Abstract
Dynamic hazard and coal spontaneous combustion are major potential safety hazards in coal mines with enriched gas and spontaneously inflammable property. However, the initial oxidizability of coal samples after dynamic hazard has yet to be understood. A simplified device for dynamic hazard simulation of coal-and-gas outburst was established. The physical and chemical changes of coal surface, and their effects on initial oxidation were analyzed. Firstly, loose coal was pressurized by gas step-by-step, and the CO content was enhanced with increase in gas pressure. Meanwhile, the specific surface area provides a hotbed for low-temperature oxidation of radicals and functional groups of coal. Finally, the mechanism of mechanical force on the increase of radical and active groups with the homolysis of covalent bond was discussed. The initial oxidizability of coal after dynamic damage was characterized by oxygen absorption. The physicochemical characteristics suitable for coal oxidation, were formed under the mechano–chemical effect. The findings presented in here add to our understanding of the influence of dynamic hazard on coal oxidation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As mining depth increases, dynamic hazard is one of the critical safety hazards in deep coal mines with enriched gas (Cao et al., 2003; Yan et al., 2020). It is a frequent hazard that could directly or indirectly affect gas explosion in gobs and other hazards (Chu et al., 2019; Hu et al., 2017). For instance, coal-and-gas outburst could both ignite and detonate gas, and cause powdered coal combustion, which may cause secondary accidents (Kong et al., 2019; Qin et al., 2016; Tang & Wang, 2018). All these hazards occur instantaneously, but it is seldom discussed in the literature whether the coal after dynamic hazard has different oxidation tendency. In addition, the oxidation capacity of pulverized coal is reflected mainly in the change of its internal molecular structure (Cao et al., 2000). By exploring micro-structural changes in coal samples after experiencing dynamic disasters, it is possible to judge whether coal has the ability of spontaneous combustion. Therefore, it is important understand the complexity of coal structure, if it has experienced a dynamic hazard.
Coal is a mixture of similar polymeric compounds with different molecular weights and chemical structures. The chemical structures change under external interference (Mathews & Chaffee, 2012). In order to reveal the maturation of coal during rapid fault slip, it is necessary to consider the influence of flash temperature and mechano–chemistry at the contact of moving particles on the reaction kinetics, because coal can mature slightly under shear (Kitamura et al., 2012). The mechanical energy generated in the process of dynamic destruction not only changes the surface area of coal, but it also forces coal to be subjected to mechano–chemical action. The mechanical energy that hits the coal surface produces an unpaired electron. The highly reactive molecules with unpaired electrons can be measured by electron paramagnetic resonance (EPR) spectrometer (Yang et al., 2020). The effect of mechano–chemistry on coal spontaneous combustion has been studied preliminarily, and the result that grinding process could produce radicals was obtained (Bolm & Hernández, 2019; Li et al., 2018). The structural changes and secondary defects of coal affected by external forces, have different effects on the formation of functional groups and the polycondensation of macro-molecular structures, while reducing the structural stability of coal (Li et al., 2012). Under the action of tectonic stress, some aliphatic side chains and oxygen-containing functional groups with low-decomposition energy are degraded into radicals (Cao et al., 2007; Song et al., 2018). Therefore, radicals are often produced when the coal structure is damaged by external forces.
Active radicals can be produced in coal under mechano–chemical action, and the oxygen-containing groups and alkyl side chains are susceptible to external forces (Qi et al., 2018). Therefore, the changes of surface functional groups after the destruction of dynamic hazard also need to be investigated. As a non-destructive detection technique, X-ray photoelectron spectroscopy (XPS) is often used to measure the form and variation of coal surface functional groups (Ge et al., 2014; Grzybek et al., 2004; Zhang et al., 2018), as well as to explore the surface characteristics of coal with different metamorphic grades (Xu et al., 2017; Zhou et al., 2015). The essence of coal oxidation that leads to spontaneous combustion is interaction between coal surface active groups and molecule oxygen (Dack et al., 1985; Seehra et al., 1988). In order to prevent the occurrence of complex hazards, it is necessary to explore the oxidation capacity of coal after the destruction of dynamic hazard, so as to prevent the spontaneous combustion caused by coal oxidation.
