Abstract
This study presents the synthesis of ZnSe nanoparticles (NPs) via precursor solution method; preparation of perovskite and ZnSe:perovskite composites (0, 2, 4, and 6 mg/ml, respectively) via solution processing method. The synthesis of these materials has been confirmed by X-ray diffraction/XRD, UV–Visible spectroscopy, steady-state photoluminescence (PL) spectroscopy, time-resolved photoluminescence (TRPL) spectroscopy, contact angle analysis and scanning electron microscopy/SEM. As-synthesized ZnSe:perovskite composites have been deployed in the fabrication of solar cells. SEM micrographs of these composites have shown a significant reduction in extrinsic defects of perovskite films on incorporation of ZnSe NPs into perovskites matrices indicating improvement in morphology. The contact angle analysis confirmed a significant decrease in the hydrophilicity as contact angle is increased by ~ 10° as compared to that of pristine perovskite. Moreover, TRPL spectroscopy confirmed a modest increase in the lifetimes and hence, reduction in the recombination rate within ZnSe:perovskite composites. Likewise, J-V curve of as-fabricated devices demonstrates an improvement in the performance with the increase in the concentration of ZnSe NPs into perovskite. The best device based on these composites has shown an efficiency of 5.98%, open circuit voltage/Voc of 0.931 V, short circuit current density/JSC of 9.82 mA/cm2 and fill factor/FF of 65.50% which has retained 79.6% of its actual efficiency after 30 days of air exposure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The introduction of perovskites to photovoltaics skyrocketed the efficiency within a short duration owing to their brilliant optical, electrical properties, easy, inexpensive, and tunable synthesis routes [1]. Therefore, perovskites have drawn attention of researchers in optoelectronic applications, particularly in photovoltaics [2]. Perovskites have opened the door for the next generation cost-effective and highly efficient photovoltaic technology [3]. The first perovskite solar cells/PSCs were introduced in 2009; since then, different research groups have been working on perovskite materials and their applications in photovoltaics [4,5,6]. Over a span of few years, PSCs have achieved 25.7% PCE and still there is big scope for the improvements of these devices [6].
The early PSCs were based on thin films of perovskites, which were deposited via different methods namely spin coating, sequential deposition of the perovskite solution and thermal evaporation [7]. Such devices have shown significant performance irrespective of few limitations like formation of defect states, extrinsic defects/grain boundaries, purity of materials and quality of films which severely affect device performance [8]. Besides good efficiency, the less environmental stability and hysteresis loss in PSCs are the big concerns [9]. Lead based perovskites (CH3NH3PbX3, where X = I, Cl, Br) are commonly used to fabricate PSCs, however, they may possess extrinsic defects (larger voids/pinholes) and toxicity [10]. These extrinsic defects can produce shunt-leakage path by connecting electron transport layer/ETL and hole transport layer/HTL directly resulting in a decrease in efficiency and stability of PSCs [11, 12].
In this context, optimization of perovskite absorber layer and use of inorganic charge transport layers may lead to the enhancement in the performance of PSCs [13, 14]. As perovskite are susceptible to external moisture and oxygen which leads to direct oxidation of absorber layer resulting in reduction in absorbance and hence degradation of device [15]. Therefore, it has become necessary to protect perovskite absorber layers from external moisture and oxygen. This problem of less stability may be overcome with optimization of absorber layer as well as charge transport layers [16]. In this regard, metal oxides, metal chalcogenides such as ZnS, ZnSe, CdS, CuInS2, TiO2, ZnO, NiOx, and SnO2, etc. may be beneficial as they have versatile properties [17,18,19,20,21,22,23]. Some polymers such as Polymethyl Methacrylate/PMMA, etc. may be applied as an anti-reflecting material which form an inter-cross-linked thin layer to protect photovoltaic devices from moisture and oxygen; hence, play an important role in passivation of surface trapping and suppression of hysteresis loss in devices [24, 25]. Therefore, the photovoltaic performance of PSCs may be improved by proper use or optimization of absorber layer and charge transport layer materials, surface/solvent engineering, and better device architectures [18, 26, 27].
