Abstract
A kind of novel ZnSnO3/SnO2 hollow urchin nanostructure was synthesized by a facile, eco-friendly two-step liquid-phase process. The structure, morphology, and composition of samples were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and nitrogen adsorption–desorption techniques. The results revealed that many tiny needle-like SnO2 nanowires with the average diameter of 5 nm uniformly grew on the surface of the ZnSnO3 hollow microspheres and the ZnSnO3/SnO2 hollow urchin nanostructures with different SnO2 content also were successfully prepared. In order to comprehend the evolution process of the ZnSnO3/SnO2 hollow urchin nanostructures, the possible growth mechanism of samples was illustrated via several experiments in different reaction conditions. Moreover, the gas-sensing performance of as-prepared samples was investigated. The results showed that ZnSnO3/SnO2 hollow urchin nanostructures with high response to various concentration levels of acetone enhanced selectivity, satisfying repeatability, and good long-term stability for acetone detection. Specially, the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure exhibited the best gas sensitivity (17.03 for 50 ppm acetone) may be a reliable biomarker for the diabetes patients, which could be ascribed to its large specific surface area, complete pore permeability, and increase of chemisorbed oxygen due to the doping of SnO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acetone, a kind of volatile organic chemicals (VOCs), is widely used in laboratories and industrial applications. When exposed to more than 173 ppm acetone, it will have a destructive effect on human health (Guo 2017). Meanwhile, acetone is also deemed to be a biomarker to determine diabetes (Zhang et al. 2017b; Lemraski and Nadimi 2017). Reported studies revealed that the acetone gas concentration in breath was dramatically different for diabetes patients and people without diabetes, whose concentration in breath was higher than 1.8 ppm and lower than 0.8 ppm, respectively (Park 2017). Thus, it is important to detect low acetone concentrations in medical analysis (Al-Hardan et al. 2013). However, the current technique in diabetes is blood test, which suffers from some disadvantages, such as being painful, inefficient, and so on (Nasution et al. 2013). It is necessary to find an appropriate substitute for blood test to enhance efficiency and alleviate the pain of patients. There are several techniques to detect the acetone in the air, such as mass spectrometry and gas chromatography that are expensive and unstable in situ and continuous tests. Therefore, semiconductor gas sensors have been widely applied in acetone detection due to its cheap, facile safety (Zhang et al. 2017b; Boeglin et al. 2006). Although the study on the semiconductor gas sensors is crucial, some drawbacks also exist, such as low selectivity between ethanol and other VOCs (Yin et al. 2017) and insufficient sensitivity (Xiao et al. 2014). Hence, the key question is acquisition of accurate acetone identification in many disruptive gases, especially ethanol, high sensitivity, and low limit of acetone detection.
It is well known that size and morphology of metal oxides have a significant effect on gas-sensing properties and their applications (Kida et al. 2013). Actually, hierarchical nanostructures exhibit well-aligned porous structure, and the growth of nano-building blocks on their surface ensures their large specific surface area. The gas-sensing performance of hierarchical nanostructures naturally attains both high gas response and rapid response speed (Lee 2009). While the aggregation between nanoparticles is strong on account of the inversely proportional relationship between the Van der Waals attraction and the particle size, on this occasion, the gas response of nanoparticles will be low and sluggish (Kim and Choi 2004; Korotchenkov 2005). In fact, many studies have been reported to assemble building blocks into different hierarchical structures, such as hollow sphere (Fan et al. 2011; Jia et al. 2017; Wang et al. 2013), 3D flower-like structure (Chen et al. 2015; Wang et al. 2016b), urchin-like structures (Kuang et al. 2015), nanorod-assembled urchins (Li and Zeng 2017), hollow urchin (Bing et al. 2014), and so on.
Furthermore, zinc stannate (ZnSnO3) and tin oxide (SnO2), as multifunctional materials, have attracted considerable attention due to their high chemical response and excellent electronic properties (Hyodo et al. 2003; Zeng et al. 2012b). As stated before, hierarchical nanostructures of metal oxides show perfect gas response. Recently, some studies have been prepared ZnSnO3/SnO2 hierarchical structures; novel core–shell microspheres (Sun et al. 2011) and hierarchical nanoflakes (Zeng et al. 2012a) and their gas-sensing of materials also have been tested both exhibiting high response to ethanol. Obviously, they may not satisfy the need in the acetone detection for diabetes therapy. There are still not enough literatures about ZnSnO3/SnO2 hierarchical structures and their studies in gas-sensing properties, especially to acetone-sensing properties. For this reason, we successfully prepared ZnSnO3/SnO2 hollow urchin nanostructures by a facile two-step liquid-phase method in this paper. Firstly, ZnSnO3 hollow microspheres were synthesized using an in situ precipitation method. Then, the tiny, well-defined SnO2 nanowires with different doping content (10, 20, 30 wt%) grew on the surface of ZnSnO3 hollow microspheres via a simple hydrothermal reaction. Moreover, the gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures was investigated showing a high response to acetone at 280 °C. It is noteworthy that 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure exhibits better acetone sensitivity compared to other samples (ZnSnO3 hollow microspheres, 20 wt%, 30 wt% ZnSnO3/SnO2 hollow urchin nanostructure), indicating that the morphology and content of materials have huge effect on their gas-sensing properties.