In this paper, simulated experiments of dynamic hazard of coal samples with different grades of metamorphism were carried out. The variation of coal surface characteristics under different gas pressures was studied. The variations of gas composition and content around the coal after stepwise pressurization, were analyzed as well. Information of radicals and surface functional groups in coal was obtained. Based on the physical and chemical properties of coal surface destroyed by dynamic hazards, the mechano–chemical effect of coal in the above process, and its influence on the initial oxidation capacity of coal were studied.
Experiments
Coal Samples and Simulation Experiment of Dynamic Hazard
The coal samples selected for the experiments were anthracite from Yangquan City in Shanxi Province (YQ), bituminous coal from Panxi Coal Mine in Anhui Province (PX), lignitous coal from Datong City in Shanxi Province (DT), and Hulunbeier in Inner Mongolia (HL). The unexposed coal samples were wrapped with plastic storage bags, before being sent to the laboratory. After being cored and grounded under the protection of nitrogen, pulverized coal samples with particle sizes of 0.18–0.25 mm were selected for the experiments. The proximate analyses of the coal samples are listed in Table 1.
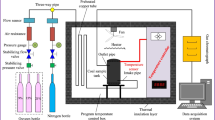
The self-designed simulation device is shown in Figure 1, and 100 g of coal sample was put into the device every time. To simplify the simulation experiment of coal-and-gas outburst, only the impact destruction of high pressure gas on coal was considered, while the horizontal and axial stress effects were ignored. Firstly, the three-way valve was turned towards the backing pump to vacuumize the specimen storehouse for three hours. Then, the three-way valve was turned towards the high-pressure cylinder of CH4, and the pressure values were set to 0.74 (i.e., the critical value of coal-and-gas outburst evaluation standard in China), 1.50, 2.25, and 3.00 MPa. Each pressure value was stabilized for two hours, so that the CH4 could be absorbed into the coal absolutely. Finally, the pressure-relief vent was opened quickly, and gas and coal powder were ejected. The ejective coal sample was sealed with nitrogen to prepare for subsequent experiments.
To lower the impact of each simulation experiment on gas composition and content, experiments in which CH4 gas pressurized the coal samples step-by-step were designed. The same coal sample was loaded at the above pressure values for two hours, respectively. Meanwhile, a small amount of gas was slowly released at each pressure value to detect its composition and content. Each experiment was performed three times to take the average composition and content.
Identification of Coal Spontaneous Combustion Tendency
The inherent property of coal oxidation ability at room temperature is the tendency of coal spontaneous combustion. Following the "Safety Regulations of Coal Mine" (a standard of China Industry Standard) with regard to determination of coal spontaneous combustion tendency, the double gas circuit of chromatographic method of fluid oxygen adsorption was adopted. The quantity of oxygen adsorption of coal per unit mass at room temperature and atmosphere pressure, could characterize the oxidation capacity of the coal. The ZRJ-2000 model coal spontaneous combustion tendency tester was used in the experiments. The measurement process was strictly in accordance with the standard "Oxygen Adsorption Identification Method with Chromatograph of Coal Spontaneous Combustion Tendency" (GB/T104-2006). The measurement range of oxygen adsorption was 0.05–4.00 ml/g, and the measurement error was ≤ 5%.
Test for Surface Area of Pores
The physical properties of the coal surface inevitable change after mechanical destruction (Jiang et al., 2002). Therefore, experiments of surface pore structure characteristics of pulverized coal were carried out. The experiments employed a BELSORP-Max automatic analyzer (Japan), for testing the specific surface area and pores volume. The temperature was set to 40 °C for 1 h to dry the water and reduce the degree of coal oxidation. After the sample was sufficiently cooled to end the degassing process, the nitrogen adsorption was used to test surface area at 77 K. The surface physical parameters were calculated using the BET (Brunauer–Emmett–Teller) equation (Brunauer et al., 1938).
EPR and XPS Spectroscopy Tests
The radicals of coal samples were measured by EPR spectroscopy, using a MS-5000 model spectrometer (German), whose parameters are listed in Table 2. The EPR spectra of a standard sample (Tempol) and the experimental samples, were tested using the same instrumental parameters. Because the spectrum parameters of Tempol are known, the EPR parameters of the experimental samples can be calculated by referring to Zhou et al. (2020).