In this study, we have focused on optimization of absorber layer and prepared ZnSe-perovskite composites (ZnSe:CH3NH3PbI3; 2, 4, and 6 mg/ml). Synthesis of these composites has been confirmed by XRD, UV–Visible spectroscopy, PL/TRPL spectroscopy, Contact angle analysis and SEM. SEM micrographs revealed significant decrease in extrinsic defects of perovskite films with the increase in the concentration of ZnSe into perovskite matrices. This decrease has clearly indicated an improvement in morphology resulting reduction in the extrinsic defects/grain boundary problem. The contact angle analysis showed a substantial decrease in the hydrophilicity as contact angle is increased by ~ 10° in comparison to that of pristine perovskite. Also, TRPL spectroscopy confirmed a small but gradual increase in the lifetimes indicating a reduction in the recombination rate within ZnSe:perovskite composites. Furthermore, as-fabricated composite materials have been deployed in the fabrication of solar cells. As-fabricated solar cells have demonstrated improved performance and have shown an efficiency of 5.98%, Voc of 0.931 V, JSC of 9.82 mA/cm2 and FF of 65.50%. Finally, the stability performance of as-fabricated solar cells has been studied. The best performing solar device retained ~ 79.6% of its actual efficiency even after 30 days exposure under ambient atmosphere.
Methodology
Synthesis of ZnSe nanoparticles/NPs
Initially, polyvinyl pyrrolidine/PVP (2 g) was dissolved into deionized water/DI (70 ml) and solution was stirred for 2 h at 70 °C. In the next step, selenium powder (0.4 mmol) was dissolved into ethylenediamine (80 ml) to get selenium ethylenediamine. Furthermore, solution of zinc nitrate (Zn(NO3)2) and selenium ethylenediamine was prepared under constant stirring and added to the PVP solution to obtain a homogeneous solution. Finally, solution was dried at 80 °C for 24 h and as-obtained powder was crushed and calcined at ~ 700 °C in a tube furnace for 90 min (under N2 atmosphere) [28].
Synthesis of CH3NH3PbI3 and ZnSe:CH3NH3PbI3 composites
Initially, Hydriodic acid/HI (dropwise) was added into methyl amine and solution was kept under stirring for 2–3 h at 0 °C (using ice-bath). Further, solution was dried at 60 °C for overnight to get precipitated and washed multiple times with di ethyl ether and ethyl alcohol. In the next step, precipitate annealed at 60 °C for overnight to get rid of impurities. Finally, white powder was obtained which indicated synthesis of methyl ammonium iodide/MAI [29].
Furthermore, 1 M of MAI and Lead Iodide/PbI2 were taken into N-N dimethyl pyrrolidone/NMP and stirred for 12 h at 60 °C to prepare Methyl ammonium lead Iodide/MALI solution (one step method) [30]. Similarly, ZnSe:CH3NH3PbI3 composites (2, 4, and 6 mg/ml) were prepared except the addition of different concentrations of ZnSe NPs during the preparation of composite solutions.
Fabrication of perovskite solar cells
The fabrication of solar cell was done in multiple steps; firstly, Fluorine doped Tin Oxide/FTO glass substrates were etched out and washed with soap, distilled water, and ethyl alcohol, and dried in the oven. In the next step, NiOX HTL was deposited onto FTO substrate and annealed at 110 °C for 2–3 min. Furthermore, photoactive layer of Methyl Ammonium Lead Iodide (CH3NH3PbI3) was deposited and annealed at 100 °C for 5 min. After this, ZnO ETL was deposited and annealed at 100 °C for 2–3 min. In the last step, bottom contact of silver/Ag metal was thermally deposited onto this substrate [31, 32]. Similar steps were followed to fabricate the ZnSe:CH3NH3PbI3 composites based devices except the deposition of ZnSe:CH3NH3PbI3 absorber layers instead of pristine CH3NH3PbI3 absorber layer.
Characterization techniques
All the prepared materials such as ZnSe NPs, MAI, MALI, MALI-Perovskite composites are confirmed using X-Ray Diffractometer (Rigaku SmartLab), UV–Visible Spectrophotometer (Perkin Elmer UV/Vis Lambda 365), Photoluminescence (PL) Spectrophotometer (Shimadzu RF-5301 PC Spectrofluorophotometer), Scanning Electron Microscope (FEI Nova Nanosem 450). Furthermore, as-fabricated solar devices based on ZnSe-perovskite composites are tested using Keithley Sourcemeter (2450 Sourcemeter) and PET Solar simulator (SS50AAA).
