Abstract
The semiconductor-based photocatalytic oxidation process is considered as one of the most economical and environmentally friendly approach for the degradation of toluene. For the sake of exploring an efficient visible-light-driven photocatalysts, the MoS2@g-C3N4 core-shell nanospheres were successfully fabricated by taking the advantages of the morphology and structure of materials tailoring and semiconductor coupling, via a two-step approach including the hydrothermal method followed by ultrasonic adhering approach. The morphology, crystallinity, composition, and optical property of as-prepared catalysts were well characterized. The core-shell structure was fabricated by using MoS2 as the template and the coating of g-C3N4 improved the separation of photoinduced charges, due to the strong electronic interaction between them. The photocatalytic activity of MoS2@g-C3N4 core-shell nanospheres had been investigated by the degradation of toluene under visible light irradiation using in situ Fourier transform infrared (FTIR) spectroscopy. The MoS2@g-C3N4 nanospheres displayed enhanced visible-light photocatalytic activity, which is about 1.3 and 9.6 times than that of g-C3N4 and MoS2 nanospheres under identical conditions. Electron spin resonance (ESR) examinations confirmed the generation of ·OH and ·O2−, which were the key reactive oxygen species involved in the photocatalytic process.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Volatile organic pollutants (VOCs), released from the extensive chemical products, have recently attracted great attention because of their environmental persistence and high toxicity (Aguero et al. 2009; Atkinson and Arey 2003; Kostiainen 1995; Sun et al. 2013; Wang et al. 2014b). Toluene, one most typical and ubiquitous hazardous components of VOCs, which is usually found in the exhaust gases from vehicles and indoor air, has severely threatened human health (Liu et al. 2015; Sun et al. 2015). There is an urgent need to develop viable elimination methods towards toluene. For the past decades, a large number of strategies have been reported as potential removal methods for toluene, such as plasma (Mista and Kacprzyk 2008), biodegradation (Malhautier et al. 2014), adsorption (Gil et al. 2014; Puértolas et al. 2014), and catalysis (Kesavan et al. 2011; Sun et al. 2014). Among them, the semiconductor-based photocatalytic oxidation process is considered as one of the most economical and environmentally friendly approach for the environmental pollutant remediation, due to the huge advantage of strong oxidizing ability, utilization of sunlight, and complete mineralization of organics (Parida et al. 2010; Wang et al. 2010; Yu et al. 2009; Yurdakal et al. 2008; AlShehri et al. 2017a; Ahmad et al. 2017; AlShehri et al. 2017b). In order to improve the utilization of sunlight and enhance the activity for the practical application, a number of attempts have focused on the development of efficient visible-light-driven photocatalysts (Hou et al. 2012; Xiong et al. 2011).
As a member of the layered transition metal dichalcogenides, MoS2 nanosheets have attracted increasing interest in catalysis, energy storage, electronics, and optoelectronics (Choi et al. 2014; Cummins et al. 2015; Hwang et al. 2011; Lukowski et al. 2013; Yu et al. 2014; Zhou et al. 2013). However, the rapid recombination of photoinduced charges and poor activity limits the practical applications of MoS2 in the photocatalytic elimination of pollutants. To date, various strategies have been put forward to address these problems. One effective strategy should be the tailor of the nanomaterials to obtain a suitable geometric structure and morphology to provide the effective carrier transfer pathways (Li et al. 2015a; Wang et al. 2013; Wang et al. 2014a). Luo et al. reported the assembling of the MoS2 nanosheets into a 3D hierarchical architecture (Zhang et al. 2014). By this approach, it can retain the large contact surface and exposes the highly active edge sites for the adsorption of substance and catalysis. Indeed, this solution improves the performance of MoS2 in lithium storage and hydrogen evolution reaction, and proves promising in photocatalysis. Another one is semiconductor coupling (Barpuzary et al. 2015; Hou et al. 2016; Jiang et al. 2015; Li et al. 2015a; Lu et al. 2016; Zhao et al. 2016). In order to improve the photocatalytic activity of MoS2, a few recipes have been developed on the coupling with graphitic carbon nitride (g-C3N4) (Li et al. 2012), BiVO4 (Li et al. 2015a), CdS (Chen et al. 2012), CoS2 (Huang et al. 2015), Fe3O4 (Lin et al. 2015), etc. Especially, g-C3N4, a typical metal-free polymeric semiconductor material has received great attentions due to its low toxicity and visible light response (Li et al. 2015b). For example, Lu et al. fabricated g-C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance (Lu et al. 2016). Hou et al. reported that MoS2/g-CN layered junctions were fabricated and used for hydrogen-evolution catalysis (Hou et al. 2013). Therefore, coupling with g-C3N4 makes MoS2 a potential material for photocatalysis. Nonetheless, 3D MoS2 hierarchical architecture modified with g-C3N4 has rarely been reported, taking into account the significant challenge to the mineralization of various VOCs.
