Abstract
The temperature behavior of bismuth orthoferrite nanoparticles obtained by the glycine-nitrate combustion method was studied by high-temperature X-ray diffractometry and complex thermal analysis. The region of stability of the material in a single-phase state was found. It was shown that the nanocrystalline BiFeO3 did not undergo decay in the temperature interval 550–780 °С. In this temperature interval, we have obtained the nanocrystalline material with average crystallite sizes 40–90 nm and average particle sizes 100–150 nm the sizes of which depend on temperature. The features of formation of BiFeO3 and the process of their sintering were studied. Results show that the crystallite growth slowed down after the amorphous phase disappeared. The sintering of nanopowder became more intense in the temperature interval 600–700 °С, but no noticeable increase in the crystallite sizes occurred. The magnetic behavior of obtained material was also discussed. It was found to be consistent with the concept of violation of the cycloidal magnetic order in bismuth orthoferrite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perovskite-like multiferroics exhibit a wide range of functional properties, which makes them attractive for microelectronic and spintronic devices (Scott 2007; Akbashev and Kaul 2011; Park et al. 2007; Wu et al. 2016). Of particular interest are the materials in which transitions to ferroelectric and magnetically ordered states occur at room temperature and above it. The efficiency of these materials depends on such factors as temperature and chemical stability, phase homogeneity, morphology, and particle and crystallite sizes (Park et al. 2007; Wu et al. 2016).
Bismuth orthoferrite (BiFeO3) is a material combining the properties of ferroelasticity, antiferromagnetism, and ferroelectricity (Wang et al. 2003; Scott 2007; Catalan and Scott 2009; Park et al. 2007; Akbashev and Kaul 2011; Wu et al. 2016; Jiagang et al. 2016). The magnetic structure of BiFeO3 is described as an antiferromagnetic order of Fe3+ ions with a space-modulated cycloid spin structure having a period of 62 ± 2 nm which is incommensurate with the crystal lattice (Sosnowska et al. 1982; Schmid 1994; Ruette et al. 2004). A weak ferromagnetism arises due to a canting of the antiferromagnetic Fe sublattices and the realization of the Dzyaloshinsky-Moriya exchange interaction (Popov et al. 1993). BiFeO3 has the G-type AFM Neel point at T N ≈360 °C and ferroelectric Сurie point at Т С ≈830 °С (Wang et al. 2003; Catalan and Scott 2009; Wu et al. 2016).
Structural transformations of BiFeO3 are described in (Catalan and Scott 2009; Arnold et al. 2009, 2010; Yaako et al. 2013). It is shown that BiFeO3 undergoes several phase transitions. In the polar phase below 830 °C, bismuth orthoferrite is in the form of an α-phase with a rhombohedral distorted perovskite crystal structure (R3c space group). In the range 830–925 °С, there exists an intermediate insulator-type β-phase with an orthorhombic (Pbnm space group) structure similar to that of GdFeO3 (Arnold et al. 2009). Near the bismuth orthoferrite decomposition temperature (925–933 °C), the β phase transforms into a metallic-type cubic γ phase of BiFeO3 (Palai et al. 2008; Arnold et al. 2009, 2010).
The preparation of nanocrystalline materials based on BiFeO3 is widely discussed in the literature (see, for example, (Carvalho and Tavares 2008; Silva et al. 2011; Ortiz-Quinonez et al. 2013; Lomanova and Gusarov 2013; Egorysheva et al. 2013; Koferstein 2014; Liu et al. 2015; Suzuki et al. 2016; Goliс et al. 2016; Vijayasundaram et al. 2016; Lomanova et al. 2016; Ganesh et al. 2017)). Basic methods used for their production are sol-gel synthesis (Egorysheva et al. 2013; Ganesh et al. 2017), chemical coprecipitation (Lomanova and Gusarov 2013), hydrothermal synthesis (Liu et al. 2015; Suzuki et al. 2016; Goliс et al. 2016), molten salt synthesis (Carvalho and Tavares 2008), and solution combustion (Ortiz-Quinonez et al. 2013; Koferstein 2014; Vijayasundaram et al. 2016; Lomanova et al. 2016). Each of these methods has its own advantages and drawbacks, so the search for conditions of a simple, effective, and environmentally safe synthesis of a single-phase material with controlled morphology and properties is still of great importance.