Experimental
Preparation of ZnSnO3/SnO2 nanocomposites
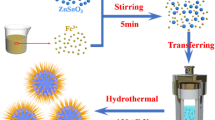
ZnSnO3/SnO2 hollow urchin nanostructures were synthesized using a facile, eco-friendly two-step liquid-phase method. Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), tin tetrachloride pentahydrate (SnCl4·5H2O), sodium hydroxide (NaOH), and trisodium citrate dihydrate (Na3C6H5O7·2H2O) were purchased from Sinopharm Chemical Reagent Co. Ltd., (Shanghai, China) and used as received without any further purification. Firstly, the ZnSnO3 hollow microspheres were synthesized in a moderate reaction. In a typical experiment, 1.785 g of Zn(NO3)2·6H2O, 2.10 g of SnCl4·5H2O, and 2.4 g of NaOH were dispersed into 140 mL water–ethanol solution (volume ratio is 2:1) by magnetic stirring for 15 min. Then, 6.0 g of NaOH was dissolved into 40 mL water–ethanol solution and slowly added it into the above solution with vigorous stirring. After that, the suspension was transferred into a 500-mL round-bottom flask and heating reflux was performed at 80 °C for 3 h. The flask cooled down naturally after the reaction. The precipitate was collected by centrifugation and washed with absolute ethyl alcohol and deionized water several times before drying at 70 °C for 12 h. The final product was calcined at 400 °C for 2 h.
Secondly, ZnSnO3/SnO2 hollow urchin nanostructures were prepared by a simple hydrothermal strategy. In detail, 0.5 g of the above ZnSnO3 powder was dispersed in 60 mL water-ethanol (volume ratio 2:1). A certain amount of Na3C6H5O7·2H2O was dissolved into the above solution. Then, different amount of SnO2 (10, 20, 30 wt%) was anchored on the surface of ZnSnO3 hollow microspheres by adding different amount of SnCl4·5H2O and NaOH into the above solution. After vigorous stirring for 10 min, the suspension was transferred into a Teflon-lined stainless steel autoclave (100 mL) with 80% degree of fill and heated at 180 °C for 12 h. Finally, the autoclave was cooled down naturally. The obtained product was centrifuged and washed with absolute ethyl alcohol and deionized water several times, and dried at 70 °C for 12 h. For convenience, the as-prepared ZnSnO3/SnO2 hollow urchin nanostructures were labeled as 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructure.
Characterization
The crystal phase of as-prepared products was observed by a D8-Advance X-ray diffractometer using high-intensity Cu Kα radiation (λ = 1.5406 Å) with the scanning range of 2θ = 10–80°. Field emission scanning electron microscopy (FE-SEM; JEOL JSM-7001F, Japan) and transmission electron microscopy (TEM; JSM-2100 (HR), Japan) with an accelerating voltage of 200 kV were applied to obtain the morphological and structural information of samples. The specific surface area and pore size distribution were analyzed on a Surface Area and Porosity System (TriStar II 3020) through nitrogen adsorption–desorption. X-ray photoelectron spectroscopy (XPS; VG ESCALAB 210) was recorded to study the surface chemical composition.
Fabrication and measurement of gas sensors
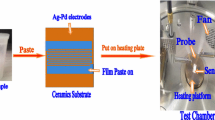
Gas sensor fabrication process was described as follows: a suitable amount of as-synthesized sample powders was dispersed in deionized water to form pastes, then pasted onto the outer surface of the ceramic tube (outer diameter = 1.35 mm, length = 4 mm) with a pair of gold electrodes printed previously. After the gas sensors with sensing film drying subsequently, they were calcined at 400 °C for 2 h. Then, a Ni–Cr alloy coil (resistance = 42 Ω) was inserted into the alumina tube as a heater to adjust the required operating temperature. In order to improve long-term stability, the sensors were aged with 220 °C for 10 days. The photograph and the schematic circuit of gas sensors were shown in Fig. 1a, b.
