59.6% for Montecristo to 11.7% for El Pinalón (Table 12). The provenance differences observed suggest that there is potential to improve pitch canker resistance in both taxonomic groups through selection and breeding. In both species, provenance differences were correlated with elevation, with lower collection sites demonstrating more resistance. For HE P. tecunumanii, the correlation between 20-week LiveStem and elevation was 0.58 (significant at α = 0.05), while in P. patula this correlation was 0.45 (significant at α = 0.05). In P. patula, there was also a moderately strong correlation between 20-week LiveStem and longitude (R = −0.70, significant at α = 0.001), with more westerly provenances demonstrating more resistance.
Discussion
Pinus patula and P. tecunumanii can easily be separated morphologically (Perry 1991) and by RAPD molecular markers (Grattapaglia et al. 1992; Furman et al. 1997, Dvorak et al. 2001). Both species appear to have evolved from an ancient P. oocarpa progenitor (Dvorak et al. 2000). The separation of P. tecunumanii into HE and LE groups was originally based on subtle morphological differences in bark thickness and patterns, cone shape, size, and abundance and needle thickness in natural stands (Dvorak 1986; Dvorak and Raymond 1991). Generally, with decreasing altitude of the collection site, needle thickness increases. Camcore has established more than 75 genetic field trials of HE and LE P. tecunumanii around the world, and from a perspective of growth and productivity, the results do not conclusively demonstrate that the two P. tecunumanii subpopulations should be kept in breeding populations (Hodge and Dvorak 1999). However, it is clear the HE and LE subpopulations react differently to pitch canker infection.
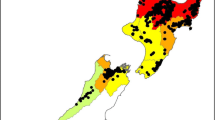
There are important differences in pitch canker resistance at the provenance level for both P. patula and HE P. tecunumanii, and the variation patterns in both species appear to be related to geography and elevation. For P. patula, provenances in the far western and northern part of the range were more resistant than those in the eastern areas (Fig. 1a). For both P. patula and HE P. tecunumanii, the lower the altitude of the provenance, the more resistant was the genetic material. These results raise an interesting question about what might be the pattern of variation among the LE P. tecunumanii provenances—although these provenances are generally very resistant, is there also a relationship between elevation and resistance within this subpopulation?
(a) Relative resistance of P. patula provenances to pitch canker. Symbol size represents LiveStem percentage 20 weeks after inoculation of 21-week-old seedlings. Provenance names associated with provenance numbers can be found in Table 1. (b) Relative resistance of high-elevation P. tecunumanii provenances to pitch canker. Symbol size represents LiveStem percentage 20 weeks after inoculation of 12-week-old seedlings. Provenance names associated with provenance numbers can be found in Table 1b
For P. patula, the most resistant provenances occur at the north and southwest tails of the natural distribution (Fig. 1a). The two northernmost populations, El Cielo and Conrado Castillo, are separated from the rest of the P. patula natural range by approximately 225 km. Based on our observations in provenance/progeny tests, they differ morphologically from other provenances from the central part of the natural range of P. patula in that their crowns are more compact, and their needles are thicker and less pendent. The other two populations in the northern part of the range that show better than average resistance to pitch canker, Pinal de Amoles and La Encarnación, exhibit no discernable external characteristic that would separate them from other P. patula populations.
Two southern populations of P. patula, Tlacuache and Yextla, exhibit higher levels of resistance than do populations in the central part of the species’ natural occurrence, and these provenances are different in several respects. First, they occur in the Sierra Madre del Sur under climatic influences from the Pacific Ocean, rather than under the effects of the more continental climates of Sierra Madre Oriental. Second, their morphology is characterized by smaller cones, shorter needles, more needles per fascicle and a higher proportion of resin canals in the internal position than do populations in the Sierra Madre Oriental (Dvorak et al. 2001). Third, RAPD molecular marker analysis indicates that all of the Yextla and some of Tlacuache trees have unusual marker patterns relative to “typical” P. patula (Dvorak et al. 2001). However, these “atypical” trees are still more closely related to P. patula than to any of the other closed-cone pine species. The significance of these marker differences between Yextla and Tlacuache and the other populations in the natural range of P. patula populations is not well understood.
For HE P. tecunumanii, the populations most susceptible to pitch canker were located in the San Cristobal highlands of Chiapas, Mexico extending into the Sierra de Chuacus and the Sierra de las Minas in north central Guatemala (Fig. 1b). These ranges are predominantly oriented in an east-west direction through both countries (and will be referred to here as the northern range). The most resistant populations of P. tecunumanii are located 60–150 km to the south in a second mountain range that runs parallel to the northern range. The southern range is geologically part of the Sierra Madre del Sur that extends from Mexico, across Guatemala and into northern El Salvador (referred here as the southern range). In the western highlands of Guatemala, distinction between the northern and southern ranges is poorly defined, but the two ranges are well separated by the Rio Motagua Valley in southeastern Guatemala.
An attractive hypothesis to explain the trend in resistance among HE P. tecunumanii is that there is more P. oocarpa in the southern than in the northern range and hybridization and introgression with HE P. tecunumanii has produced resistant populations. High levels of introgression might also explain why in DNA bulking assessments by species using RAPD markers, P. oocarpa and P. tecunumanii show only slight genetic separation (Dvorak et al. 2001), but with no species-specific markers to reliably distinguish between the two groups (Furman and Dvorak 2005). However, the introgression theory is not congruent with the fact that several HE populations of P. tecunumanii in the northern range, like San Lorenzo and Cabrican, occur sympatrically or in close proximity to P. oocarpa stands yet exhibit moderate to high susceptibility to pitch canker.