Herein, we demonstrate a simple hydrothermal method to synthesize MoS2 nanospheres assembles with nanosheets and construct the MoS2@g-C3N4 core-shell nanostructures by the ultrasonic adhering approach. By building the core-shell structures, it can integrate different semiconductors into one system and form favorable interface, which can significantly improve the activity including long-term photostability of core and decrease of recombination of photogenerated carriers (Ghosh Chaudhuri and Paria 2012). The crystal structure, morphology, and optical properties of the as prepared MoS2@g-C3N4 core-shell nanospheres were comparatively investigated. As expected, the MoS2@g-C3N4 core-shell nanospheres exhibit enhanced photocatalytic activity comparing with MoS2 and g-C3N4. In addition, the reactive oxide species during the toluene degradation over MoS2@g-C3N4 have been monitored and the proposed mechanisms of toluene degradation have been discussed.
Experimental section
Synthesis of MoS2@g-C3N4 nanospheres
The MoS2@g-C3N4 core-shell nanospheres were fabricated via a two-step approach including the hydrothermal method followed by ultrasonic adhering approach (Pan et al. 2012; Zhang et al. 2015). The synthesis procedure is illustrated in Fig. 1. Firstly, MoS2 nanospheres were fabricated by the hydrothermal method reported previously with modifications. In a typical case, 0.3 g Na2MoO4·2H2O, 0.6 g NH2CSNH2, and poly(vinylpyrrolidone) (PVP) were dissolved in 80 mL deionized water with magnetic stirring for 15 min under the room temperature, followed by transferring into 100 mL Teflon-lined autoclave. After that, the container was placed into an oven and heated at 200 °C for 12 h. After cooling to room temperature naturally, the obtained MoS2 was collected by centrifugation and dried at 80 °C for 6 h. Secondly, 0.005 g g-C3N4, which was obtained from the calcination of melamine at 550 °C for 2 h, was dispersed in 20 mL methanol with ultrasound for 100 min. After 0.045 g MoS2 was added, the mixed solution were stirred in a fume hood until the methanol was volatilized completely. The MoS2@g-C3N4 was obtained by drying at 70 °C for 8 h.
Characterization
The sample morphology was observed by scanning electron microscope (SEM) (JEOLJSM-6360LV microscope) with an accelerating voltage of 30.0 kV and transmission electron microscopy (TEM, FEI Tecnai G2) at an acceleration voltage of 200 kV, respectively. The crystalline structure of samples were determined by X-ray diffraction (XRD) on Empyrean X-ray diffractometer using Cu Kα radiation in the range of 10–80°. The chemical valences and composition of the sample were measured by an X-ray photoelectron spectroscopy (XPS) (Perkin-Elmer PHI 5600). All the binding energies were referenced to C 1 s at 285.6 eV. UV-vis diffusive reflectance spectra (DRS) of the as-prepared materials were recorded using UV-vis absorption spectrophotometer (JASCO, UV-550) in the spectral range of 200–800 nm. The FTIR spectra were collected by the FTIR spectrometer (Bruker VERTEX 70-FTIR). To investigate the behavior of the electron–hole pairs, the photoluminescence (PL) characteristics were collected on a Hitachi F-4500 fluorescence spectrophotometer at room temperature with the excitation wavelength of 325 nm. Electron spin resonance (ESR) signals of radicals spin-trapped by spin-trap reagent 5, 5-dimethyl-1-pirroline-N-oxide (DMPO) were examined with a Bruker A200 spectrometer. The concentration of DMPO was 0.1 mol/L, the volume of reactor was 5 mL, and the relevant irradiation source was a 500-W high pressure xenon lamp (Shanghai Lansheng Light, China). The parameters for the ESR spectrometer were the following: center field = 3400 G, frequency = 9.46 GHz, microwave power = 20.02 mW.
Photocatalytic activity
Photocatalytic reaction of the materials was evaluated by degradation of toluene under visible light in a home-made in situ quartz infrared reaction cell (Shen et al. 2012). Mixing up the 8-mg catalyst with 35 mg KBr were tableted and settled in the sample holder, followed by sealing the reaction tube. Then, 2 μL of liquid toluene was injected into the reactor by using a microsyringe. Then, after the adsorption–desorption equilibrium in the reactor for 1 h, a 500-W xenon lamp (XQ-500 W) with a 400-nm UV-cutoff filter as the light source was turned on. In situ FTIR spectra during the photocatalytic reaction on MoS2@g-C3N4 nanospheres were recorded using a FTIR spectrometer (Bruker VERTEX 70-FTIR) over accumulative 64 scans with a resolution of 4 cm−1 in the range of 4000–600 cm−1.