The gas-sensing properties of sensors were tested using a commercial WS-30A static gas-sensing measurement system (Zhengzhou Winsen Electronics Technology Co. Ltd., China). The tested gases were injected into the chamber by a microinjector and the electrical response was recorded by a computer. The gas response (S) was defined as the ratio of (R a/R g) the resistance of the sensor in air (R a) to that in target gases (R g). The response time τres and recovery time τrec were defined as the time taken by the sensors to achieve 90% of the total resistance variation when gas was turned on and off, respectively.
Results and discussion
Structural and morphological characteristics
Crystal structures of the as-prepared nanocomposites are investigated by XRD. The XRD pattern of ZnSnO3 hollow microspheres is shown in Fig. 2a. Only one broad characteristic peak at around 2θ = 32.411° can be observed, which may be attributed to self-assembled oriented growth of the hollow microspheres and their grain size variation (Zhang et al. 2017a; Ghammraoui et al. 2012). Figure 2b shows the XRD pattern of ZnSnO3/SnO2 hollow urchin nanostructures. After the restructuring of materials, more characteristic peaks of ZnSnO3 hollow microspheres that are agreed well with their standard diffraction pattern (JCPDS No. 11-0274) can be observed in Fig. 2b. Moreover, the residual peaks are located at 26.611°, 33.893°, 54.757°, and 61.870°, which are readily indexed to (110), (101), (220), and (310) crystal planes of SnO2 (JCPDS No. 41-1445), respectively. Notably, the peaks at 26.611° and 33.893° both become broad, which may be ascribed to the fact that the crystalline sizes of SnO2 nanoparticles diminish in hydrothermal reaction (Yin et al. 2014). No other diffraction peaks of impurities can be found, indicating the purity of samples.
Figure 3 shows the typical SEM images of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructure. As shown in Fig. 3a, the diameter of ZnSnO3 microsphere is about 1.3 μm and its surface is coarse. Obviously, the ZnSnO3 microsphere is made up of many aggregated ZnSnO3 nanoparticles with the average size being about 150 nm. The broken part of the ZnSnO3 microsphere demonstrates its hollow structure and also shows that the shell thickness of the hollow microsphere is about 100 nm. As shown in Fig. 3b, many great needle-like SnO2 nanowires are anchored on the surface of ZnSnO3 hollow microspheres. Meanwhile, the shell thickness (about 100 nm) of ZnSnO3/SnO2 nanocomposites has no obvious changes compared to the ZnSnO3 hollow microspheres. But their shells turn into looser, which can be attributed to the restructuring of ZnSnO3 hollow microspheres into hollow urchin nanostructures. Fig. 3c, d show that 20 and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures are assembled more needle-like SnO2 nanowires on the surface of ZnSnO3 hollow microspheres when increasing the doping concentrations of SnO2. Actually, the amount of SnO2 nanowires may play an important role in the sensitivity of materials due to their effect on surface area and permeability of hierarchical nanostructures (Lee 2009). The average diameters of 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures are 1.2 ± 0.1, 1.1 ± 0.1, and 1.6 ± 0.1 μm, respectively.
The morphologies and microstructures of the ZnSnO3 hollow microspheres and 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure were further observed by the TEM technique. Figure 4a shows the typical images of ZnSnO3 hollow microspheres, confirming their hollow structure according to their dark edges and bright center. It also reveals that the ZnSnO3 hollow microspheres are surrounded by many aggregated ZnSnO3 nanoparticles, which have an average size of about 150 nm, in consistence with the SEM measurement. For newly prepared ZnSnO3/SnO2 hollow urchin nanostructure, the typical TEM images are shown in Fig. 4b, c. As can be seen from Fig. 4b, the hollow urchin nanostructure displays the dark contour at their edges with many tiny needle-like nanowires and bright contours at their centers, indicating the hollow nature of ZnSnO3/SnO2 nanostructure. It also reveals that the ZnSnO3 hollow microspheres serve as the inner core of the ZnSnO3/SnO2 hollow urchin nanostructure and the SnO2 nanowires are anchored on their surface. The larger magnification TEM images of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure in Fig. 4c present the tiny needle-like SnO2 nanowires branched out with the average diameter of 5 nm, which are helpful to enhance the specific surface area of nanostructure. In addition, the corresponding selected area electron diffraction (SAED) pattern aiming at the nanowires is shown in Fig. 4d. It is composed of different diffraction circles, demonstrating the polycrystalline nature of SnO2.