Summary
There is significant and important provenance variation for resistance to pitch canker among P. patula and high-elevation P. tecunumanii. These differences should be considered when making selection and breeding decisions in these species. In South Africa, some organizations that use P. patula as the primary commercial species are considering the possibility of crossing P. patula with LE P. tecunumanii or P. oocarpa to develop pitch canker resistant commercial hybrids. It may be worthwhile to focus on hybrids using material from more resistant provenances.
References
Balocchi CE (1997) Radiata pine as an exotic species. In: 24th Proc. SFTIC. Orlando, FL. Univ. of Florida, pp 11–17
Birks JS, Barnes RD (1991) Genetic control of wood quality in Pinus patula. Final Report, ODA Research Scheme R4616, Oxford Forestry Institute, University of Oxford, UK. 29 p
Burdon RD (1977) Genetic correlation as a concept for studying genotype-environment interaction in forest tree breeding. Silvae Genet 26:168–175
Dvorak WS (1986) Provenance /progeny testing of Pinus tecunumanii. In: Proc. IUFRO. A joint meeting of working parties on Breeding theory, progeny testing and seed orchards. Oct. 13–17. Williamsburg, VA. North Carolina State University. Raleigh, NC, pp 299–309
Dvorak WS, Raymond RH (1991) The taxonomic status of closely related closed cone pines in Mexico and Central America. New Forests 4:291–307
Dvorak WS, Jordan AP, Hodge GR, Romero JL (2000) Assessing evolutionary relationships in the Oocarpae and Australes subsections using RAPD markers. New Forests 20:163–192
Dvorak WS, Jordan AP, Romero JL, Hodge GR, Furman BJ (2001) Quantifying the geographic range of Pinus patula var. longipedunculata in southern Mexico using morphologic and RAPD marker data. South African Forest J 19:219–30
Dwinell LD, Barrows-Broaddus JB, Kulman EG (1985) Pitch canker: A disease complex of southern pines. Plant Dis 69:270–276
Furman BJ, Dvorak WS, O’Malley DM (1997) Analysis of genetic relationships of Central American and Mexican pines using RAPD markers that distinguish species. Mol Ecol 6(4):321–331
Furman BJ, Dvorak WS (2005) Population level analysis to identify species diagnostic RAPD markers for classification of Central American and Mexican pines. Forest Genet 12:67–78
Grattapaglia D, O’Malley D, Dvorak W (1992) Phylogenetic analysis of Central American and Mexican pines using RAPD markers on bulked DNA samples. In: Proc. IUFRO Resolving Tropical Forest Resource Concerns through Tree Improvement, Gene Conservation and Domestication of New Species. Cartegena and Cali, Colombia, pp 132–147
Gordon TR, Storer AJ, Wood DL (2001) The pitch canker epidemic in California. Plant Dis 85(11):1128–1139
Guerra-Santos J (1999) Pitch canker on Monterey pine in Mexico. In: Devey ME, Matheson AC, Gordon TR (eds) Current and potential impacts of pitch canker in radiata pine. Proc. Impact Monterey Workshop. California, USA, pp 58–61
Hepting GH, Roth ER (1946) Pitch canker, a new disease of some southern pines. J For 44:742–744
Hodge GR, Dvorak WS (1999) Genetic parameters and provenance variation of Pinus tecunumanii in 78 international trials. Forest Genet 6(3):157–180
Hodge GR, Dvorak WS (2000) Differential responses of Central American and Mexican pine species and Pinus radiata to infection by the pitch canker fungus. New Forests 19:241–258
McRae CH, Rockwood DL, Blakeslee GM (1987) Pitch canker resistant Slash pine identified by greenhouse screening. In: Proc. 19th Southern For. Tree Impr. Conf. College Station Texas, June 16–18, pp 132–139
Nirenberg HI, O’Donnell K (1998) New Fusarium species and combinations with the Gibberella fujikuroi species complex. Mycologia 90:434–458
Oak SW, Blakeslee GM, and Rockwood DL (1987) Pitch canker resistant slash pine identified by greenhouse screening. In: Proc. 19th Southern For. Tree Impr. Conf., College Station Texas, June 16–18, pp 132–139
Perry Jr JP (1991) The pines of Mexico and Central America. Timber Press, Inc., Portland, OR, p 231
SAS Institute 1989. SAS/STAT User’s Guide 4th edn., vol 2. SAS Institute, Cary, North Carolina, 846 pp
Vilogen A, Wingfield MJ, Marasas WFO (1994) First report of Fusarium subglutinans f. sp. pini on pine seedlings in South Africa. Plant Dis 78:309–312
Wingfield MJ, Jacobs A, Coutinho TA, Ahumada R, Wingfield BD (2002) First report of the pitch canker fungus, Fusarium circinatum, on pines in Chile. Plant Pathol 51:397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hodge, G.R., Dvorak, W.S. Variation in pitch canker resistance among provenances of Pinus patula and Pinus tecunumanii from Mexico and Central America. New Forests 33, 193–206 (2007). https://doi.org/10.1007/s11056-006-9023-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-006-9023-6