Results and discussion
Characterization of MoS2@g-C3N4 core-shell nanospheres
The MoS2@g-C3N4 core-shell nanospheres are synthesized by the two-step procedure. Firstly, the MoS2 nanospheres has been fabricated via the hydrothermal method according to the reported method with miner modification (Zhang et al. 2015). The morphology of the as-prepared MoS2 has been characterized by the SEM (Fig. 2a). The SEM image clearly confirms that the MoS2 samples are spherical in shape, with the average diameter of about 166 nm. The MoS2@g-C3N4 core-shell nanospheres are synthesized by a facile ultrasonic adhering approach (Fig. 2b). With the coating of g-C3N4 shell, the diameter increases to 250 nm. The thickness of g-C3N4 shell can be calculated as about 42 nm by comparing the diameters of MoS2 and MoS2@g-C3N4. The TEM image in Fig. 2c reveals that the MoS2 nanospheres are shown to adopt uniform radial orientation and are constructed of ultrathin nanosheets. It needs to point out that the PVP surfactant absorbed on the surface of MoS2 could not only prevent the aggregation of MoS2 nanosheets, but also drive the oriented assembly of the nanosheets into spheres (Zhang et al. 2015). It can cleanly see the g-C3N4 nanosheets on the surface from the TEM image of MoS2@g-C3N4 (Fig. 2d). The core-shell structure can be conformed from the HRTEM images in Fig. 2e. The existence of MoS2 nanosheets with radial orientation and amorphous g-C3N4 indicated the cover of g-C3N4 on the surface of MoS2 spheres. In addition, the spheral distribution of C and N elements of the EDX mapping (Fig. 2f) can also conform the configuration of core-shell structure. The results of EDX mapping and spectrum (Fig. 2g) reveals that the core-shell nanospheres are composed of Mo, S, C, N, and Si elements (Si signal originates from the underlying substrates in characterization). Moreover, no other elements could be detected within the sensitivity of the apparatuses, indicating high purity of the MoS2@g-C3N4 composites.
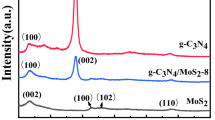
The crystallinity and phase purity of the obtained g-C3N4, MoS2 and MoS2@g-C3N4 composites are analyzed by XRD technique (Fig. 3a). The XRD pattern of the MoS2 samples corresponds to hexagonal MoS2, which is consistent with the reference values from JCPDS (37-1492) (Zhang et al. 2015). The diffraction peaks at 11.8°, 32.7°, and 57.2° could be ascribed to (002), (100), (101), and (110), respectively. No other peaks could be found. At the same time, the diffraction peaks for the g-C3N4 sample shows two diffraction peaks at 27.3° and 13.3°, which result from the (002) and (100) crystal planes, respectively (Dang et al. 2015). It can be seen that all the characteristic diffraction peaks of MoS2 can be observed in the composites, indicating that the g-C3N4 coated has no impact on the crystalline structure of MoS2. Meanwhile, the existence of the weak diffraction peak appearing at 27.3° indicates that the g-C3N4 has been successfully compounded with MoS2. However, the diffraction peak of g-C3N4 at 13.3° is too close to the peak of MoS2 at 11.8° to distinguish. In addition, no other significant peaks were detected in the MoS2@g-C3N4 composites, indicating the composites contain g-C3N4 and MoS2 only.
Figure 3b displays the Fourier transform infrared (FTIR) spectra of g-C3N4, MoS2, and MoS2@g-C3N4 core-shell nanospheres. The typical stretching modes revealed a typical molecular structure of g-C3N4, which was also supported by the FTIR bands at 1641, 1570, 1461, and 1411 cm−1 corresponding to typical stretching vibration modes of heptazine-derived repeating units. In addition, the bands at 1321 and 1243 cm−1 of g-C3N4 represent stretching vibration of connected units of C–N(–C)–C or C–NH–C, meanwhile, the intense band at 809 cm−1 is assigned to the out-of-plane bending vibration of heptazine rings (Liu et al. 2011). For pure MoS2, the characteristic peak at 478 cm−1 can be observed, which represents the bridging Mo–S bond (Mo–S vibration perpendicular to the basal plane). The peak at 647 cm−1 and 562 cm−1 can be assigned to S–H vibrations and S–S bonding, respectively (Burman et al. 2016). The typical vibration band of g-C3N4 at 1570, 1243, and 809 cm−1 and MoS2 at 649, 564, and 478 cm−1 in the composites indicate the hybridization in MoS2@g-C3N4 nanospheres, while the shift strongly suggests chemical interactions between the core and shell.
The optical properties of as-prepared g-C3N4, MoS2, and MoS2@g-C3N4 core-shell nanospheres are shown in Fig. 3c by DRS analysis. As shown in the results, the MoS2 nanospheres have significant absorption in UV light and visible light regions, which could be deduced from the color of black. However, due to the nature of rapid recombination of photoinduced charges, MoS2 showed poor photocatalytic activity. The pure g-C3N4 exhibits an absorption edge at about 500 nm due to the intrinsic band gap absorption, which can hardly improve the light absorption in the visible light region. Nevertheless, with MoS2 coating with g-C3N4, the photoinduced charges transfer between the core and shell could improve the separation of the generated electron-hole pairs. According to the DRS results, the obtained band gap energy (Eg) for g-C3N4 can be calculated as 2.73 eV by Tauc’s plots (Pradhan et al. 2013), while the one of pure MoS2 is 1.67 eV, which is consistent with the previous report (Zou et al. 2015). For the MoS2@g-C3N4 composites, the value of the band gap energy is 1.98 eV. The result indicates that using MoS2@g-C3N4 nanospheres as a core can not only control the morphology of g-C3N4, but also enhance the visible light utilization.