The chemical composition and valence state of elements of the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure were determined using the XPS technique. Figure 5 shows the typical XPS spectra of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure. The survey spectrum of sample is shown in Fig. 5a, which reveals the presence of Zn, Sn, C, and O elements. The high-resolution spectrum of Zn 2p is shown in Fig. 5b, displaying two peaks of Zn 2p3/2 and Zn 2p1/2 located at 1021.63 and 1044.68 eV, respectively, which are assignable to lattice zinc ions. Figure 5c shows the Sn 3d5/2 and Sn 3d 3/2 located at 486.58 and 495.03 eV, respectively, which are attributed to the Sn in ZnSnO3 crystal lattice (Jain et al. 2011). The O 1s XPS spectrum of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure and ZnSnO3 hollow microspheres is also exhibited in Fig. 5d and its inset, respectively. It could be found that both of the two broadened peaks of them can be divided into three peaks, indicating that the existence of three kinds of oxygen species in the samples, which is corresponded to the O2− species in the lattice (OL), oxygen vacancies or defects (OV), and chemisorbed or dissociated (OC) oxygen species, respectively (Dupin et al. 2000; Khan et al. 2017). For ZnSnO3 hollow microspheres, the three peaks locate at 529.99, 531.12, 532.23 eV, which are assigned to OL, OV, and OC, respectively (Han et al. 2009). Simultaneously, the three distinct oxygen peaks of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure are locate at 529.90, 531.25, and 532.13 eV, respectively. It seems that the binding energies of these peaks between two samples changed rarely. However, the amount of each oxygen species of ZnSnO3 hollow microspheres and 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure varies greatly. The relative percentages of each oxygen species of them are displayed in Table 1. Compared with ZnSnO3 hollow microspheres, there is a decrease in OL concentration for 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure, whereas an increase in OV (from 26.93 to 74%) and OC concentrations (from 6.9 to 13.8%), suggesting the increase of oxygen defects and chemisorbed oxygen in amount via the anchoring of SnO2 nanowires on the surface of ZnSnO3 hollow microspheres.
The surface structural characteristics and pore structural information of the ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructures with different amount of SnO2 were further performed via nitrogen adsorption and desorption measurements. Figure 6 presents the measured results of ZnSnO3 hollow microspheres (a), 10 wt% (b), 20 wt% (c), and 30 wt% (d) ZnSnO3/SnO2 hollow urchin nanostructures. As observed in Fig. 6, ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructures with different SnO2 content all exhibit a type IV isotherm with a H3-type hysteresis loop, demonstrating the presence of mesopores in the tested samples (Kruk and Jaroniec 2001; Sun et al. 2016). It is worth mentioning that the H3-type hysteresis loop of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure in its isotherm is found more clearer compared with that of other samples, which may be associated with its more completely mesoporous structure. The pore size distributions of four samples are shown in the insets of Fig. 6a–d, which reveal the correspondingly maximum peak pore diameter of samples. And their calculated values are listed in Table 2. It is found the maximum peak pore diameter of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures is 2.10, 7.61, 4.27, and 3.67 nm, respectively, which can further confirm that 10 wt% ZnSnO3/SnO2 hollow urchin is a more completely mesoporous material among the four samples. In addition, the Brunauer–Emmett–Teller (BET) surface areas of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures are calculated to be 23.71, 55.21, 89.57, and 87.12 m2/g, respectively. Based the on pore size distribution and the BET surface area information of four samples, the newly as-prepared ZnSnO3/SnO2 hollow urchin nanostructures with different SnO2 content (10, 20, 30 wt%) are larger in maximum peak pore diameter and BET surface area compared with those of ZnSnO3 hollow microspheres, probably leading to higher gas-sensing performance of materials. The contradiction of variation trend between the maximum peak pore diameter and BET surface area among ZnSnO3/SnO2 hollow urchin nanostructures (10, 20, 30 wt%) may be related to the anchoring amount of SnO2 nanowires on the ZnSnO3 hollow microsphere surface. The pore structure and the specific surface area are vital characters for enhancing the sensitivity of materials (Jin et al. 2015).
Formation mechanism of ZnSnO3/SnO2 hollow urchin nanostructure
To facilitate understanding of the evolution process of ZnSnO3/SnO2 hollow urchin nanostructures, the morphological evolutions of ZnSnO3/SnO2 nanocomposites at different reaction conditions, including reaction temperature and the change of Sn4+ concentration, have been carried out. As shown in Fig. 7a–c, when adjusting the reaction temperature at 120, 150, and 180 °C but keeping other experimental conditions fixed, the obtained morphologies of samples show substantially different which changes from a concave cubic with rough surfaces (120 °C), gradually a more smooth cubic (150 °C) to a hollow urchin nanostructure (180 °C) finally. It is demonstrated that the reaction temperature plays an important role in forming ZnSnO3/SnO2 hollow urchin nanostructure. Furthermore, to explore the effect of Sn4+ and trisodium citrate dihydrate (Na3C6H5O7·2H2O) on the morphologies of samples, we choose to add the trisodium citrate dihydrate (Na3C6H5O7·2H2O) into the reaction mixture but not to add the Sn4+. The SEM image is shown in Fig. 7d, displaying a structure of hollow microsphere with a diameter of 1.3 μm that is similar to the structure of ZnSnO3 hollow microspheres as stated in Fig. 3a, but making the surface more rough, suggesting the surface activity function of trisodium citrate dihydrate. Reflected from the side, Sn4+ may be a key factor for the formation of ZnSnO3/SnO2 hollow urchin nanostructure.