Results and discussion
Figure 1 (SEM micrograph of ZnSe) displays the nanoscale morphology (average particle size; 51 nm) of as-prepared ZnSe nanoparticles/NPs. Further, XRD pattern (Fig. 2) confirms the synthesis of ZnSe NPs and suggests the presence of (110), (220), (311), (400), (102), and (203) crystal planes [JCPDS-79–2205] [28]. Moreover, UV visible spectroscopy was used to record the absorbance–wavelength spectrum (Fig. 3). The absorbance spectrum shows a peak corresponding to 280 nm, further Tauc Plot was used to determine bandgap of ZnSe NPs (Fig. 4) which was found to be 4.43 eV which is an agreement with the reported results [33]. In the next step, we performed Photoluminescence (PL) spectroscopy to study the emission spectrum of ZnSe NPs. The (PL) emission spectrum (Fig. 5) shows a peak at 407 nm, whereas the peak at ~ 500 nm can be attributed to the trap states [34]. Therefore, the SEM micrographs, XRD patterns, (UV–Visible) absorbance spectrum, and (PL) emission spectrum of ZnSe evidently confirm the synthesis of ZnSe NPs. Furthermore, as-synthesized ZnSe NPs have been used to prepare different ZnSe-CH3NH3PbI3 composites which are deployed as absorber layers in the fabrication of peroskite solar cells/PSCs.
Figure 6 (a, b, c, and d) displays SEM micrographs of four different perovskite-ZnSe composites based absorber layers deployed in as-fabricated solar cells. Furthermore, using ImageJ (https://imagej.nih.gov/ij/) software, the grain size of perovskite-ZnSe composites based absorber layers are calculated. The average grain size of ZnSe:CH3NH3PbI3 composite (0, 2, 4, and 6 mg/ml, respectively) films are found to be increasing with the increase of ZnSe NPs into perovskite matrices (Fig. 6) which are 263, 738, 984, and 1364 nm, respectively. This increase in grain size may be owing to the prolonged crystallization/aggregation of perovskite film in the presence of ZnSe NPs [34] and due to the larger dipole moment (42 D) of ZnSe NPs in comparison to that of CH3NH3 (2.2 D) [35, 36]. ZnSe NPs have improved morphology of ZnSe:perovskite composite films by filling pinholes/voids. As morphology of films depends on coverage ratio, crystallinity, and crystal size, therefore, larger grain size of perovskite composite films increases optical performance resulting increase in the performance of the PSCs.
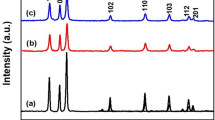
Figure 7 demonstrates XRD patterns of perovskite:ZnSe composites which clearly shows the role of the incorporation of ZnSe NPs into perovskite matrices. XRD patterns of CH3NH3PbI3:ZnSe composites (0, 2, 4, and 6 mg/ml, respectively) consist of CH3NH3PbI3 diffraction peaks corresponding to (110), (220), (202), (310), (400) and (404) planes [37, 38]. The positions of CH3NH3PbI3 diffraction peaks are unchanged even after incorporation of ZnSe NPs into perovskite matrices indicating that MALI has well-preserved crystal structure. Figure 7 does not show the diffraction peaks corresponding to ZnSe NPs which is probably due to the less amount of ZnSe NPs present in the films. Therefore, it is quite difficult to detect the diffraction peaks of ZnSe NPs present in CH3NH3PbI3:ZnSe composite films than that of the crystallite sites of CH3NH3PbI3 (Fig. 7). Furthermore, Table 1 shows a comparative study on FWHM (full width of half maximum) and diffraction peak intensities correspond to (110) and (220) diffraction peaks. It is obvious from Table 1 that the diffraction peak intensities increase with the increase in the concentration of ZnSe NPs into CH3NH3PbI3:ZnSe composite films resulting improvement in the morphology. Hence, the crystallinity of the perovskite phase has been improved drastically. Conversely, FWHM of diffraction peaks has been seen decreasing with the increase in the concentration of ZnSe NPs indicating the formation of larger crystalline domains of perovskite present in CH3NH3PbI3:ZnSe composites. The crystalline domain sizes (calculated from the Scherrer equation) of CH3NH3PbI3:ZnSe composites (0, 2, 4, and 6 mg/ml) are found to be 4.66 Å, 5.15 Å, 5.57 Å and 6.78 Å, respectively.