The surface elements and functional groups of coal samples were tested by the ESCALAB 250Xi X-ray photoelectron spectrometer (USA) of the Advanced Analysis & Computation Center of China University of Mining and Technology. The changes of functional groups were analyzed according to the wide and narrow spectra. The wide-spectrum scanning range was 0–1350 eV, and the narrow-spectrum C element scanning range was 280–300 eV. Physical displacement is generated during the test of non-conductive samples. Therefore, the binding energies were calibrated based on the C–C bond at 284.8 eV. Functional group peaks were fitted using the XPSPEAK 4.1 software.
Results and Discussions
Step-by-Step Change of CO Gas Around Pressurized Coal
Figure 2 displays the results obtained from the analysis of the content of CO gas. There is a clear increasing trend with increase in CH4 gas pressure. Because the specimen storehouse was previously evacuated and there is no CO gas in the atmosphere, the change of CO gas can reflect the condensation of the active groups in coal (Hou et al., 2017). It is apparent from Figure 2 the very small range in cumulative CO content of all the coal samples. That is because no outburst of coal and CH4 gas occurred, and the active groups in coal were not exposed to oxygen. In an oxygen-free environment, CO gas can be generated from the loose coal loaded step-by-step. This is mostly because mechanical energy promotes the deformation of coal molecules during the dynamic destruction, resulting in changes in chemical structure and the production of CO (Xu et al., 2014; Yu et al., 2017). Because the evolution of microscopic functional groups is often accompanied by the formation of gaseous or liquid products (Ma et al., 2016), there is a significant positive correlation between the generation of CO and active groups.
Effect of Dynamic Destruction on Coal Surface
The surface physical parameters of coal samples that were damaged under different pressures are shown in Table 3, including the BET specific surface area and pore volume at different partial pressures P/P0. With successive increases in gas pressure, the increase in the BET specific surface area of low-metamorphic DT and HL coal samples was significantly less, compared to those of high-metamorphic PX and YQ coal samples. The pore volume showed a similar trend. Pore volume and surface area are important factors that affect heat and mass exchange during coal oxidation. Greater surface area and pore volume provide favorable places for the physicochemical adsorption of oxygen. This is more beneficial to the composite reaction of coal and oxygen.
Regression analysis was used to predict the BET specific area and pore volume as a function of pressure. It can be seen in Figure 3 that the BET specific area and pore volume of low-metamorphic DT and HL coal samples showed an approximately linear increasing trend with increasing pressure. However, the trends of high-metamorphic PX and YQ coal samples increased exponentially with pressure. What is more important is that, the correlation coefficients R of the fitted straight lines were all > 0.95, and all confidence probability P-values of curve fitting were less than 0.01, indicating statistically significant relationships of specific area and pore volume with pressure.
The differences in results for the DT and HL coal samples and the PX and YQ coal samples, may be related to the content of dry ash-free fixed carbon in coals with different grades of metamorphism (Jiang et al., 2002). The values of dry and ash-free fixed carbon of the YQ and PX coal samples were 84.54% and 75.42%, respectively, while those of the DT and HL coal samples were 50.38% and 54.43%, respectively. The brittleness of fixed carbon in coals with low grade of metamorphism makes it easier to destroy carbon under external high-pressure gas damage; thus, increasing the surface area and pore volume of coal. After the pressure increased to 3.0 MPa, the increments of BET surface area of the coal samples were 0.8902 m2/g (YQ), 1.5918 m2/g (PX), 6.2751 m2/g (DT), and 5.9269 m2/g (HL). Among the coal samples, the increment for PX coal was the most significant. This will provide a hotbed for low-temperature oxidation of coal, which can finally lead to coal spontaneous combustion.