The PL spectra are employed to reveal the transfer and recombination processes of the photogenerated charges during the photocatalysis. It needs to note that the MoS2 has no photoluminescence property during the room-temperature PL analysis (Li et al. 2015a). Figure 3d presents the PL spectra of g-C3N4 and MoS2@g-C3N4 nanospheres, while that of MoS2 is absent to avoid misunderstanding. The PL spectrum of g-C3N4 shows a strong emission centered at around 443 nm, meanwhile, the relative intensity of the peak for MoS2@g-C3N4 nanospheres deceased obviously. It means that the coating of g-C3N4 onto MoS2 could suppress the recombination of photogenerated charges due to the formation of core-shell nanostructure. The decreasing of recombination of photogenerated charges may enhance the photocatalytic activity of hybrids, which would be conformed following.
To investigate the electronic interactions between MoS2 and g-C3N4, the XPS analysis has been employed on MoS2@g-C3N4 (Fig. 4). The survey spectra shown in Fig. 4a demonstrate that Mo, S, C, and N elements exist in MoS2@g-C3N4 composites, which is consistent with chemical composition in Fig. 1e. It is proposed that the C 1s peak at around 284.8 eV can be assigned to the signal from carbon contained in the apparatus and is used for calibration. Figure 4b displays the XPS spectra of N 1s. As for the pure g-C3N4, the binding energies at 398.8 and 400.6 eV can be attributed to the presence of C–N–C groups and tertiary nitrogen N–(C)3 groups (Li et al. 2012). However, both of the N peaks are positively shifted after covering onto the MoS2, which indicates that there are strong electronic interactions between the MoS2 and g-C3N4. In addition, for the pure MoS2, the binding characteristic orbital of S 2s, S 2p1/2, S 2p3/2, Mo 3d3/2, and Mo 3d5/2 peaks is observed with peak locations at 225, 162, 160.8, 231.1, and 227.9 eV, respectively, which are all consistent with the reported values for MoS2 (Wang et al. 2014a; Yan et al. 2013). After g-C3N4 covering onto the MoS2, S 2s, S 2p1/2, S 2p3/2, Mo 3d3/2, and Mo 3d5/2 peaks shift to 225.6, 162.7, 161.4, 232.4, and 228.4 eV, respectively. It is clear that the binding peaks of the core-shell nanospheres shift to a higher binding energies, which also confirms strong electronic interactions involving MoS2 and g-C3N4 in the core-shell nanospheres (Feng et al. 2015; Zhao et al. 2016). It needs to point out that the weak peak around 168 eV in Fig. 4d can be attributed to the sulfate species from the oxidation of sulfur. The oxidation of sulfur was exacerbated during the evaporation of methanol for the coating of g-C3N4, which caused the increasing of the peak (Li et al. 2016).
Photocatalytic degradation of toluene
The in situ FTIR technique can provide a real-time monitoring of transient events during the photocatalytic degradation of toluene, and the surface-adsorbed species of catalysts can be used to analyze the reaction mechanism (Li et al. 2011). A set of FTIR spectra yielded during the photocatalytic degradation of toluene over the MoS2@g-C3N4 composites under visible-light irradiation is shown in Fig. 5.
FTIR spectra in the range of 3200–2300 cm−1 (a), 1800–1300 cm−1 (b), and 3900–3500 cm−1 (c) collected as a function of time for the photodegradation of toluene over the MoS2@g-C3N4 under visible light irradiation. d Conversion of toluene over the KBr, g-C3N4, MoS2, and MoS2@g-C3N4 under visible light irradiation and e the related kinetics of toluene degradation by different catalysts. f Cycling runs in the photocatalytic degradation of toluene over the MoS2@g-C3N4
Before visible light irradiation (t = 0), the characteristic peaks of toluene at 3076, 3040, 2936, 2881, 1609, and 1499 cm−1 can be seen from Fig. 5a. The bands at 3076 and 3040 cm−1 are assigned to the C–H stretching mode of the aromatic ring (Liu and Jin 2015), while the bands at 2936 and 2881 cm−1 are attribute to the C–H stretching mode of methyl stretching vibration (Maira et al. 2001). In addition, the bands at 1609 and 1499 cm−1 are due to the vibrations of aromatic ring. As the irradiation time extended, all the intensity of characteristic peaks of toluene are gradually decreasing. The decreasing peak indicates the degradation of toluene over MoS2@g-C3N4 during the photocatalytic reaction. Meanwhile, some new peaks located at 1748, 1732, 1717, 1541, 1506, 1473, 1455, 1396, 1362, 1338, and 1315 cm−1 appeared during the photocatalytic reaction proceeding in Fig. 5b. The bands located at 1473 and 1455 cm−1 are related to the characteristic peaks of benzyl alcohol and bands at 1362 cm−1 and 1338 cm−1 attributed to the υ(O-H) stretching vibration of hydroxy in benzyl alcohol. The bands observed at 1506, 1541, and 1717 cm−1 are attributed to the υ(C=O) stretching vibration of carbonyl compounds in benzaldehyde (Belver et al. 2003; Cao et al. 2000). The 1748, 1732, 1396, and 1315 cm−1 are the characteristic peaks of benzoic acid (Domi et al. 2003). In addition, the bands at 2360 and 2340 cm−1 indicate the generation of carbon dioxide, while the bands appearing in the 3750–3600 cm−1 range are related to hydroxyl groups on the catalyst surface (Fig. 5c). According to the above results and previous reports (Ardizzone et al. 2008; Augugliaro et al. 1999), the intermediate products in the photodegradation of toluene include benzyl alcohol, benzaldehyde, and benzoic acid, followed by mineralizing into carbon dioxide and water finally.