The phase diagram of the Bi2O3-Fe2O3 system was repeatedly refined in the region of existence of bismuth orthoferrite (Koizumi et al. 1964; Speranskaya et al. 1965; Morozov et al. 2003; Maitre et al. 2004; Palai et al. 2008; Perejon et al. 2014; Rojac et al. 2014). Nevertheless, there is still no unambiguous opinion regarding temperature ranges of stability of different BiFeO3 phases. It is shown that this compound has a peritectic-type decomposition in the region 934-961 °С. The possibility of a metastable existence of BiFeO3 and a change in its composition with increasing temperature were considered in (Morozov et al. 2003; Selbach et al. 2008).
It was shown in (Rojac et al. 2014) that a spontaneous decomposition of BiFeO3 occurs in the range 447–767 °C. The thermodynamic calculations presented in (Mikhailov et al. 2011) indicate that the synthesis of bismuth orthoferrite should be carried out at temperatures below 730 °C. As shown in Lomanova and Gusarov (2013), the formation of BiFeO3 by chemical coprecipitation is accompanied by the appearance of impurities in the interval 450–500 °C. According to (Egorysheva et al. 2013), a necessary factor for obtaining nanocrystalline bismuth orthoferrite under such conditions is a short-term isothermal holding at 600 °C for 10–15 min.
As a rule, impurities (parasitic phases) appear at grain boundaries during BiFeO3 synthesis (Morozov et al. 2003; Valant et al. 2007; Thrall et al. 2008; Rojac et al. 2014; Cheng et al. 2015). They are typically Bi24FeO39 (sillenite-type structure) and Bi2Fe4O9 (mullite-type structure) compounds. The presence of impurities in a nanocrystalline material can significantly change its functional properties (Bajpai et al. 2014; Oyarzun et al. 2015; Zhang et al. 2015). Even a small amount of impurities increases the magnetization of BiFeO3 in several times (Ortiz-Quinonez et al. 2013; Koferstein 2014).
The papers (Thrall et al. 2008; Cheng et al. 2015) describe in situ study of formation of phases at BiFeO3 synthesis by X-ray diffractometry. It was shown that the amounts of bismuth and iron oxides at the nucleation of BiFeO3 decrease with the equal rates. In addition, the iron oxide starts to react with Bi2O3 after the bismuth oxide has been transformed from monoclinic form to cubic one, which slows down the nucleation process. The activation temperature of sintering is reciprocal to the heating rate, which is important for the nucleation, phase stability, and transformation process. One of the most rapid reactions for nanocrystalline BiFeO3 synthesis is solution combustion. It is convenient for synthesis of nanocrystalline perovskite-like materials, because it substantially increases the components contacting in the reaction zone and thereby increases the nucleation (Aruna and Mukasyan 2008; Ostroushko and Russkikh 2017).
Specific features of synthesis of perovskites, including BiFeO3, under the conditions of solution combustion were discussed in Ortiz-Quinonez et al. (2013), Koferstein (2014), Priyadharsini et al. (2014), Vijayasundaram et al. (2016), Lomanova et al. (2016), Wu et al. (2004), Popkov and Almjasheva (2014), and Tugova et al. (2017). It was shown that the use of glycine as a fuel component made it possible to obtain nanocrystalline BiFeO3 with a high degree of phase homogeneity (Ortiz-Quinonez et al. 2013; Lomanova et al. 2016). The optimal temperature for the BiFeO3 nucleation by the glycine-nitrate combustion was indicated to be 500 ± 50 °С which coincides with the value obtained by «soft chemistry» methods, (Carvalho and Tavares 2008; Lomanova and Gusarov 2013; Egorysheva et al. 2013; Ortiz-Quinonez et al. 2013). Since crystallite sizes can affect not only properties, but also the stability of compounds and their structural modifications (Popkov and Almjasheva 2014), it was of interest to study the thermal stability of BiFeO3 nanocrystals obtained by the glycine-nitrate combustion method. For the effective synthesis control, the peculiarities of phase transformation, nucleation, and nanocrystal growth processes should be worked out in detail by in situ X-ray diffractometry method.
The goals of our work were in situ high-temperature XRD study of the temperature behavior of nanocrystalline bismuth orthoferrite, obtained by the glycine-nitrate combustion method, determination of the region of its thermal stability, and magnetic characterization of obtained single-phase material.
Experimental
The synthesis of BiFeO3 nanoparticles was performed by the glycine-nitrate combustion. The purity of initial reagents of bismuth nitrate, i.e., pentahydrate (Bi(NO3)3·5H2O), iron nitrate nonahydrate (Fe(NO3)3·9H2O), glycine (С2H5NO2), and diluted nitric acid (HNO3, 64%), were 99.8% and higher.