Analysis of Dynamic Destruction affecting Radicals in Coal
As gas pressure increased, the area of the EPR spectrum has improved to a certain extent and its intensity was different with different metamorphic grades of coal (Fig. 4). The g factor represents the position when the external magnetic field H resonates with unpaired electron, which is one of the important parameters reflecting the molecular structure of the substance measured (Carr et al., 1995). The results obtained from the analysis of the g factor are presented in Table 4. It gradually decreased as the grade of coal metamorphism increased, which means that the orbit-spin coupling was weakened. This may be due to the decrease in the number of heterocyclic functional groups, and the increase in the aromatic structure of macro-molecules with increasing coal rank (Tadyszak et al., 2015). Therefore, it can be seen from the variation of the g factor that the coals with lower grade of metamorphism possess more heteroatoms, and the greater g factor of low-rank coal indicates stronger orbit spin coupling. Meanwhile, it is reflected that the g factor of low-metamorphic coal is higher than that of high-metamorphic coal. The linewidth reflects the interaction among the radicals, and/or between the radicals and the micro-crystalline structure of coal, i.e., energy exchange (Dack et al., 1985; Tadyszak et al., 2015). Generally, both the number of heteroatoms and types of radicals in coal decrease with increase of coal metamorphism. Therefore, the interaction between unpaired electrons increases and results in narrowing of the linewidth of the EPR spectra. Accordingly, the linewidth of the coal samples decreased gradually with increase in metamorphic grade. In terms of radical concentration in raw coal, it increases with the increase of metamorphic grade. It’s obvious that the concentration of original radicals in DT and HL lignite, is much lower than that of PX bitumite and YQ anthracite (Table 4).
It is apparent that the g values of the four coal samples varied inconspicuously to a certain range with increase in gas pressure (Fig. 5). This indicates that the impact of high-pressure gas can change few radical species, and the g value is consistent with that of coal at room temperature. For the DT and HL lignites, the g values remained relatively high level under the impact of gas pressure. With respect to coal rank, the g factor varied as follows: DT and HL coal > PX coal > YQ coal. Because there are more heteroatoms and oxygen-containing functional groups in low-rank coals, the g values of these radicals are relatively large; thus, the g values for the DT and HL coal samples are high. In contrast, the high-metamorphic PX and YQ coal samples contain lots of ordered aromatic radicals with low g values.
High-pressure gas affected the concentrations of radicals in coal (Fig. 6), which are different from g factor. Therefore, with increase in gas pressure, the concentrations of radicals in coal showed a gradual rising tendency. After being destroyed by gas pressure of 0.74 MPa, the radical concentrations of the DT and HL coal samples increased from the initial concentrations to 3.83 × 1017 spin/g and 4.44 × 1017 spin/g, respectively. These increases in concentrations of radicals, directly affirm that the chemical bonds in the coal were broken after the dynamic destruction. This unambiguously shows that mechanical action generates radicals, and it is direct evidence for chain scission. According to the Chinese regulation, the critical pressure value of coal-and-gas outburst is 0.74 MPa, the concentrations of radicals in the DT and HL coal samples after experiencing the critical pressure increased by 3.23% and 1.37%, respectively. Similarly, the PX and YQ coal samples showed increases of 7.26% and 4.69%, respectively. Among them, the radical concentration increment of the PX bituminous coal was higher than those of the others. When gas pressure is low, the change of radical concentration in the low-rank DT and HL coal sample was inconspicuous. As the pressure was increased further, the concentrations of radicals increased significantly, and the internal central bond was vulnerable to fracture, due to mechanical stress (Hou et al., 2017). However, the high-rank PX and YQ coal samples showed a large increase, when the pressure increased initially. When the surface of coal was destroyed by stretching, the pore diameter and surface area of coal surface increased, and the weak covalent bond was induced mechanically to homolysis to form radicals (Baláž & Dutková, 2009). After the pressure was increased to 3.0 MPa, the increments of radical concentrations of the DT, HL, PX, and YQ coal samples were 0.47 × 17 spin/g, 0.53 × 17 spin/g, 1.08 × 17 spin/g, and 0.88 × 1017 spin/g, respectively. Under the impact of coal-and-gas outburst, PX coal had the highest increase in absolute value of radical concentration. It may be that the PX coal sample, compared with the other coal samples, contained abundant weak bonds (fatty side chains and bridge bonds) that were easy to fracture.
Influence of Dynamic Failure on Surface Functional Groups of Coal
Figure 7 shows the wide-scan XPS spectra of raw coal samples. It can be seen that the coal samples were dominated by the characteristic peaks of O and C elements. In order to quantify the variation of functional groups in coal destroyed by high-pressure impact, the XPSPEAK 4.1 software was applied to peak fitting of the C 1 s spectra. Figure 8 shows the fitting process of the original C 1 s spectra of the four coal samples, in which the binding energies of four main functional groups were C–C/C–H (aromatic and aliphatic carbon) at about 284.8 eV, C–O (alcohol, phenol, or ether carbon) at about 285.5 eV, C=O (carbonyl carbon) at about 286.3 eV, and O=C–O (carboxyl carbon) at about 289.0 eV (Xi et al., 2019; Xia et al., 2018).