UV–Visible spectroscopic technique (Fig. 8) is used to record optical absorbance of perovskite:ZnSe composites in the range of 400–800 nm. Figure 8 has shown that the incorporation of ZnSe NPs enhances the optical absorbance of the perovskite (composite) films. It is also clear from literature that ZnSe NPs absorb in the range less than 400 nm [38,39,40,41]. Therefore, higher absorbance of ZnSe:CH3NH3PbI3 composite films does not correspond to the absorbance of ZnSe NPs in the range of 400–800 nm, however, ZnSe NPs show high reflectance in this range [40]. Moreover, ZnSe NPs present in ZnSe:CH3NH3PbI3 composite films act as the network of reflection centers in the range 400–800 nm which further increases the light trapping in ZnSe:CH3NH3PbI3 composite films. Therefore, enhancement in the light trapping boosts the probability of light absorption by the perovskite and hence increases the absorbance of perovskite:ZnSe composite films [40].
The photo-physics of absorber layers has been studied using PL (emission) spectra of FTO/CH3NH3PbI3:ZnSe (0, 2, 4, 6 mg/ml, respectively) composite films (Fig. 9) which are peaked at 762 nm (Fig. 9) indicating that the optical bandgaps of the multilayers are being controlled by CH3NH3PbI3 layer [41]. An obvious quenching is observed for each composite films (Fig. 9) owing to the introduction of ZnSe. The maximum PL quenching is observed for the highest concentrated (6 mg/ml) perovskite:ZnSe composite film by improving morphology of perovskite:ZnSe composite films by forming larger grains which reduces extrinsic defects by filling the pinholes/voids. Mahmoudi et al. also reported that perovskite/NiO-graphene composites filled pinholes and voids of the perovskite films and hence improvement in morphology of perovskite films which further reduced charge recombination at interfaces. This reduction in charge recombination at interfaces increased overall performance of PSCs [42]. This study also shows reduction in extrinsic defects which indicates improvement in morphology of perovskite:ZnSe composite films by reducing the probability of charge recombination at the interfaces [43]. Hence, the drastic PL quenching in FTO/CH3NH3PbI3:ZnSe composite (2, 4, and 6 mg/ml, respectively) films is observed in comparison to that of pristine CH3NH3PbI3. This increase in the PL quenching with the increase in the concentration of ZnSe NPs into the perovskite NPs further indicates the increased charge recombination in photoactive layers owing to the aggregation of ZnSe NPs in CH3NH3PbI3:ZnSe composite films providing the required sites for charge recombination.
Figure 10 depicts an important physical property of the as-fabricated CH3NH3PbI3:ZnSe composites i.e., contact angle analysis which shows small contact angle and high surface wettability [36]. However, the incorporation of ZnSe NPs into perovskites reduces the hydrophilicity of the perovskite-ZnSe composites [44]. The results clearly demonstrate that the contact angle increases significantly as concentration of ZnSe NPs into perovskite matrices increases, and comes to be 33.92°, 35.03°, 40.31°, and 43.52° for perovskite-ZnSe composites (0, 2, 4, and 6 mg/ml, respectively). It is obvious from the obtained results that the pristine perovskite has a very high hydrophilicity [45]; nevertheless, this hydrophilicity considerably decreases with an increase in ZnSe NPs concentration into perovskite, indicating an improvement in environmental stability. Moreover, Fig. 11 (TRPL spectroscopy) revealed the charge carrier dynamics of perovskite-ZnSe composites. It is evident from Fig. 11 that the average lifetime of pure perovskite (deposited onto FTO substrate) is determined to be 21.49 ns; however, in case of FTO/NiOx/perovskite lifetime was found to be 21.115 ns. It is also found that the average lifetimes of perovskite-ZnSe composites (2, 4, and 6 mg/ml) are 21.206, 21.310, and 21.412 ns, respectively. The lifetimes of perovskite-ZnSe composites have shown a modest increase with the increase in the ZnSe NPs concentration into perovskite which may be due to the low concentration of ZnSe NPs into perovskite and carrier recombination process/charge carrier trapping at perovskite-NiOx interface as charge carriers are not transferred to glass substrate easily [46, 47]. However, from these findings it is obvious that the recombination rate decreased as ZnSe NPs concentration in perovskite increased, leading to an increase in the separation rate and, thus, an increase in charge carrier mobility [46, 47] (Fig. 12).