On the basis of the above investigations and SEM and TEM observations, the possible formation processes were put forward in Fig. 8. Firstly, the Zn2+ and Sn4+ in the strong alkaline solution form the ZnSn(OH)6, and the second addition of OH− converts ZnSn(OH)6 solid microspheres to ZnSn(OH)6 hollow microspheres; finally, the ZnSnO3 hollow microspheres synthesized via the thermal treatment. Secondly, the crucial SnO2 nanowire nucleation occurs on the surface of ZnSnO3 hollow microspheres through the reaction between Sn4+ and OH− with the help of trisodium citrate dihydrate as the surfactant. The suitable hydrothermal reaction conditions provide a soft reaction environment for the growth of SnO2 nanowires. At last, the unique ZnSnO3/SnO2 hollow urchin nanostructures are successfully prepared.
Gas-sensing performance
The unique ZnSnO3/SnO2 hollow urchin nanostructures with high specific surface area and effective gas diffusion may be superb materials for gas-sensing applications. Based on this, the acetone gas-sensing properties of them have been investigated. For the purpose of comparison, ZnSnO3 hollow microspheres are also fabricated into gas sensors.
It is well known that the optimal operating temperature is an important stimulation to enhance the chemical activity of gas-sensing materials, which can be interpreted as the balance between gas diffusion rate and surface chemical activity reaching at optimal operating temperature (Zhang et al. 2016; Yamazoe et al. 2003). In order to determine the optimal operating temperature, the response of ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructures with different SnO2 content is tested in the range of 220–340 °C to 50 ppm acetone. As shown in Fig. 9, the response of all samples increases firstly with the increase in operating temperature, reaches maximum at 280 °C, and then decreases with the further increase in operating temperature. Therefore, the optimal operating temperature of 280 °C is determined and is applied to the following investigations. Moreover, all of the ZnSnO3/SnO2 hollow urchin nanostructures exhibit higher response than ZnSnO3 hollow microspheres; especially, the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure attains the highest response value. At optimal working temperature of 280 °C, the ZnSnO3/SnO2 hollow urchin nanostructures with different doping ratio all possess high response to 50 ppm acetone, indicating their good reproducibility. It is well believed that doping of SnO2 nanowires on the ZnSnO3 hollow microspheres greatly contributes to the improvement of gas sensitivity and reproducibility.
The response and recovery characteristics are significant parameters to evaluate the gas-sensing properties. Figure 10 shows the response and recovery transients of ZnSnO3 hollow microspheres and the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure to 50 ppm acetone at 280 °C. Obviously, ZnSnO3 hollow microspheres and the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure all exhibit rapid response upon exposure to 50 ppm acetone and their response time is both equal to 3 s. When sensors are exposed to atmosphere, they return to initial state quickly. The recovery time of ZnSnO3 hollow microspheres and the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure is 13 and 11 s, respectively. The shortening recovery time of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure may be related to its looser structure with the larger micropores providing an effective gas diffusion, which is consistent with the results of Fig. 6.
Figure 11 shows the dynamic response and recovery curves of ZnSnO3 hollow microspheres and 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure to different acetone concentrations in the range of 1–400 ppm at 280 °C. Notably, the response values of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure to low-concentration acetone go up sharply and are higher than that of ZnSnO3 hollow microspheres, suggesting the great contribution of newly prepared ZnSnO3/SnO2 hollow urchin nanostructure to reduce the limit of acetone detection. To a certain value, the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure (17.03) is two times higher than ZnSnO3 hollow microspheres (6.40) to 50 ppm acetone. And its response is also immensely higher in comparison with the response of ZnSnO3 hollow microspheres.
In short, the broadening of various levels in acetone detection is significant to fabricating reliable sensors (Nasution et al. 2013). There is no doubt that the gas-sensing properties of ZnSnO3/SnO2 hollow urchin nanostructure have been enhanced due to its distinct nanostructure.