As shown in Figure 9a, the relative contents of C–C/C–H groups in the DT and HL coal samples fluctuated within a certain range with increase in gas pressure, whereas those of the PX and YQ coal samples show an overall gradual increasing trend. The mechanical stress of the dynamic impact induces easily the homolysis of the central bond C–C, because of the viscous flow and the mechanical stress on the central bond, which also lead to the increase of C–C/C–H groups (Beyer & Clausen-Schaumann, 2005). Figure 9b shows the changing trend of COOH groups with gas pressure of dynamic destruction. The relative percentage of COOH group on the coal surface showed a gradually increasing trend, which indicates that certain contents of carboxyl groups accumulated on the surface area of coal. In polymer solids, the molecule bonds break without oxygen and could form the vinyl (R-CH=CH2), vinylene (R2C=CH2), and methyl (R-CH3) endgroups; under atmospheric conditions, aldehyde (R-CHO) groups could also form (Zhurkov & Korsukov, 1974). The increase in relative contents of carboxyl groups means that part of the C–C/C-H groups were transformed into carboxyl groups under mechanical destruction or oxidation. Due to the inevitable contact with oxygen on the coal surface during the XPS test, a certain amount of active groups were oxidized quickly, which may have caused the consumption of the active C–C/C–H groups in coal. Therefore, it is reasonable to speculate that the content of C–C/C–H groups on the surface of coal increased with increase in gas pressure. Under the destruction of dynamic hazard, the content of active C–C/C–H group on coal surface increased, and it was consistent with the increase in radical concentration. The consumption of C–C/C–H group, and the accumulation of COOH group in the DT and HL coal samples were higher than those in the other coal samples, reflecting that low-rank coals have higher probability to oxidize, compared to high-metamorphic coals.
Structure Change and Initial Oxidizability of Coal after Dynamic Hazard
Mechano–chemistry is a branch of solid state chemistry in which weak chemical bonds between molecules are broken by mechanical forces, and this can cause chemical reactions and phase transitions (Beyer & Clausen-Schaumann, 2005; Davis et al., 2009; Friščić et al., 2020; Kaupp, 2009). Coal-and-gas outburst is a complex dynamic evolution process, which includes destruction of mechanical shearing and stretching (Wang et al., 2018). As shown in Figure 10, the physical and chemical changes took place during the dynamic rupture process. Macroscopically, the specific surface area of coal increased, and high-energy milling produced gas such as CO. The CO is mainly the product of ductile deformation (Wang et al., 2019), Microscopically, mechanical strain energy breaks the C–C covalent bond to form radicals. The mechano–chemical activation increases the surface area and lowers the coherent energy of coal, so that radicals could be generated easily (Peter Baláž, 2008). In general, mechanical forces reduce barriers to specific reactions whether single molecule reactions such as chain rupture or bimolecular reaction like radical conversion (Beyer & Clausen-Schaumann, 2005). The physical structure and chemical properties of the coal surface changed after the dynamic hazard. The breakage of old bonds and the formation of new ones liberated the CO. Compared with the original coal samples, more radicals and functional groups were present in the coal samples after the damage. Meanwhile, mechanical force decreases the barrier of active group reaction, and improves the possibility of oxidation. That is to say, the radicals detected by EPR spectroscopy indicate that coal has the ability to transform them into active radicals within a suitable environment. If they gather in large quantities and are exposed to the air, the safety risk of oxidizability will be increased.
To quantify further, the oxidation capacity on the coal surface, the index of oxygen absorption of coal at room temperature and normal pressure was used to measure its initial oxidation capacity. The oxygen absorption of coal with different metamorphic grades could be obtained from the experiments of spontaneous combustion tendency of coal. The oxygen absorption capacities of the raw DT, HL and PX and YQ coal samples were 1.14 cm3/g, 1.30 cm3/g, 0.59 cm3/g, and 0.74 cm3/g, respectively. The classification of coal spontaneous combustion tendency in the standard of "Oxygen Adsorption Identification Method with Chromatograph of Coal Spontaneous Combustion Tendency" is shown in Table 5. According to this classification standard, the raw DT and HL coal samples belong to the I type easy self-combustion coal, whereas the raw PX coal sample belongs to the II type normal self-combustion coal. Because the total sulfur of YQ coal sample was higher than 2.00%, and its dry ash-free base volatile matter ≤ 18%, it also belongs to the II type normal self-combustion coal.