Figure 5d displays the photocatalytic degradation of toluene over different samples under visible light irradiation. The blank experiment (only with 45 mg KBr) with the toluene conversion of about 3.7% means that the degradation over KBr could be neglected. From the results, it can be seen that the removal efficiency of toluene over MoS2@g-C3N4 was as high as 81% after 8 h visible light irradiation time, while there are only 70 and 15% for that of MoS2 nanospheres and g-C3N4, respectively. The excellent photocatalytic performance of MoS2@g-C3N4 nanospheres could be attracted to the fabrication of core-shell nanostructures. Figure 5e shows the related kinetic curves of the different catalysts. The linear transformation of a pseudo-first-order kinetic model was used to fit the degradation data: –ln (Ct/C0) = kt, where Ct is the concentrations of gaseous toluene at time t, C0 is the initial concentration after equilibrium adsorption, and k is the apparent first-order reaction rate constant (Sun et al. 2014). The kinetic constants for g-C3N4 and MoS2 nanospheres, and MoS2@g-C3N4 core-shell nanospheres are 0.0217, 0.1566, and 0.2081 h−1, respectively. With kinetic constant of 9.59 and 1.32 times than that of g-C3N4 and MoS2, MoS2@g-C3N4 nanospheres show enhanced photocatalytic activity.
In order to examine the stability of the MoS2@g-C3N4, the photocatalysis process was repeated three times with the same photocatalyst tablet. After three recycles for the photodegradation of toluene, the MoS2@g-C3N4 did not exhibit significant loss of activity, as shown by Fig. 5f, which confirms the excellent stability of the photocatalyst.
ESR had been employed to confirm the formation of hydroxyl radicals (·OH) and superoxide anion radicals (·O2−) during photocatalytic oxidation. From the ESR spin-trap signals (with DMPO) shown in Fig. 6a, four characteristic peaks with intensity ratio of 1:2:2:1 for the DMPO-·OH were detected with suspensions of the MoS2@g-C3N4 core-shell nanospheres under the visible light illumination. Meanwhile, six characteristic peaks of the DMPO-·O2− were detected in Fig. 6b. It is confirmed that spin-trapped DMPO-·OH and DMPO-·O2− adducts are formed in the process of photocatalytic degradation of toluene during photocatalysis. In contrast, there is no signals of ·OH and ·O2− that were detected in the dark. The ESR result indicates that the active species ·OH and ·O2− were produced in the MoS2@g-C3N4 core-shell nanospheres under visible light irradiation, which induced the degradation of organic pollutants.
Based on the above discussion, a possible photocatalytic mechanism of MoS2@g-C3N4 nanospheres based on the electronic structures, a schematic illustration of interparticle photo-generated charge transfer behavior is shown in Fig. 7. Under the visible light irradiation (λ > 400 nm), photons can be absorbed by both MoS2 and g-C3N4 to induce h+ and e− (Eqs. (1) and (2)). The photogenerated e− migrates to MoS2 from g-C3N4, while the h+ transfers through the opposite direction from MoS2 to g-C3N4. Therefore, the transfer of photogenerated charges could improve the separation of them effectively. Some of the photoinduced h+ accumulated in g-C3N4 could interact with surface-bound H2O or OH− to produce the ·OH species (Eq. (3)), while the stored e− in the conduction band of MoS2 can react with the adsorbed O2 molecules to generate ·O2− (Eq. (4)). In addition, the OH• radicals are thus likely formed via indirect routes from ·O2− (Eq. (5)) (Muñoz-Batista et al. 2016). Then, the toluene react with the highly oxidative species, ·OH and ·O2− and ·H2O2−, with the intermediate products of benzyl alcohol, benzaldehyde, and benzoic acid, followed by mineralizing into carbon dioxide and water finally (Eq. (6)). According to the above analysis, the relevant reactions at the nanospheres surface can be expressed as follows:
Conclusion
In summary, we have successfully synthesized MoS2@g-C3N4 core-shell nanospheres via a facile hydrothermal and ultrasonic adhering approach using MoS2 nanospheres as the template. The MoS2@g-C3N4 core-shell nanospheres extended the photo-response range and decreased the recombination rate of the photoinduced electron-hole pairs, therefore exhibited enhanced photocatalytic activity under the visible light irradiation, compared to g-C3N4 flake and pure MoS2 nanospheres. Benefitting from the reactive species such as ·OH and ·O2− generated during the photocatalytic process, toluene can be mineralized into carbon dioxide and water. The MoS2@g-C3N4 core-shell nanospheres are expected to have promising applications in mineralization of volatile organic pollutants, and further be employed as an efficient visible-light-responsive photocatalyst towards practical application.