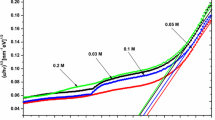
Figure 13 demonstrates J-V characteristics of the solar devices having the structures FTO/NiOx/CH3NH3PbI3:ZnSe (0, 2, 4, and 6 mg/ml, respectively). The schematic diagram as well as energy level diagram of as-fabricated solar devices are shown in Fig. 12 (a), and (b), respectively. Furthermore, Table 2 demonstrates photovoltaic parameters such as VOC, Jsc, PCE, FF, Series Resistance/Rs and Shunt Resistance/Rsh for as-fabricated devices. Table 2 also reveals that all the photovoltaic parameters (except Rsh) are found to be increasing with the increase in the concentration of ZnSe NPs into perovskite matrices owing to the improved optical properties and morphology [43]. The incorporation of ZnSe NPs facilitates crystallization and growth of bigger perovskite grains resulting in filling of the pinholes/voids by effectively reducing the charge recombination or charge trapping at the grain boundaries. This reduction in charge recombination helps in easy movement of charges towards the electrodes resulting increase in the Jsc of the devices. Moreover, larger grain size of the perovskite:ZnSe composites reduces series resistance/Rs and increases fill factor which is due to the less hindrance experienced by the charges [43]. The decrease in pinholes/voids and cracks increases the value of shunt resistance/Rsh [11, 12]. The conductivity and electron mobility are also improved with the increase in the concentration of ZnSe NPs into perovskite:ZnSe composite films by increasing Jsc and decreasing Rs of as-fabricated devices [11, 12].
Figure 14 displays stability responses of as-fabricated solar devices; the best device retained 79.6% of its original efficiency even after 30 days under ambient atmosphere. Whereas solar devices possessing pristine absorber layer has retained just 20.7% of the original efficiency after the same period of 30 days. Moreover, it has been supported by the literature that the use of inorganic charge transport, i.e., ZnO and NiOx enhances the stability of PSCs [13, 14]. In this work, we have deployed ZnO as ETL and NiOx as HTL (Fig. 12); this is because of the low stability of the PCBM (ETL) and PEDOT:PSS (HTL) based PSCs owing to the hygroscopic and acidic natures of PCBM and PEDOT:PSS [13]. Moreover, use of PCBM leads to the diffusion of electrode materials into the PCBM layer up to 10 nm during vacuum evaporation [48]. This diffusion of PCBM into electrode increases the probability of the exposure of electrode material with perovskite and hence increases the risk of chemical reaction between electrode and perovskite films under humid conditions which may trigger faster device degradation. In this context, inorganic charge transport layers such as highly air stable ZnO, and NiOx could be the good options. ZnO entirely separates the perovskite with electrode material [13] due to its scavenging effect [49, 50]. Conversely, highly stable NiOx may replace hydrophilic and acidic PEDOT:PSS [51, 52]. Therefore, use of ZnO and NiOx as ETL, and HTL, respectively improves the stability of PSCs. Likewise, incorporation of ZnSe NPs into perovskite matrices improves morphology of as-fabricated solar devices by filling pinholes/voids. Pinholes/voids leads to direct oxidation absorber layers resulting in rapid degradation of absorber layer [53,54,55,56]. Therefore, filling of pinholes/voids is important for the fabrication of stable and efficient solar cells. In this context, both the strategies (use of inorganic charge transport layers and incorporation of ZnSe NPs into perovskite) which were adopted by us have played a significant role which is further justified with the available literature [49, 50, 53,54,55,56].
Conclusion
The incorporation of ZnSe NPs into perovskite matrices significantly enhanced overall performance of perovskite:ZnSe (CH3NH3PbI3:ZnSe; 0, 2, 4, and 6 mg/ml, respectively) composites based solar devices. Furthermore, such fabricated perovskite solar cells/PSCs demonstrated that employment of CH3NH3PbI3:ZnSe photoactive layers improved visible absorbance, and increased grain sizes and hence reduction in extrinsic defects of CH3NH3PbI3 films which further increased performance of PSCs. Moreover, highly stable inorganic carrier transport layers also played an important role in performance increase. As-fabricated PSCs have shown optimized performance; the best device has shown an efficiency of 5.98% and retained 79.6% of the original value even after 30 days under ambient atmosphere.
Data availability
All data generated or analyzed during present study are included in this manuscript [No supplementary files].