In order to further illustrate the relationship between the response of samples and gas concentrations, Fig. 12a displays the dependence of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructure gas response to different acetone concentrations at the optimal operating temperature. According to Fig. 12a, it can be found that all ZnSnO3/SnO2 sensors present superiority over ZnSnO3 hollow microspheres in their acetone response. In particular, the 10 wt% ZnSnO3/SnO2 hollow urchin sensors exhibit the highest response among other sensors, which can be ascribed to their synergistic effect of surface structure and pore structure, from what brings into surface chemical reaction and gas diffusion process, respectively (Wang et al. 2015b). Simultaneously, the response of ZnSnO3/SnO2 hollow urchin sensors exhibits good linear relationship in the range of 1–20 ppm, which is well agreed with the linear regression equation: y = a × x + b, regression coefficient a = 0.428, 0.316, and 0.312, and intercept b = 1.562, 1.759, and 1.408 for 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures, respectively. The residuals of them are also listed in Table 3 to support this. The linear relationship of gas response and gas concentrations indicates the wide detection scope of ZnSnO3/SnO2 hollow urchin sensors (Ren et al. 2016).
a Dependence of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructure gas response to different acetone concentrations at 280 °C. b Response of ZnSnO3 hollow microspheres and 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures to lower acetone concentrations at 280 °C
Moreover, considering that acetone detection is potential application in diabetes therapy (Likhodii et al. 2002), and the acetone concentration of diabetic patient in exhaled air is reported to be more than 1.8 ppm (Deng et al. 2004), we believe that the higher response to lower-concentration acetone is vital to acetone detection for diabetes therapy (Wang et al. 2014). Hence, the response of all samples to acetone in low concentration (1–50 ppm) is shown in Fig. 12b. It is obvious that the response of ZnSnO3 hollow microspheres in low concentrations rises slowly, which have no response to 1 ppm acetone (the response value is deemed to 1) and the response to 5 ppm acetone is only 1.52. With the increase in gas concentration, its response values reach to the maximum of 6.40 to 50 ppm acetone. On the contrary, the response of ZnSnO3/SnO2 hollow urchin nanostructures (10, 20, 30 wt%) increases rapidly with the increase of concentrations in the range of 1–50 ppm. The response of 10, 20, and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures to 1 ppm acetone is 1.69, 1.65, and 1.52, respectively. When exposed to 5 ppm acetone, the measured response of them reaches to 3.84, 3.69, and 3.06, respectively, which are all higher than the response of ZnSnO3 hollow microspheres. Especially, the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure exhibits highest response not only in high concentration but also in low concentration for acetone test, indicating that the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure will be a promising candidate as a diabetes biomarker at various levels of acetone concentrations.
For the practical application, the selectivity is one remarkable parameter to measure the quality of gas sensors. Therefore, the selectivity of ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructure (10, 20, 30 wt%) was checked by exposing to different gases including ethanol, acetone, ammonia, toluene, and methanol with same concentration of 50 ppm at 280 °C. Figure 13 shows the bar graph of selectivity of gas sensors fabricated by ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructures. One can find that ZnSnO3/SnO2 hollow urchin nanostructures (10, 20, 30 wt%) are all selectively responsive to 50 ppm acetone with the response values of 17.03, 15.75, and 14.07, respectively. At the same time, the selectivity values of ZnSnO3/SnO2 hollow urchin nanostructures (10, 20, 30 wt%) display the same trend that the order of their response values is acetone, ethanol, ammonia, methanol, and toluene from high to low, whereas the order of ZnSnO3 hollow microsphere response values is ethanol (7.521), acetone (6.4), ammonia (6.246), methanol (3.066), and toluene (1.887). Clearly, the ZnSnO3 hollow microspheres have bad selectivity to ethanol, which have no obvious difference in ethanol, acetone, and ammonia response. It seems that the selectivity of ZnSnO3/SnO2 hollow urchin nanostructures is better than that of ZnSnO3 hollow microspheres. For the same kind of gas, the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure exhibits the highest response compared with the ZnSnO3 hollow microspheres and other ZnSnO3/SnO2 hollow urchin nanostructures (20, 30 wt%), which corresponds to the previous investigations. That is to say, 10 wt% ZnSnO3/SnO2 hollow urchin not only has high response to acetone with different concentrations but also possesses enhanced selectivity to the reducing gases, especially to acetone. It is a good signal for the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure being deemed as a reliable diabetes biomarker.
In addition, repeatability is also an important factor needed to be considered to evaluate the application of gas sensor. In order to inspect the repeatability of fabricated gas sensors, three temporal curves of 10 wt% ZnSnO3/SnO2 gas sensor are conducted. As shown in Fig. 14a, the sensor maintains its initial response (17.03) without significant decrease upon three consecutive exposures to 50 ppm acetone at 280 °C, indicating its satisfying repeatability. Figure 14b depicts response values of 10 wt% ZnSnO3/SnO2 gas sensor vs time to 50 ppm acetone at 280 °C. It can be known that the maximum deviations of response are less than 8%, which confirms that the 10 wt% ZnSnO3/SnO2 gas sensor has good long-term stability for acetone detection.