The preparation process used to obtain the sample in the initial state (combustion product) was as follows: 3 mol of Bi(NO3)3·5H2O, 3 mol of Fe(NO3)3·9H2O, and 10 mol of glycine were dissolved in a mixture of 50 mL of deionized water and 5 mL of HNO3 under magnetic stirring at room temperature. Glycine is an organic fuel, which provides an opportunity for the redox reactions to occur between the reagents during the combustion process. The obtained solution was heated at 240 °C until the entire solvent evaporated and a combustion reaction occurred.
The elemental composition and morphology of the sample were determined by scanning electron microscopy (SEM) and energy dispersive X-ray microanalysis (FEI Quanta 200 SEM with the EDAX attachment); the error in the elemental content depended on the atomic number and was ± 0.3% on the average.
A specific surface area of the sample was determined by BET (Brunauer–Emmett–Teller) method from the nitrogen isothermal adsorption data measured by an ASAP 2020 Micromeritics automated system at 77 K.
The density was measured by the helium pycnometry method (Ultrapycnometer 1000, Quanta Chrome). The specific surface area and pycnometry density were performed successively after sample heat treatment at 400, 550, 650, 750, 820 °С in a Wisetherm furnace in the isothermal exposure—cooling mode. Particle sizes in the sample were determined by two methods: using the values of their density and specific surface area (the calculation was carried out for a spherical particle shape) and by SEM data.
The changes induced in the sample by thermal treatment were studied by high-temperature X-ray diffractometry (HT-XRD) by using Shimadzu XRD-7000 with an HTK–1200N high-temperature attachment (Anton Paar). The CuKα radiation was used. The material of the tray in which the sample was placed (α-Al2O3) was used as the internal standard. To calculate unit cell parameters, the PDWin 4.0 software package was used. Average crystallites sizes were determined according to diffraction peaks width (by means of the Scherrer equation).
The thermal behavior was investigated by a NETZSCH STA 429 thermal analyzer which could perform simultaneously studies by differential scanning calorimetry (DSC), thermogravimetry (TG), and mass-spectrometry (MS). This experiment was carried out in the temperature range from room temperature to 820 °C in air; the heating rate was 5 °C/min.
The dilatometric analysis was carried out by a NETZSCH DIL 402 E dilatometer (Germany) at a heating rate of 5 K/min in air for the sample in the form of a tablet.
A WISSEL spectrometer with a 57Со/Rh source was used for the Mössbauer examination of samples. The measurements were performed in the absorption geometry at room temperature. The isomer shift (IS) was determined relative to α-Fe.
Magnetic measurements were performed by a PPMS vibration magnetometer of Quantum Design at the temperature 400 K and in the range of fields 0–140 kOe. Temperature and field dependences of the specific magnetization (M) in a constant field H = 500 Oe in the zero field cooling (ZFC) and field cooling (FC) mode were studied.
Results and discussion
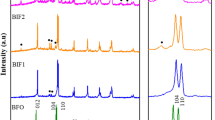
The EDS analysis of the combustion product and the sample after its study by HT-XRD showed that, within the error of the method, the Bi:Fe ratio was 1:1, i.e., it corresponded to the BiFeO3 stoichiometry. As follows from the XRD patterns of the combustion product, the sample mainly consisted of a mixture of an Х-ray amorphous phase and a small amount of an impurity phase with a peak at 2θ = 28.1° (see Fig. 1). A low content of this phase did not allow its unambiguous identification because reflexes of several bismuth-containing phases could give rise to the peak at 2θ≈28° at the XRD patterns. The main phases were Bi2Fe4O9 (PDF 20-836), Bi25FeO39 (PDF 46-416), BiO (PDF 2754), and Bi6(NO3)4(OH)2O6·2H2O (PDF 28654). The signal at 2θ = 28.1° was closest to the intense peak of Bi2Fe4O9 at 2θ = 28.20° and Bi25FeO39 at 2θ = 27.97°. In Ortiz-Quinonez et al. (2013), Bi2Fe4O9 was indicated as the most probable phase in the BiFeO3 synthesis. The data of (Lomanova and Gusarov 2013) showed that if the phases based on Bi2Fe4O9 and Bi25FeO39 are present at the initial stage of synthesis of bismuth orthoferrite, they do not disappear and are also present at subsequent stages of heat treatment. The BiO oxide formation in the reaction system at these temperatures is unlikely. The HT-XRD data (Fig. 2) show that the peaks caused by the impurity phase disappear on the diffractogram after the sample loses mass at ~ 500 °C. All these facts suggest that bismuth hydroxonitrate can be the most probable impurity phase that can be formed from the residual amounts of undecomposed bismuth nitrate in the process of combustion. Since the composition of this impurity is not known exactly, we shall refer to it below as a by-product.