References
Rong SS, Faheem MB, Li YB (2021) Perovskite single crystals: synthesis, properties, and applications. J Electron Sci Technol 19(2):100081
Zhang J, Shum PP, Su L (2022) A review of geometry-confined perovskite morphologies: from synthesis to efficient optoelectronic applications. Nano Res 1–30
Green MA, Ho-Baillie A (2017) Perovskite solar cells: the birth of a new era in photovoltaics. ACS Energy Lett 2(4):822–830
Kojima A, Teshima K, Shirai Y, Miyasaka T (2009) Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc 131:6050–6051
Mujahid M, Chen C, Zhang J, Li C, Duan Y (2021) Recent advances in semitransparent perovskite solar cells. InfoMat 3(1):101–124
National Renewable Energy Laboratory, https://www.nrel.gov/pv/cell-efficiency.html, accessed June 2022
Abbas M, Zeng L, Guo F, Rauf M, Yuan XC, Cai B (2020) A critical review on crystal growth techniques for scalable deposition of photovoltaic perovskite thin films. Materials 13(21):4851
Mehdizadeh-Rad H, Mehdizadeh-Rad F, Zhu F, Singh J (2021) Heat mitigation in perovskite solar cells: the role of grain boundaries. Sol Energy Mater Sol Cells 220:110837
Wu F, Pathak R, Qiao Q (2021) Origin and alleviation of JV hysteresis in perovskite solar cells: a short review. Catal Today 374:86–101
Chen Y, Yang X, Liu P, Wang W, Ran R, Zhou W, Shao Z (2021) Improving moisture/thermal stability and efficiency of CH3NH3PbI3-based perovskite solar cells via gentle butyl acrylate additive strategy. Solar RRL 5(3):2000621
Lin L, Jones TW, Yang TCJ, Duffy NW, Li J, Zhao L, Chi B, Wang X, Wilson GJ (2021) Inorganic electron transport materials in perovskite solar cells. Adv Func Mater 31(5):2008300
Elsmani MI, Fatima N, Jallorina MPA, Sepeai S, Su’ait MS, Ahmad Ludin N, Mat Teridi MA, Sopian K, Ibrahim MA (2021) Recent issues and configuration factors in perovskite-silicon tandem solar cells towards large scaling production. Nanomaterials 11(12):3186
Yang J, Xu J, Zhang Q, Xue Z, Liu H, Qin R, Zhai H, Yuan M (2020) An efficient and stable inverted perovskite solar cell involving inorganic charge transport layers without a high temperature procedure. RSC Adv 10(32):18608–18613
Tara A, Bharti V, Sharma S, Gupta R (2022) Computational approach to explore suitable charge transport layers for all inorganic CsGeI3 perovskite solar cells. Opt Mater 128:112403
Sutherland LJ, Weerasinghe HC, Simon GP (2021) A review on emerging barrier materials and encapsulation strategies for flexible perovskite and organic photovoltaics. Adv Energy Mater 11(34):2101383
Sebastian V, Kurian J (2021) Simulation and optimization studies on CsPbI3 based inorganic perovskite solar cells. Sol Energy 221:99–108
Chen M, Wang J, Yin F, Du Z, Belfiore LA, Tang J (2021) Strategically integrating quantum dots into organic and perovskite solar cells. J Mater Chem A 9(8):4505–4527
Jin M, Li H, Lou Q, Du Q, Huang Q, Shen Z, Li F, Chen C (2022) Toward high-efficiency stable 2D/3D perovskite solar cells by incorporating multifunctional CNT:TiO2 additives into 3D perovskite layer. EcoMat 4(2):e12166
Valadi K, Gharibi S, Taheri-Ledari R, Akin S, Maleki A, Shalan AE (2021) Metal oxide electron transport materials for perovskite solar cells: a review. Environ Chem Lett 19(3):2185–2207
Chen C, Zhai Y, Li F, Tan F, Yue G, Zhang W, Wang M (2017) High efficiency CH3NH3PbI3:CdS perovskite solar cells with CuInS2 as the hole transporting layer. J Power Sources 341:396–403
Guo M, Li F, Ling L, Chen C (2017) Electrochemical and atomic force microscopy investigations of the effect of CdS on the local electrical properties of CH3NH3PbI3:CdS perovskite solar cells. J Mater Chem C 5(46):12112–12120
Zheng J, Zhu L, Shen Z, Li F, Ling L, Li H, Chen C (2022) Effects of the incorporation amounts of CdS and Cd(SCN2H4)2Cl2 on the performance of perovskite solar cells. Int J Miner Metall Mater 29(2):283–291