References
Aguero FN, Barbero BP, Gambaro L, Cadús LE (2009) Catalytic combustion of volatile organic compounds in binary mixtures over MnOx/Al2O3 catalyst. Appl Catal, B 91:108–112
Ahmad T, Phul R, Alam P, Lone IH, Shahazad M, Ahmed J, Ahamad T, Alshehri SM (2017) Dielectric, optical and enhanced photocatalytic properties of CuCrO2 nanoparticles. RSC Adv 7:27549–27557
AlShehri SM, Ahmed J, Ahamada T, Almaswari BM, Khan A (2017a) Efficient photodegradation of methylthioninium chloride dye in aqueous using barium tungstate nanoparticles. J Nanopart Res 19:289
AlShehri SM, Ahmed J, Alzahrania AM, Ahamada T (2017b) Synthesis, characterization, and enhanced photocatalytic properties of NiWO4 nanobricks. New J Chem 41:8178–8186
Ardizzone S, Bianchi CL, Cappelletti G, Naldoni A, Pirola C (2008) Photocatalytic degradation of toluene in the gas phase: relationship between surface species and catalyst features. Environ Sci Technol 42:6671–6676
Atkinson R, Arey J (2003) Atmospheric degradation of volatile organic compounds. Chem Rev 103:4605–4638
Augugliaro V, Coluccia S, Loddo V, Marchese L, Martra G, Palmisano L, Schiavello M (1999) Photocatalytic oxidation of gaseous toluene on anatase TiO2 catalyst: mechanistic aspects and FT-IR investigation. Appl Catal, B 20:15–27
Barpuzary D, Banik A, Gogoi G, Qureshi M (2015) Noble metal-free counter electrodes utilizing Cu2ZnSnS4 loaded with MoS2 for efficient solar cells based on ZnO nanowires co-sensitized with CuInS2–CdSe quantum dots. J Mater Chem A 3:14378–14388
Belver C, López-Muñoz MJ, Coronado JM, Soria J (2003) Palladium enhanced resistance to deactivation of titanium dioxide during the photocatalytic oxidation of toluene vapors. Appl Catal, B 46:497–509
Burman D, Ghosh R, Santra S, Guha PK (2016) Highly proton conducting MoS2/graphene oxide nanocomposite based chemoresistive humidity sensor. RSC Adv 6:57424–57433
Cao L, Gao Z, Suib SL, Obee TN, Hay SO, Freihaut JD (2000) Photocatalytic oxidation of toluene on nanoscale TiO2 catalysts: studies of deactivation and regeneration. J Catal 196:253–261
Chen G, Li D, Li F, Fan Y, Zhao H, Luo Y, Yu R, Meng Q (2012) Ball-milling combined calcination synthesis of MoS2/CdS photocatalysts for high photocatalytic H2 evolution activity under visible light irradiation. Appl Catal, A 443–444:138–144
Choi MS, Qu D, Lee D, Liu X, Watanabe K, Taniguchi T, Yoo WJ (2014) Lateral MoS2 p–n junction formed by chemical doping for use in high-performance optoelectronics. ACS Nano 8:9332–9340
Cummins DR, Martinez U, Kappera R, Voiry D, Martinez-Garcia A, Jasinski J, Kelly D, Chhowalla M, Mohite AD, Sunkara MK, Gupta G (2015) Catalytic activity in lithium-treated core–shell MoOx/MoS2 nanowires. J Phys Chem C 119:22908–22914
Dang X, Zhang X, Chen Y, Dong X, Wang G, Ma C, Zhang X, Ma H, Xue M (2015) Preparation of β-Bi2O3/g-C3N4 nanosheet p–n junction for enhanced photocatalytic ability under visible light illumination. J Nanopart Res 17:93
Domi et al (2003) Silica-supported palladium nanoparticles show remarkable hydrogenation catalytic activity. J Mol Catal A Chem 197:185–191
Feng JX, Ding LX, Ye SH, He XJ, Xu H, Tong YX, Li GR (2015) Co(OH)2 @PANI hybrid nanosheets with 3D networks as high-performance electrocatalysts for hydrogen evolution reaction. Adv Mater 27:7051–7057
Ghosh Chaudhuri R, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112:2373–2433
Gil RR, Ruiz B, Lozano MS, Martín MJ, Fuente E (2014) VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem Eng J 245:80–88
Hou Y, Zuo F, Dagg A, Feng P (2012) Visible light-driven alpha-Fe2O3 nanorod/graphene/BiV1-xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett 12:6464–6473
Hou Y, Laursen AB, Zhang J, Zhang G, Zhu Y, Wang X, Dahl S, Chorkendorff I (2013) Layered nanojunctions for hydrogen-evolution catalysis. Angew Chem Int Ed 52:3621–3625
Hou H, Gao F, Wang L, Shang M, Yang Z, Zheng J, Yang W (2016) Superior thoroughly mesoporous ternary hybrid photocatalysts of TiO2/WO3/g-C3N4 nanofibers for visible-light-driven hydrogen evolution. J Mater Chem A 4:6276–6281
Huang J, Hou D, Zhou Y, Zhou W, Li G, Tang Z, Li L, Chen S (2015) MoS2 nanosheet-coated CoS2 nanowire arrays on carbon cloth as three-dimensional electrodes for efficient electrocatalytic hydrogen evolution. J Mater Chem A 3:22886–22891