Furthermore, a comparison of acetone gas-sensing performance of the previously reported gas sensors as well as the as-fabricated ZnSnO3/SnO2 was listed in Table 4. The ZnSnO3/SnO2 gas sensor exhibits the highest response to acetone when the variation temperature is less than 20% (Wang et al. 2016a, b; Li and Zeng 2017). The obtained results demonstrate that the ZnSnO3/SnO2 gas sensor is extremely promising for the detection of acetone.
Gas-sensing mechanism
It is believed that the gas-sensing mechanism of n-type semiconducting metal oxides can be explained by the surface depletion models (Barsan and Weimar 2001). ZnSnO3 and SnO2, as two kinds of typical semiconductor metal oxides, their response–recovery mechanism also can be comprehended in terms of the modulation of depletion layers that are mainly caused by oxygen adsorption (Liu et al. 2017). Figure 15 displays the detailed transformation processes of depletion layers for ZnSnO3 hollow microspheres and ZnSnO3/SnO2 hollow urchin nanostructure gas sensors. For ZnSnO3 hollow microspheres, when they are exposed to air, oxygen molecules absorb on the surface and ionize to chemisorbed oxygen species (O2 −, O−, and O2−) by trapping electrons from the ZnSnO3 conduction band. In this process, the electron depletion layers will be formed, leading to increase of the resistance. The reaction can be depicted as follows (Li et al. 2016):
When the ZnSnO3 hollow microspheres are exposed to acetone, the acetone will react with absorbed O−; the former electrons trapped by chemisorbed oxygen species naturally are released back to the conduction of ZnSnO3, resulting in the decrease of resistance. The reaction is as follows (Lian et al. 2017):
According to the above reactions, it is easily found that how to release more electrons from the material surface or interface to their conduction reducing the resistance of materials is significant to enhance the sensitivity of gas sensors. In other words, the key element to obtain highly sensitive gas-sensing materials is more adequate electron depletion layers.
It is worth mentioning that ZnSnO3/SnO2 hollow urchin gas sensors may have more complete electron depletion layers compared to ZnSnO3 hollow microsphere gas sensors. It can be attributed to the following aspects. Firstly, due to many tiny needle-like SnO2 nanowires anchoring on the surface of ZnSnO3/SnO2 nanostructures; the specific surface area is immensely improved, leading to more active sites forming on the surface of sensors. It is well acknowledged that more oxygen molecules absorb on the surface and more exhaustive electron depletion layers form (Ngo and Hur 2016), leading to high response of ZnSnO3/SnO2 nanostructures. In addition, the shell thickness (about 100 nm) of ZnSnO3/SnO2 nanostructures is nearly 20 times of the average diameter of tiny needle-like SnO2 nanowires, indicating that the gas diffusion to the whole sensing surface is not to be hampered (Yang et al. 2017). It can be seen as the reason of the fast response of ZnSnO3/SnO2 nanostructures. Secondly, for the ZnSnO3/SnO2 hollow urchin nanostructures in our work, the unique porous structures with a looser equipment urge the oxygen molecules to pass in and out from a part of ZnSnO3/SnO2 hollow urchin freely. That is to say, another electron depletion layer of the inner surface of ZnSnO3/SnO2 hollow urchin also generates. Thirdly, the Schottky junctions form owing to the different work function of ZnSnO3 and SnO2 (Bai et al. 2015). The electron migration at the interface between ZnSnO3 and SnO2 will happen, leading to thicker electron depletion layers on the interface (Wang et al. 2017). Finally, when acetone is introduced, the materials present conduction state with sharp decrease of the resistance. The gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures also enhances. Moreover, the measured results show that the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure exhibits higher response to acetone than 20 and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures. It can be related to the following two factors. One is that the redundant doping will decrease the quantity of conductive ions to cut down the response of sensors (Wang et al. 2015a). Another factor is that the sensitivity is controlled by gas diffusion rate at high T o, which is larger pore size and higher response of sensors (Wang et al. 2015a). As stated in Fig. 6, the pore size of 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure is 7.61 nm, larger than that of 20 and 30 wt% ZnSnO3/SnO2 hollow urchin nanostructures, which further confirms the above theory. Thus, 10 wt% doping concentration is deemed to be optimal.