According to HT-XRD (Fig. 1), the phase state of the sample varies only slightly in the region 100–500 °С. The DSC curve (Fig. 2a) exhibits an endothermic peak accompanied by a sharp loss of mass at about 180 °С. According to the mass-spectrometric analysis of the gases released during this process (Fig. 2b), this effect is associated with the dehydration process. In the region 350–500 °С, in addition to the peaks of the X-ray amorphous phase and byproduct, the peak characteristic of BiFeO3 appears on the X-ray diffraction patterns.
The phase analysis of the sample above 400 °C shows a decrease in the amounts of the X-ray amorphous and impurity phases (Fig. 1) which is accompanied, as indicated by TG, by a loss of mass (Fig. 2a). At 450 °C the intensity of the BiFeO3 peaks sharply grows, while the amounts of the amorphous phase and byproduct decrease and go to zero at 550 °C. A nearly complete disappearance of the peaks due to the by-product in this temperature region shows that the substance of this phase, like that of the amorphous phase, is spent on the formation of crystals of the main phase.
As temperature is further increased, the recrystallization occurs and sample sintering becomes intense. This manifests itself in an increase in the crystallite and particles sizes of BiFeO3 and a decrease in the relative linear dimension of the sample. These processes are evident from the SEM images (Fig. 3) and dilatometric curve (Fig. 4). Figure 3 presents SEM images of the sample after its successive heat treatment at 550, 650, 750, and 820 °С. The sample morphology at all stages of synthesis can be described as a network of sintered particles the sizes of which grow with increasing temperature. The particles of material with average sizes 0.1–1.5 μm are composed of crystallites; average size d is 40–90 nm depending on the temperature. Characteristics of the sample after its heat treatment are given in Table 1.
As shown in Lomanova and Gusarov (2013) and Lomanova et al. (2016), the intensification of the bismuth orthoferrite formation under conditions of «soft chemistry» and glycine-nitrate combustion in the temperature range 450–500 °С is associated with the transition of the particle surface to a liquid-like state that activates a mass transfer in the reaction system. The DSC curve exhibits an exothermic peak at about 450 °С which is apparently attributable to the crystallization of BiFeO3 from the amorphous phase observed immediately after the end of the mass loss by the sample.
According to the HT-XRD data, the peaks observed in the region 450–780 °С are attributed to the pure rhombohedrally distorted perovskite BiFeO3 (PDF 20-169). This correlates with the data of (Ortiz-Quinonez et al. 2013) showing that single-phase BiFeO3 is obtained by the glycine-nitrate combustion in the vicinity of 600 °С. It was found in (Rojac et al. 2014) that BiFeO3 ceramic partially decomposed in the range 447–767 °С to form Bi2Fe4O9 and Bi25FeO39. However, according to our HT-XRD experiment, no formation of these phases was observed in this temperature interval, and above 550 °С the sample remained a single-phase one.
The difference between our results and work (Rojac et al. 2014) are probably because of specificity of sample preparation at the initial stage. As it was shown in Thrall et al. (2008) and Cheng et al. (2015) by in situ X-ray diffractometry, the impurity phases Bi25FeO39 and Bi2Fe4O9 are the intermediate phases in the BiFeO3 ceramic formation process. In contrast to the conventional ceramic technique, the solution combustion method is based on a rapid chemical reaction with such rate and temperature that the formation of impurity phases is unlikely for kinetic reasons.
The use of solution combustion process increases contacting in the initial composition, which intensifies the nucleation and enhance the homogeneity in the material (Aruna and Mukasyan 2008; Ostroushko and Russkikh 2017). Also, the formation of impurity phases (which is possible for the cooled reacting system) may not take place during in in situ analysis of the materials.
The temperature dependences of the average crystallite size determined from the XRD pattern broadening (Fig. 5 a, d(T) curve) and the BiFeO3 unit cell volume v(T) = V(Т)/Z (Fig. 5 b, v(T) curve) monotonically increase to 780 °C. This temperature corresponds to the beginning of the endothermic peak on the DSC curve with a maximum at 810 ± 5 °C (Fig. 2a). Analysis of the d(T) curve shows that above 600 °C, the crystallite growth somewhat slows down. A sharp decrease in the specific surface area Ssurf of the powder and, hence, an increase in the particle size are also observed in this temperature region (see Table 1). Above 650 °C, particle sizes increase due to the coalescence of crystallites, the crystallite size varying only slightly. The dilatometric data reveal a further reduction in the linear sample dimensions attributable to its sintering (Fig. 4).