The variations of oxygen absorption of coal after dynamic hazard are shown in Figure 11. The oxygen absorption of the low-rank DT and HL coal samples increased slowly with increase in pressure, while that of PX coal sample shows a nearly-exponential increasing trend. The variation range of the YQ anthracite sample was lower than that of the PX coal sample, but higher than those of the low-rank DT and HL coal samples. Because the volatile matter Vdaf of the DT, HL and PX coal samples was > 18%, only the oxygen absorption of the raw PX coal sample belongs to class II type normal self-combustion tendency, but after dynamic destruction, it belongs to I type easy self-combustion coal, indicating its greater tendency to be oxidized. The YQ coal sample belongs to volatile Vdaf ≤ 18%, and it showed I type self-combustion tendency, only after the outburst pressure reached 2.25 MPa. Because the oxygen adsorption of coal mainly occurs on its surface, the damage of dynamic hazard is mainly reflected on the surface of coal. Therefore, the surface area of the PX coal sample changed drastically compared to the other coal sample, which is consistent with the amount of oxygen absorption. This proves that the surface of coal was destroyed by the dynamic hazard, and the surface area and the possibility of oxygen adsorption of coal increased. Finally, the functional groups and radicals on the surface of coal were changed.
Conclusions
To model the process of coal-and-gas outburst, macro- and micro-experiments on coal after dynamic hazard were carried out. The variations of pore structure and active groups on coal surface were obtained. In addition, a quantitative approach is proposed to evaluate the risks of oxidizability on coal surface. The main conclusions are as follows.
With increase in loading pressure, the cumulative CO content in coal was detected, and it was enhanced within a small range with increase in gas pressure. After the pressure was increased to 3.0 MPa, the increments of BET surface areas of the DT, HL, PX, and YQ coal samples were 0.8902 m2/g, 1.5918 m2/g, 6.2751 m2/g, and 5.9269 m2/g, respectively. The BET specific surface area of coal increased, which provides a suitable site for the physical and chemical adsorption of oxygen.
The maximum increments of radical concentrations of the DT, HL, PX, and YQ coal samples were 0.47 × 17 spin/g ,0.53 × 17 spin/g, 1.08 × 17 spin/g, and 0.88 × 1017 spin/g, respectively. The resulting surface functional groups suggest that, with increase in gas pressure, the C–C/C–H groups in coal increased, and their absolute increments of radical concentrations increased as well.
The possible oxidizability of coal is characterized by the amount of oxygen absorbed by coal. Generally, the physicochemical characteristics of coal after the mechano–chemical effect, indicate the increasing capacity of coal oxidation. This may lead to increasing the risk of the surface area and the possibility of oxygen adsorption of coal.
References
Baláž, P., & Dutková, E. (2009). Fine milling in applied mechanochemistry. Minerals Engineering, 22(7–8), 681–694.
Baláž, P. (2008). Mechanochemistry in nanoscience and minerals. Springer.
Beyer, M. K., & Clausen-Schaumann, H. (2005). Mechanochemistry: The mechanical activation of covalent bonds. Chemical Reviews, 105(8), 2921–2948.
Bolm, C., & Hernández, J. G. (2019). Mechanochemistry of gaseous reactants. Angewandte Chemie (international Ed. in English), 58(11), 3285–3299.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of American Chemical Society, 60(2), 309–319.
Cao, D., Li, X., & Zhang, S. (2007). Influence of tectonic stress on coalification: Stress degradation mechanism and stress polycondensation mechanism. Science in China Series D: Earth Sciences, 50(1), 43–54.
Cao, Y., Davis, A., Liu, R., Liu, X., & Zhang, Y. (2003). The influence of tectonic deformation on some geochemical properties of coals—a possible indicator of outburst potential. International Journal of Coal Geology, 53, 69–79.