Zhu L, Chen C, Li F, Shen Z, Weng Y, Huang Q, Wang M (2019) Enhancing the efficiency and stability of perovskite solar cells by incorporating CdS and Cd(SCN2H4)2Cl2 into the CH3NH3PbI3 active layer. J Mater Chem A 7(3):1124–1137
Kim H, Lee KS, Paik MJ, Lee DY, Lee SU, Choi E, Yun JS, Seok SI (2022) Polymethyl methacrylate as an interlayer between the halide perovskite and copper phthalocyanine layers for stable and efficient perovskite solar cells. Adv Funct Mater 32(13):2110473
Zhao Y, Heumueller T, Zhang J, Luo J, Kasian O, Langner S, Kupfer C, Liu B, Zhong Y, Elia J, Osvet A, A. (2022) A bilayer conducting polymer structure for planar perovskite solar cells with over 1,400 hours operational stability at elevated temperatures. Nat. Energy 7(2):144–152
Cho SH, Byeon J, Jeong K, Hwang J, Lee H, Jang J, Lee J, Kim T, Kim K, Choi M, Lee YS (2021) Investigation of defect-tolerant perovskite solar cells with long-term stability via controlling the self-doping effect. Adv Energy Mater 11(17):2100555
Altinkaya C, Aydin E, Ugur E, Isikgor FH, Subbiah AS, De Bastiani M, Liu J, Babayigit A, Allen TG, Laquai F, Yildiz A (2021) Tin oxide electron-selective layers for efficient, stable, and scalable perovskite solar cells. Adv Mater 33(15):2005504
Salem A, Saion E, Al-Hada NM, Kamari HM, Shaari AH, Radiman S (2017) Simple synthesis of ZnSe nanoparticles by thermal treatment and their characterization. Results Phys 7:1175–1180
Etgar L, Gao P, Xue Z, Peng Q, Chandiran AK, Liu B, Nazeeruddin MK, Gratzel M (2012) Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J Am Chem Soc 134(42):17396–17399
Dang Y, Liu Y, Sun Y, Yuan D, Liu X, Lu W, Liu G, Xia H, Tao X (2015) Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm 17(3):665–670
Liu Z, He T, Wang H, Song X, Liu H, Yang J, Liu K, Ma KH (2017) Improving the stability of the perovskite solar cells by V2O5 modified transport layer film. RSC Adv 7(30):18456–18465
Tian S, Li J, Li S, Bu T, Mo Y, Wang S, Li W, Huang F (2019) A facile green solvent engineering for up-scaling perovskite solar cell modules. Sol Energy 183:386–391
Senthilkumar K, Kalaivani T, Kanagesan S, Balasubramanian V, Balakrishnan J (2013) Wurtzite ZnSe quantum dots: synthesis, characterization and PL properties. J Mater Sci: Mater Electron 24(2):692–696
Pradhan N, Goorskey D, Thessing J, Peng X (2005) An alternative of CdSe nanocrystal emitters: pure and tunable impurity emissions in ZnSe nanocrystals. J Am Chem Soc 127(50):17586–17587
Zhou S, Zhang W, Lin P, Tian L, Li X, Jiang Y, Du L, Zhou X, Wen F, Duan G, Yu L (2021) Controllable perovskite crystallization via platelet-like PbI2 films from water processing for efficient perovskite solar cells. J Alloys Compd 885:160900
Yan W, Li S, Zhang Y, Yao Q (2010) Effects of dipole moment and temperature on the interaction dynamics of titania nanoparticles during agglomeration. J Phys Chem C 114:10755–10760
Frost JM, Butler KT, Walsh A (2014) APL Mater 2:081506
Afzaal M, Yates HM (2017) Growth patterns and properties of aerosol-assisted chemical vapor deposition of CH3NH3PbI3 films in a single step. Surf Coat Technol 321:336–340
Ahmad S, Abbas H, Khan MB, Nagal V, Hafiz AK, Khan ZH (2021) ZnO for stable and efficient perovskite bulk heterojunction solar cell fabricated under ambient atmosphere. Sol Energy 216:164–170
Manzoor S, Zhengshan JY, Ali A, Ali W, Bush KA, Palmstrom AF, Bent SF, McGehee MD, Holman ZC (2017) Improved light management in planar silicon and perovskite solar cells using PDMS scattering layer. Sol Energy Mater Sol Cells 173:59–65
Liu Z, He T, Wang H, Jain SM, Liu K, Yang J, Zhang N, Liu H, Yuan M (2018) Improvement in the performance of inverted planar perovskite solar cells via the CH3NH3PbI3-xClx:ZnO bulk heterojunction. J Power Sources 401:303–311