As follows from Morozov et al. (2003), Maitre et al. (2004), Palai et al. (2008), Arnold et al. (2009), and Arnold et al. (2010), the peak at 820 ± 10 °С on the DSC curve can be attributed to the ferroelectric-paraelectric phase transition in BiFeO3. The positions of the X-ray peaks at 820 °C on the diffractogram (Fig. 1) point to the formation of the β-BiFeO3 phase. Figure 5b shows both the experimental and literature data on the β-BiFeO3 parameters for 820 °С (Palai et al. 2008; Arnold et al. 2009, 2010). Various ideas about the structure of this intermediate phase are put forward in the literature. As shown in (Arnold et al. 2009), a coexistence of the α and β phases can be observed near the α → β transition. A discrepancy between the literature and our data can be explained by an insufficient precision of the X-ray data in our study and the effect of temperature broadening of XRD patterns on the cell parameter estimates. On the whole, the v(T) experimental dependence (Fig. 5b) is in reasonable agreement with the literature data.
The sample in the initial state (combustion product) and after heat treatment at 350 and 550 °C, i.e., at temperatures belonging to the temperature interval in which an intense growth of crystallites is observed, was studied by the Mössbauer spectroscopy. The spectra shown in Fig. 6 are consistent with the data on the change in the BiFeO3 crystallite sizes. The initial state of bismuth orthoferrite synthesis is characterized by crystallite sizes less than 60 nm, which correlates with the doublets of the superparamagnetic phase in the Mössbauer spectra (see Fig. 6). At an annealing temperature of 550 °С, sextets appear in the Mossbauer spectrum, which points to the onset of a magnetic order. As can be concluded from the isomeric shift (Table 2), iron is in the Fe3+ state in all samples. The parameters of the spectra measured at all the stages of sample synthesis are typical of BiFeO3 and agree with the data given in (Park et al. 2007; Ortiz-Quinonez et al. 2013) for nanoparticles of this compound (see Table 2).
The magnetic characteristics of BiFeO3 belong to the most important properties of the material; moreover, they can be regarded as one of the labeling attributes of this substance. It is well known that the magnetic ordering in it governing by the antiferromagnetic type of interaction between iron ions and in a bulk sample appears as a spin cycloid with a period λ = 62 ± 2 nm (Sosnowska et al. 1982). However, in a nanocrystalline sample (when λ is smaller or at least comparable with a particle size), the cycloid get distorted. Also, there is a possible contribution to the magnetic moment of nanoparticle from surface and defects. So, the magnetic behavior of BiFeO3 in nanophase becomes more complicate and should be examined after its synthesis. Here, we concentrate on the main macroscopic magnetic parameters which are indicative for the system under discussion.
As shown in Ortiz-Quinonez et al. (2013) and Koferstein (2014), magnetic properties of bismuth orthoferrite are sensitive even to small amounts of impurities. For this reason, magnetic characteristics of the material with an average crystallite size of 70 ± 5 nm were heat treated at 550 °C for 1 h, i.е., at the temperature below that of bismuth oxide evaporation and possible appearance of impurity phases in the reaction system.
It is believed that the spin cycloid in BiFeO3 can be distorted by decreasing the crystallite sizes or applying a strong magnetic field H (Kadomtseva et al. 2006; Tokunaga et al. 2010).
For this reason, magnetic measurements were performed up to H = 140 kOe. The field dependence of magnetization M(H) recorded at room temperature is presented in Fig. 7a. This curve shows the hysteresis typical of the magnetically ordered state of the substance (see inset a).
It can be seen that magnetic saturation is not achieved even in very high fields, and at H > 30 kOe, the magnetization behavior becomes close to a linear one, which was approximated (the dashed line) by the function M = M0 + aH, where M = 0.02, a = 0.006. At H > 75 kOe, the deviation from the straight line is observed (see inset b). A similar feature is described in Tokunaga et al. (2010), the authors of which attributed the «metamagnetic» transition observed under similar conditions to the cycloid distortion. The mechanism of this effect is still a subject of discussion (Kadomtseva et al. 2006).
The temperature dependences M(T) recorded in ZFC and FC modes (Fig. 8) demonstrate a discrepancy, which is typical for nanopowdered magnetic materials. Since in our case the characteristic crystallite sizes are close to the spin cycloid period, the existence of a nonzero magnetic moment of individual particles even in low H is presumably associated with this dimensional effect.
Conclusions
The temperature behavior of bismuth orthoferrite nanoparticles obtained by the glycine-nitrate combustion method was studied. The region of stability of the material in a single-phase state was found. As evidenced by high-temperature diffractometry and complex thermal analysis, nanocrystalline BiFeO3 did not undergo decay in the temperature interval 550–780 °С.