Cao, Y., Mitchell, G. D., Davis, A., & Wang, D. (2000). Deformation metamorphism of bituminous and anthracite coals from China. International Journal of Coal Geology, 43, 227–242.
Carr, R. M., Kumagai, H., Peake, B. M., Robinson, B. H., Clemens, A. H., & Matheson, T. W. (1995). Formation of free radicals during drying and oxidation of a lignite and a bituminous coal. Fuel, 74(3), 389–394.
Chu, T., Li, P., & Chen, Y. (2019). Risk assessment of gas control and spontaneous combustion of coal under gas drainage of an upper tunnel. International Journal of Mining Science and Technology, 29(3), 491–498.
Dack, S. W., Hobday, M. D., Smith, T. D., & Pilbrow, J. R. (1985). E.p.r study of organic free radicals in Victorian brown coal. Fuel, 64(2), 219–221.
Davis, D. A., Hamilton, A., Yang, J., Cremar, L. D., Van Gough, D., Potisek, S. L., et al. (2009). Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature, 459(7243), 68–72.
Friščić, T., Mottillo, C., & Titi, H. M. (2020). Mechanochemistry for synthesis. Angewandte Chemie (international Ed. in English), 59(3), 1018–1029.
Ge, T., Zhang, M., & Cai, C. (2014). XPS analysis of surface texture of coking coal in Fenxi County. Asian Journal of Chemistry, 26(6), 1741–1744.
Grzybek, T., Pietrzak, R., & Wachowska, H. (2004). The comparison of oxygen and sulfur species formed by coal oxidation with O-2/Na2CO3 or peroxyacetic acid solution. XPS Studies. Energy & Fuels, 18(3), 804–809.
Hou, Q., Han, Y., Wang, J., Dong, Y., & Pan, J. (2017). The impacts of stress on the chemical structure of coals: A mini-review based on the recent development of mechanochemistry. Science Bulletin, 62(13), 965–970.
Hu, X. C., Yang, S. Q., Liu, W. V., Zhou, X. H., Sun, J. W., & Yu, H. (2017). A methane emission control strategy in the initial mining range at a spontaneous combustion-prone longwall face: A case study in coal 15, Shigang Mine, China. Journal of Natural Gas Science and Engineering, 38, 504–515.
Jiang, X. M., Zheng, C. G., Yan, C., Liu, D. C., Qiu, J. R., & Li, J. B. (2002). Physical structure and combustion properties of super fine pulverized coal particle. Fuel, 81(6), 793–797.
Kaupp, G. (2009). Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm, 11(3), 388–403.
Kitamura, M., Mukoyoshi, H., Fulton, P. M., & Hirose, T. (2012). Coal maturation by frictional heat during rapid fault slip. Geophysical Research Letters, 39(16), L16302.
Kong, B., Wang, E., Lu, W., & Li, Z. (2019). Application of electromagnetic radiation detection in high-temperature anomalous areas experiencing coalfield fires. Energy, 189, 116144.
Li, J., Li, Z., Yang, Y., Wang, C., & Sun, L. (2018). Experimental study on the effect of mechanochemistry on coal spontaneous combustion. Powder Technology, 339, 102–110.
Li, X., Ju, Y., Hou, Q., & Lin, H. (2012). Spectra response from macromolecular structure evolution of tectonically deformed coal of different deformation mechanisms. Science China Earth Sciences, 55(8), 1269–1279.
Ma, L., Wang, D., Wang, Y., Xin, H., Dou, G., & Xu, C. (2016). Experimental investigation on a sustained release type of inhibitor for retarding the spontaneous combustion of coal. Energy & Fuels, 30(11), 8904–8914.
Mathews, J. P., & Chaffee, A. L. (2012). The molecular representations of coal—A review. Fuel, 96, 1–14.
Qi, X., Chen, L., Xin, H., Ji, Y., Bai, C., Song, R., et al. (2018). Reaction mechanism and thermodynamic properties of aliphatic hydrocarbon groups during coal self-heating. Energy & Fuels, 32(10), 10469–10477.
Qin, B. T., Li, L., Ma, D., Lu, Y., Zhong, X. X., & Jia, Y. W. (2016). Control technology for the avoidance of the simultaneous occurrence of a methane explosion and spontaneous coal combustion in a coal mine: A case study. Process Safety and Environmental Protection, 103, 203–211.