Hwang H, Kim H, Cho J (2011) MoS2 nanoplates consisting of disordered graphene-like layers for high rate lithium battery anode materials. Nano Lett 11:4826–4830
Jiang W, Liu Y, Zong R, Li Z, Yao W, Zhu Y (2015) Photocatalytic hydrogen generation on bifunctional ternary heterostructured In2S3/MoS2/CdS composites with high activity and stability under visible light irradiation. J Mater Chem A 3:18406–18412
Kesavan L, Tiruvalam R, Rahim MHA, bin Saiman MI, Enache DI, Jenkins RL, Dimitratos N, Lopez-Sanchez JA, Taylor SH, Knight DW, Kiely CJ, Hutchings GJ (2011) Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 331:195–199
Kostiainen R (1995) Volatile organic compounds in the indoor air of normal and sick houses. Atmos Environ 29:693–702
Li X, Zou X, Qu Z, Zhao Q, Wang L (2011) Photocatalytic degradation of gaseous toluene over Ag-doping TiO2 nanotube powder prepared by anodization coupled with impregnation method. Chemosphere 83:674–679
Li J, Shen B, Hong Z, Lin B, Gao B, Chen Y (2012) A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem Commun 48:12017–12019
Li H, Yu K, Lei X, Guo B, Fu H, Zhu Z (2015a) Hydrothermal synthesis of novel MoS2/BiVO4 hetero-nanoflowers with enhanced photocatalytic activity and a mechanism investigation. J Phys Chem C 119:22681–22689
Li X, Hartley G, Ward AJ, Young PA, Masters AF, Maschmeyer T (2015b) Hydrogenated defects in graphitic carbon nitride nanosheets for improved photocatalytic hydrogen evolution. J Phys Chem C 119:14938–14946
Li G, Sun J, Hou W, Jiang S, Huang Y, Geng J (2016) Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium–sulfur batteries. Nat Commun 7:10601
Lin T, Wang J, Guo L, Fu F (2015) Fe3O4@MoS2 core–shell composites: preparation, characterization, and catalytic application. J Phys Chem C 119:13658–13664
Liu B, Jin N (2015) The typical environmental system optimization problems solved by computer software. In: 2015 7th international conference on modelling, identification and control (ICMIC), 18–20 Dec. 2015, pp 1–6
Liu J, Zhang T, Wang Z, Dawson G, Chen W (2011) Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J Mater Chem 21:14398
Liu B, Li X, Zhao Q, Ke J, Liu J, Liu S, Tadé M (2015) Photocatalytic degradation of gaseous toluene with multiphase TixZr1−xO2 synthesized via co-precipitation route. J Colloid Interface Sci 438:1–6
Lu X, Jin Y, Zhang X, Xu G, Wang D, Lv J, Zheng Z, Wu Y (2016) Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance. Dalton Trans 45:15406–15414
Lukowski MA, Daniel AS, Meng F, Forticaux A, Li L, Jin S (2013) Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 Nanosheets. J Am Chem Soc 135:10274–10277
Maira AJ, Coronado JM, Augugliaro V, Yeung KL, Conesa JC, Soria J (2001) Fourier transform infrared study of the performance of nanostructured TiO2 particles for the photocatalytic oxidation of gaseous toluene. J Catal 202:413–420
Malhautier L, Quijano G, Avezac M, Rocher J, Fanlo JL (2014) Kinetic characterization of toluene biodegradation by Rhodococcus erythropolis: towards a rationale for microflora enhancement in bioreactors devoted to air treatment. Chem Eng J 247:199–204
Mista W, Kacprzyk R (2008) Decomposition of toluene using non-thermal plasma reactor at room temperature. Catal Today 137:345–349
Muñoz-Batista MJ, Fontelles-Carceller O, Ferrer M, Fernández-García M, Kubacka A (2016) Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl Catal, B 183:86–95
Pan C, Xu J, Wang Y, Li D, Zhu Y (2012) Dramatic activity of C3N4/BiPO4 Photocatalyst with core/shell structure formed by self-assembly. Adv Funct Mater 22:1518–1524
Parida KM, Sahu N, Tripathi AK, Kamble VS (2010) Gold promoted S,N−doped TiO2: an efficient catalyst for CO adsorption and oxidation. Environ Sci Technol 44:4155–4160
Pradhan GK, Padhi DK, Parida KM (2013) Fabrication of α-Fe2O3 nanorod/RGO composite: a novel hybrid photocatalyst for phenol degradation. ACS Appl Mater Interfaces 5:9101–9110
Puértolas B, García-Andújar L, García T, Navarro MV, Mitchell S, Pérez-Ramírez J (2014) Bifunctional Cu/H-ZSM-5 zeolite with hierarchical porosity for hydrocarbon abatement under cold-start conditions. Appl Catal, B 154–155:161–170