The features of formation of BiFeO3 crystallites and the process of their sintering were studied. It was found that synthesis of bismuth orthoferrite nanoparticles was not accompanied by the appearance of impurity phases based on Bi24FeO39 and Bi2Fe4O9 in the reaction zone up to the temperature 780 °С. The BiFeO3 crystallite growth slowed down at temperatures above 600 ± 50 °С after the amorphous phase disappeared in the system. The nanopowder sintering became more intense due to particle growth in the temperature interval 600–700 °С, but no noticeable increase in the crystallite sizes occurred.
The magnetic behavior of obtained single-phase material with a crystallite size of 70 ± 5 nm was discussed. Magnetic characteristics of the nanopowder were measured. Results show magnetic behavior to be consistent with the concept of violation of the cycloidal magnetic order in bismuth orthoferrite.
References
Akbashev AR, Kaul AR (2011) Structural and chemical aspects of the design of multiferroic materials. Russ Chem Rev 80(12):1159–1179. https://doi.org/10.1070/RC2011v080n12ABEH004239
Arnold DC, Knight KS, Morrison FD, Lightfoot P (2009) Ferroelectric-paraelectric transition in BiFeO3: crystal structure of the orthorhombic phase. Phys Rev Lett 102(2):027602–027612. https://doi.org/10.1103/PhysRevLett.102.027602
Arnold DC, Knight KS, Catalan G, Redfern SAT, Scott JF, Lightfoot P, Morrison FD (2010) The 2β-to-γ transition in BiFeO3: a powder neutron diffraction study. Adv Funct Mater 20(13):2116–2123. https://doi.org/10.1002/adfm.201000118
Aruna ST, Mukasyan AS (2008) Combustion synthesis and nanomaterials. Curr Opin Solid State Mater Sci 12(3-4):44–50. https://doi.org/10.1016/j.cossms.2008.12.002
Bajpai OP, Kamdi JB, Selvakumar M, Ram S, Khastgir D, Chattopadhyay S (2014) Effect of surface modification of BiFeO3 on the dielectric, ferroelectric, magneto-dielectric properties of polyvinylacetate/BiFeO3 nanocomposites. Express Polym Lett 8(9):669–681. https://doi.org/10.3144/expresspolymlett.2014.70
Carvalho TT, Tavares PB (2008) Synthesis and thermodynamic stability of multiferroic BiFeO3. Mater Lett 62(24):3984–3986. https://doi.org/10.1016/j.matlet.2008.05.051
Catalan G, Scott JF (2009) Physics and applications of bismuth ferrite. Adv Mater 21(24):2463–2485. https://doi.org/10.1002/adma.200802849
Cheng GF, Ruan YJ, Liu W, Wu XS (2015) Effect of temperature variation on the phase transformation in the reaction sintering of BiFeO3 ceramics. Mater Lett 143:330–332. https://doi.org/10.1016/j.matlet.2014.12.121
Egorysheva AV, Kuvshinova TB, Volodin VD, Ellert OG, Efimov NN, Skorikov VM, Baranchikov AE, Novotortsev VM (2013) Synthesis of high-purity nanocrystalline BiFeO3. Inorgan Mater 49(3):310–314. https://doi.org/10.1134/S0020168513030035
Ganesh RS, Sharma SK, Sankar S, Divyapriya B, Durgadevi E, Raji P, Ponnusamy S, Muthamizhchelvan C, Hayakawa Y, Kim DY (2017) Microstructure, structural, optical and piezoelectric properties of BiFeO3 nanopowder synthesized from sol-gel. Curr Appl Phys 17(3):409–416. https://doi.org/10.1016/j.cap.2016.12.008
Goliс DL, Radojkoviс A, Cirkovi J, Dapcevi A, Pajic D, Tasic N, Savic SM, Pocuca-Nesi M, Markovic S, Brankovic G, Stanojevic ZM, Brankovic Z (2016) Structural, ferroelectric and magnetic properties of BiFeO3 synthesized by sonochemically assisted hydrothermal and hydro-evaporation chemical methods. J Eur Ceram Soc 36:1623–1631
Jiagang W, Fan Z, Xiao D, Zhu J, Wang J (2016) Multiferroic bismuth ferrite-based materials for multifunctional applications: ceramic bulks, thin films and nanostructures. Prog Mater Sci 84:335–402