Wang L, McCleese C, Kovalsky A, Zhao Y, Burda C (2014) Femtosecond time-resolved transient absorption spectroscopy of CH3NH3PbI3 perovskite films: evidence for passivation effect of PbI2. J Am Chem Soc 136(35):12205–12208
Mahmoudi T, Wang Y, Hahn YB (2021) Highly stable perovskite solar cells based on perovskite/NiO-graphene composites and NiO interface with 25.9 mA/cm2 photocurrent density and 20.8% efficiency. Nano Energy 79:105452
Yazdi MRA, Rahimzadeh A, Clouqi Z, Miao Y, Eslamian M (2018) Viscosity, surface tension, density and contact angle of PbI2, PbCl2 and methyl ammonium lead halide perovskite solutions used in perovskite solar cells. AIP Adv 8:025109
Bi D, Gao P, Scopelliti R, Oveisi E, Luo J, Grätzel M, Hagfeldt A, Nazeeruddin MK (2016) High-performance perovskite solar cells with enhanced environmental stability based on amphiphile-modified CH3NH3PbI3. Adv Mater 28(15):2910–2915
Stranks SD, Eperon GE, Grancini G, Menelaou C, Alcocer MJ, Leijtens T, Herz LM, Petrozza A, Snaith HJ (2013) Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342(6156):341–344
Chen H, Ding X, Pan X, Hayat T, Alsaedi A, Ding Y, Dai S (2018) Comprehensive studies of air-brush spray deposition used in fabricating high-efficiency CH3NH3PbI3 perovskite solar cells: combining theories with practices. J Power Sources 402:82–90
Xu X, Sun Y, He D, Liang Z, Liu G, Xu S, Li Z, Zhu L, Pan X (2021) Grain size control for high-performance formamidinium-based perovskite solar cells via suppressing heterogenous nucleation. J Mater Chem C 9(1):208–213
Zhang S, Liu Z, Zhang W, Jiang Z, Chen W, Chen R, Huang Y, Yang Z, Zhang Y, Han L, Chen W (2020) Barrier designs in perovskite solar cells for long-term stability. Adv Energy Mater 10(35):2001610
Ahmad AA, Al-Bataineh QM, Alsaad AM, Samara TO, Al-izzy KA (2020) Optical properties of hydrophobic ZnO nano-structure based on antireflective coatings of ZnO/TiO2/SiO2 thin films. Physica B Condens Matter 593:412263
Han J, Kwon H, Kim E, Kim DW, Son HJ, Kim DH (2020) Interfacial engineering of a ZnO electron transporting layer using self-assembled monolayers for high performance and stable perovskite solar cells. J Mater Chem A 8(4):2105–2113
Xia Y, Yan G, Lin J (2021) Review on tailoring PEDOT:PSS layer for improved device stability of perovskite solar cells. Nanomaterials 11(11):3119
Abbas H, Ahmad S, Parvaz M, Khan MB, Khan MS, Khan A, Alshahrie A, Khan ZH (2022) Surface optimization of metal halide perovskite solar cells using ZnS nanorods. J Mater Sci: Mater Electron 33(27):21576–21587
Liu Z, Wang L, Xie X, Xu C, Tang J, Li W (2022) High-performance Ruddlesden-Popper two-dimensional perovskite solar cells with solution processed inorganic charge transport layers. Phys Chem Chem Phys 24:15912–15919
Hoseinpour V, Shariatinia Z, Mahmoodpour S (2022) Surface passivation boosted performances of perovskite solar cells assembled under ambient conditions. Opt Mater 131:112746
Latypova AF, Maskaev AV, Gutsev LG, Emelianov NA, Kuznetsov IE, Kuznetsov PM, Nikitenko SL, Baskakova YV, Akkuratov AV, Komissarova EA, Frolova LA (2022) Side chain engineering and film uniformity: two key parameters for the rational design of dopant-free polymeric hole transport materials for efficient and stable perovskite solar cells. Mater Today Chem 26:101218
Author information
Authors and Affiliations
Contributions
All authors contributed to the present study and helped in material preparation, data collection, and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbas, H., Khan, M.S., Ahmad, S. et al. Reduction of extrinsic defects in ZnSe:perovskite composites based solar devices. J Nanopart Res 24, 270 (2022). https://doi.org/10.1007/s11051-022-05644-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05644-8