Shen Y, Zhao Q, Li X, Yuan D, Hou Y, Liu S (2012) Enhanced visible-light induced degradation of benzene on Mg-ferrite/hematite/PANI nanospheres: in situ FTIR investigation. J Hazard Mater 241–242:472–477
Sun Z, Li X, Guo S, Wang H, Wu Z (2013) One-step synthesis of Cl−-doped Pt(IV)/Bi2WO6 with advanced visible-light photocatalytic activity for toluene degradation in air. J Colloid Interface Sci 412:31–38
Sun J, Li X, Zhao Q, Ke J, Zhang D (2014) Novel V2O5/BiVO4/TiO2 nanocomposites with high visible-light-induced photocatalytic activity for the degradation of toluene. J Phys Chem C 118:10113–10121
Sun J, Li X, Zhao Q, Tade MO, Liu S (2015) Quantum-sized BiVO4 modified TiO2 microflower composite heterostructures: efficient production of hydroxyl radicals towards visible light-driven degradation of gaseous toluene. J Mater Chem A 3:21655–21663
Wang Q, Zhang M, Chen C, Ma W, Zhao J (2010) Photocatalytic aerobic oxidation of alcohols on TiO2: the acceleration effect of a brønsted acid. Angew Chem Int Ed 49:7976–7979
Wang M, Li G, Xu H, Qian Y, Yang J (2013) Enhanced lithium storage performances of hierarchical hollow MoS2 nanoparticles assembled from nanosheets. ACS Appl Mater Interfaces 5:1003–1008
Wang PP, Sun H, Ji Y, Li W, Wang X (2014a) Three-dimensional assembly of single-layered MoS2. Adv Mater 26:964–969
Wang S, Zhang L, Long C, Li A (2014b) Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents. J Colloid Interface Sci 428:185–190
Xiong W, Zhao Q, Li X, Zhang D (2011) One-step synthesis of flower-like Ag/AgCl/BiOCl composite with enhanced visible-light photocatalytic activity. Catal Commun 16:229–233
Yan Y, Xia B, Ge X, Liu Z, Wang J-Y, Wang X (2013) Ultrathin MoS2 nanoplates with rich active sites as highly efficient catalyst for hydrogen evolution. ACS Appl Mater Interfaces 5:12794–12798
Yu J, Yue L, Liu S, Huang B, Zhang X (2009) Hydrothermal preparation and photocatalytic activity of mesoporous Au–TiO2 nanocomposite microspheres. J Colloid Interface Sci 334:58–64
Yu L, Lee YH, Ling X, Santos EJG, Shin YC, Lin Y, Dubey M, Kaxiras E, Kong J, Wang H, Palacios T (2014) Graphene/MoS2 hybrid technology for large-scale two-dimensional electronics. Nano Lett 14:3055–3063
Yurdakal S, Palmisano G, Loddo V, Augugliaro V, Palmisano L (2008) Nanostructured rutile TiO2 for selective photocatalytic oxidation of aromatic alcohols to aldehydes in water. J Am Chem Soc 130:1568–1569
Zhang L, Wu HB, Yan Y, Wang X, Lou XW (2014) Hierarchical MoS2 microboxes constructed by nanosheets with enhanced electrochemical properties for lithium storage and water splitting. Energy Environ Sci 7:3302–3306
Zhang S, Chowdari BVR, Wen Z, Jin J, Yang J (2015) Constructing highly oriented configuration by few-layer MoS2: toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 9:12464–12472
Zhao W, Liu Y, Wei Z, Yang S, He H, Sun C (2016) Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4@p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl Catal, B 185:242–252
Zhou M, Lou XW, Xie Y (2013) Two-dimensional nanosheets for photoelectrochemical water splitting: possibilities and opportunities. Nano Today 8:598–618
Zou X, Dong Y, Li X, Zhao Q, Cui Y, Lu G (2015) Inorganic–organic photocatalyst BiPO4/g-C3N4 for efficient removal of gaseous toluene under visible light irradiation. Catal Commun 69:109–113
Funding
This work was supported financially by the Major Program of the National Natural Science Foundation of China (No. 21590813), the National Natural Science Foundation of China (Nos. 21377015 and 21577012), the Key Project of the National Ministry of Science and Technology (No. 2016YFC0204204), the Program of Introducing Talents of Discipline to Universities (B13012), the Fundamental Research Funds for the Central Universities (DUT16RC(3)095), and the Key Laboratory of Industrial Ecology and Environmental Engineering, China Ministry of Education.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, X., Xiong, W., Li, X. et al. Fabrication of MoS2@g-C3N4 core-shell nanospheres for visible light photocatalytic degradation of toluene. J Nanopart Res 20, 243 (2018). https://doi.org/10.1007/s11051-018-4340-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4340-1