Kadomtseva AM, Popov Yu F, Pyatakov AP, Vorob’ev GP, Zvezdin АК, Viehland D (2006) Phase transitions in multiferroic BiFeO3 crystals, thin-layers, and ceramics: enduring potential for a single phase, room-temperature magnetoelectric ‘holy grail’. Phase Transit 79(12):1019–1042. https://doi.org/10.1080/01411590601067235
Koizumi H, Nirizaki N, Ikeda T (1964) An x-ray study on Bi2O3-Fe2O3 system. Jpn Appl Phys 3:495–496
Koferstein R (2014) Synthesis, phase evolution and properties of phase-pure nanocrystalline BiFeO3 prepared by a starch-based combustion method. J Alloy Compd 590:324–330. https://doi.org/10.1016/j.jallcom.2013.12.120
Liu Z, Liang S, Li S, Zhu Y, Zhu X (2015) Synthesis, microstructural characterization, and dielectric properties of BiFeO3 microcrystals derived from molten salt method. Ceram Int 41:S19–S25. https://doi.org/10.1016/j.ceramint.2015.03.244
Lomanova NA, Gusarov VV (2013) Influence of synthesis temperature on BiFeO3 nanoparticles formation. Nanosyst: Phys Chem Math 4:696–705
Lomanova NA, Tomkovich MV, Sokolov VV, Gusarov VV (2016) Special features of formation of nanocrystalline BiFeO3 via the glycine-nitrate combustion method. Russ J Gen Chem 86(10):2256–2262. https://doi.org/10.1134/S1070363216100030
Maitre A, Francois M, Gachon JC (2004) Experimental study of the Bi2O3–Fe2O3 pseudo-binary system. J Phase Equilib Diffus 25(1):59–67
Mikhailov AV, Gribchenkova NA, Kolosov EN, Kaul’ AR, Alikhanyan AS (2011) Mass spectrometric investigation of vaporization in the Bi2O3-Fe2O3 system. Russ J Phys Chem A 85(1):26–30. https://doi.org/10.1134/S0036024411010183
Morozov MI, Lomanova NA, Gusarov VV (2003) Specific features of BiFeO3 formation in a mixture of bismuth(III) and iron(III) oxides. Russ J Gen Chem 73:1772–1776
Ortiz-Quinonez JL, Diaz D, Zumeta-Dube I, Arriola-Santamaria H, Betancourt I, Santiago-Jacinto P, Nava-Etzana N (2013) Easy synthesis of high-purity BiFeO3 nanoparticles: new insights derived from the structural, optical, and magnetic characterization. Inorg Chem 52:10306–10317
Ostroushko AA, Russkikh OV (2017) Oxide material synthesis by combustion of organic-inorganic compositions. Nanosyst: Phys Chem Math 8(4):476–502
Oyarzun S, Tamion A, Tournus F, Dupuis V, Hillenkamp M (2015) Size effects in the magnetic anisotropy of embedded cobalt nanoparticles: from shape to surface. Sci Rep 5:14749–14745
Palai R, Katiyar RS, Schmid H, Tissot P, Clark SJ, Robertson J, Redfern SAT, Scott JF (2008) The beta phase of multiferroic bismuth ferrite and its beta-gamma metal-insulator transition. Phys Rev B 77(1):014110. https://doi.org/10.1103/PhysRevB.77.014110
Park T-J, Papaefthymiou GC, Viescas AJ, Moodenbaugh AR, Wong SS (2007) Size-dependent magnetic properties of single-crystalline multiferroic BiFeO3 nanoparticles. Nano Lett 7(3):766–772. https://doi.org/10.1021/nl063039w
Perejon A, Sanchez-Jimenez PE, Criado JM, Perez-Maqueda LA (2014) Thermal stability of multiferroic BiFeO3: kinetic nature of the β−γ transition and peritectic decomposition. J Phys Chem C 118(45):26387–26395. https://doi.org/10.1021/jp507831j
Popov YF, Zvezdin AK, Vorob’ev GP, Kadomtseva AM, Murashev VA, Rakov DN (1993) Linear magnetoelectric effect and phase transitions in bismuth ferrite BiFeO3. JETP Lett 57:69–73
Popkov VI, Almjasheva OV (2014) Yttrium orthoferrite YFeO3 nanopowders formation under glycine-nitrate combustion conditions. Russ J Appl Chem 87(2):167–171. https://doi.org/10.1134/S1070427214020074
Priyadharsini P, Pradeep A, Murugesan C, Md Gazzali PM, Chandrasekaran G (2014) Phase evolution in BiFeO3 nanoparticles prepared by glycine-assisted combustion method. Combust Sci Technol 186(3):297–312. https://doi.org/10.1080/00102202.2013.859682
Rojac T, Bencan A, Malic B, Tutuncu G, Jones JL, Daniels JE, Damjanovic D (2014) BiFeO3 сeramics: processing, electrical, and electromechanical properties. J Am Ceram Soc 97(7):1993–2011. https://doi.org/10.1111/jace.12982