Seehra, M. S., Ghosh, B., Zondlo, J. W., & Mintz, E. A. (1988). Relationship of coal extraction with free radicals and coal macerals. Fuel Processing Technology, 18(n), 279–286.
Song, Y., Jiang, B., & Han, Y. (2018). Macromolecular response to tectonic deformation in low-rank tectonically deformed coals (TDCs). Fuel, 219, 279–287.
Tadyszak, K., Augustyniak-Jabłokow, M. A., Więckowski, A. B., Najder-Kozdrowska, L., Strzelczyk, R., & Andrzejewski, B. (2015). Origin of electron paramagnetic resonance signal in anthracite. Carbon, 94, 53–59.
Tang, Y., & Wang, H. (2018). Development of a novel bentonite-acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technology, 323, 486–494.
Wang, C., Yang, S., Li, X., Jiang, C., & Li, M. (2018). Study on the failure characteristics of concrete specimen under confining pressure. Arabian Journal for Science and Engineering, 44(5), 4119–4129.
Wang, J., Guo, G.-J., Han, Y., Hou, Q., Geng, M., & Zhang, Z. (2019). Mechanolysis mechanisms of the fused aromatic rings of anthracite coal under shear stress. Fuel, 253, 1247–1255.
Xi, X., Jiang, S., Zhang, W., Wang, K., Shao, H., & Wu, Z. (2019). An experimental study on the effect of ionic liquids on the structure and wetting characteristics of coal. Fuel, 244, 176–183.
Xia, W., Li, Y., & Niu, C. (2018). Effects of high-temperature oxygen-deficient oxidation on the surface properties of sub-bituminous coal. Energy Sources, Part a: Recovery, Utilization, and Environmental Effects, 41(9), 1110–1115.
Xu, C., Zhou, G., & Qiu, H. (2017). Analysis of the microscopic mechanism of coal wettability evolution in different metamorphic states based on NMR and XPS experiments. RSC Advances, 7(76), 47954–47965.
Xu, R., Li, H., Guo, C., & Hou, Q. (2014). The mechanisms of gas generation during coal deformation: Preliminary observations. Fuel, 117, 326–330.
Yan, F., Xu, J., Peng, S., Zou, Q., Li, Q., Long, K., & Zhao, Z. (2020). Effect of capacitance on physicochemical evolution characteristics of bituminous coal treated by high-voltage electric pulses. Powder Technology, 367, 47–55.
Yang, Y., Pan, J., Wang, K., & Hou, Q. (2020). Macromolecular structural response of Wender coal under tensile stress via molecular dynamics. Fuel, 265, 116938.
Yu, M., Xie, J., & Jia, H. (2017). Release laws of CO produced by coal structure destruction under mechanical force and modification method of the index for predicting coal spontaneous combustion. Journal of China University of Mining and Technology, 46(4), 762–768.
Zhang, L., Li, Z., He, W., Li, J., Qi, X., Zhu, J., et al. (2018). Study on the change of organic sulfur forms in coal during low-temperature oxidation process. Fuel, 222, 350–361.
Zhou, B., Yang, S., Wang, C., Hu, X., Song, W., Cai, J., et al. (2020). The characterization of free radical reaction in coal low-temperature oxidation with different oxygen concentration. Fuel, 262, 116524.
Zhou, G., Xu, C., Cheng, W., Zhang, Q., & Nie, W. (2015). Effects of oxygen element and oxygen-containing functional groups on surface wettability of coal dust with various metamorphic degrees based on XPS experiment. Journal of Analytical Methods in Chemistry, 2015, 467242.
Zhurkov, S. K., & Korsukov, V. E. (1974). Atomic mechanism of fracture of solid polymers. Journal of Polymer Science: Polymer Physics Edition, 12, 385–398.
Acknowledgments
This research was supported by the National Key R&D Program of China (2018YFC0807900) and “Double First Rate” Independent Innovation Project of CUMT (2018ZZCX05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, J., Yang, S., Zheng, W. et al. Risk Assessment of Oxidizability of Coal after Dynamic Hazard and Its Effect on Functional Groups and Radicals. Nat Resour Res 30, 4533–4545 (2021). https://doi.org/10.1007/s11053-021-09941-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-021-09941-2