Schmid H (1994) Multi-ferroic magnetoelectrics. Ferroelectrics 162:19–25
Ruette B, Zvyagin S, Pyatakov AP, Bush A, Li JF, Belotelov VI, Zvezdin AK, Viehland D (2004) Magnetic-field-induced phase transition in BiFeO3 observed by high-field electron spin resonance: cycloidal to homogeneous spin order. Phys Rev B 69:064114–064117
Selbach SM, Tybell T, Einarsrud M-A, Grande T (2008) The ferroic phase transitions of BiFeO3. Adv Mater 20(19):3692–3696. https://doi.org/10.1002/adma.200800218
Silva J, Reayes A, Esparza H, Camacho H, Fuentes L (2011) BiFeO3: a review on synthesis, doping and crystal structure. Integr Ferroelectr 126:47–59
Scott JF (2007) Data storage: Multiferroic memories. Nat Mater 6(4):256–257. https://doi.org/10.1038/nmat1868
Sosnowska I, Peterlin-Neumaier T, Steichele EJ (1982) Spiral magnetic ordering in bismuth ferrite. Phys C Solid State Phys 15:4835–4846
Speranskaya EI, Skorikov VM, Rode EY, Terekhova VA (1965) The phase diagram of the system bismuth oxide – ferric oxide. Bull Acad Sci U.S.S.R 5(87):3–4
Suzuki K, Tokudome YK, Tsuda H, Takahashi M (2016) Morphology control of BiFeO3 aggregates via hydrothermal synthesis. J Appl Crystallogr 49(1):168–174. https://doi.org/10.1107/S1600576715023845
Thrall M, Freer R, Martin C, Azough F, Patterson B, Cernik RJ (2008) An in situ study of the formation of multiferroic bismuth ferrite using high resolution synchrotron X-ray powder diffraction. J Eur Ceram Soc 28(13):2567–2572. https://doi.org/10.1016/j.jeurceramsoc.2008.03.029
Tokunaga M, Azuma M, Shimakawa Y (2010) High-field study of strong magnetoelectric coupling in single-domain crystals of BiFeO3. J Phys Soc Jpn 79:064713–064715
Tugova E, Yastrebov S, Karpov O, Smith R (2017) NdFeO3 nanocrystals under glycine nitrate combustion formation. J Cryst Grow 467:88–92. https://doi.org/10.1016/j.jcrysgro.2017.03.022
Valant M, Axelsson A-K, Alford N (2007) Peculiarities of a solid-state synthesis of multiferroic polycrystalline BiFeO3. Chem Mater 19(22):5431–5436. https://doi.org/10.1021/cm071730+
Vijayasundaram SV, Suresh G, Kanagadurai R (2016) Chemically synthesized phase-pure BiFeO3 nanoparticles: influence of agents on the purity. Nano-Struct and Nano-Obj 8:1–6
Wang J, Neaton JB, Zheng H, Nagarajan V, Ogale SB, Liu B, Viehland D, Vaithyanathan V, Schlom DG, Waghmare UV, Spaldin NA, Rabe KM, Wuttig M, Ramesh R (2003) Epitaxial BiFeO3 multiferroic thin film heterostructure. Science 299(5613):1719–1722. https://doi.org/10.1126/science.1080615
Wu L, Yu JC, Zhang L (2004) Selective self-propagating combustion synthesis of hexagonal and orthorhombic nanocrystalline yttrium iron oxide. J Solid State Chem 177(10):3666–3674. https://doi.org/10.1016/j.jssc.2004.06.020
Wu J, Fan Z, Xiao D, Zhu J, Wang J (2016) Multiferroic bismuth ferrite-based materials for multifunctional applications: ceramic bulks, thin films and nanostructures. Prog Mater Sci 84:335–402. https://doi.org/10.1016/j.pmatsci.2016.09.001
Yaako MK, Tai MFM, Deni MSM, Chandra A, Lu L, Yahya MZA (2013) First principle study on structural, elastic and electronic properties of cubic BiFeO3. Ceram Int 39:283–286
Zhang W, Zhou Z, Zhong Y, Zhang T, Huang Y (2015) The effect of surface and interface on Neel transition temperature of low-dimensional antiferromagnetic materials. AIP Adv 5:117228
Funding
This work was financially supported by the Russian Science Foundation (No. 16-13-10252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lomanova, N.A., Tomkovich, M.V., Sokolov, V.V. et al. Thermal and magnetic behavior of BiFeO3 nanoparticles prepared by glycine-nitrate combustion. J Nanopart Res 20, 17 (2018). https://doi.org/10.1007/s11051-018-4125-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-018-4125-6