Conclusions
In summary, the unique ZnSnO3/SnO2 hollow urchin nanostructures with high specific surface area and perfect permeability have been successfully prepared by a facile, eco-friendly two-step liquid-phase process. Many tiny needle-like SnO2 nanowires with the average diameter of 5 nm uniformly grow on the surface of ZnSnO3 hollow microspheres. The contrast experiments in different reaction conditions were investigated to understand the evolution process of the hollow urchin nanostructures. The sensors based on ZnSnO3/SnO2 hollow urchin nanostructures with different SnO2 content exhibit enhanced performance to various concentration levels of acetone, better selectivity, satisfying repeatability, and good long-term stability for acetone detection. The enhancement in gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures may be attributed to high specific surface area, looser structure with larger pore structure, and the increase of chemisorbed oxygen due to doping SnO2. The results also show that the 10 wt% ZnSnO3/SnO2 hollow urchin nanostructure as a superb gas-sensing material (17.03 for 50 ppm acetone) is hopeful to become the ideal candidate as a reliable diabetes biomarker.
References
Al-Hardan NH, Abdullah MJ, Aziz AA (2013) Performance of Cr-doped ZnO for acetone sensing. Appl Surf Sci 270:480–485. https://doi.org/10.1016/j.apsusc.2013.01.064
Bai SL, Chen C, Luo RX, Chen AF, Li DQ (2015) Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sensors Actuators B Chem 216:113–120. https://doi.org/10.1016/j.snb.2015.04.036
Barsan N, Weimar U (2001) Conduction model of metal oxide gas sensors. J Electroceram 7(3):143–167. https://doi.org/10.1023/A:1014405811371
Bing YF, Zeng Y, Liu C, Qiao L, Sui YM, Zou B, Zheng WT, Zou GT (2014) Assembly of hierarchical ZnSnO3 hollow microspheres from ultra-thin nanorods and the enhanced ethanol-sensing performances. Sensors Actuators B Chem 190:370–377. https://doi.org/10.1016/j.snb.2013.08.015
Boeglin ML, Wessels D, Henshel D (2006) An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ Res 100(2):242–254. https://doi.org/10.1016/j.envres.2005.04.004
Chen C, Li GZ, Li JH, Liu YL (2015) One-step synthesisof 3D flower-like Zn2SnO4 hierarchical nanostructures and their gas sensing properties. Ceram Int 41(1):1857–1862. https://doi.org/10.1016/j.ceramint.2014.09.136
Deng CH, Zhang J, Yu XF, Zhang W, Zhang XM (2004) Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J Chromatogr B 810(2):269–275. https://doi.org/10.1016/S1570-0232(04)00657-9
Dupin JC, Gonbeau D, Vinatier P, Levasseur A (2000) Systematic XPS studies of metal oxides hydroxides and peroxides. Phys Chem Chem Phys 2(6):1319–1324. https://doi.org/10.1039/a908800h
Fan HT, Zeng Y, Xu XJ, Lv N, Zhang T (2011) Hydrothermal synthesis of hollow ZnSnO3 microspheres and sensing properties toward butane. Sensors Actuators B Chem 153(1):170–175. https://doi.org/10.1016/j.snb.2010.10.026
Ghammraoui B, Rebuffel V, Tabary J, Paulus C, Verger L, Duvauchelle P (2012) Effect of grain size on stability of X-ray diffraction patterns used for threat detection. Nucl Instrum Methods Phys Res A 683:1–7. https://doi.org/10.1016/j.nima.2012.04.034
Guo WW (2017) One-pot synthesis of urchin-like ZnO nanostructure and its enhanced acetone gas sensing properties. J Mater Sci Mater Electron 28(1):963–972. https://doi.org/10.1007/s10854-016-5615-x
Han XG, He HZ, Kuang Q, Zhou X, Zhang XH, Xu T, Xie ZX, Zheng LS (2009) Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J Phys Chem C 113(2):584–589. https://doi.org/10.1021/jp808233e
Huang JR, Xu XJ, Gu CP, Wang WZ, Geng BY, Sun YF, Liu JH (2012) Size-controlled synthesis of porous ZnSnO3 cubes and their gas-sensing and photocatalysis properties. Sensors Actuators B Chem 171-172:572–579. https://doi.org/10.1016/j.snb.2012.05.036
Hyodo T, Abe S, Shimizu Y, Egashira M (2003) Gas-sensing properties of ordered mesoporous SnO2 and effects of coatings thereof. Sensors Actuators B Chem 93(1-3):590–600. https://doi.org/10.1016/S0925-4005(03)00208-9
Jain VK, Kumar P, Kumar M, Jainc P, Bhandari D, Vijay YK (2011) Study of post annealing influence on structural, chemical and electrical properties of ZTO thin films. J Alloys Compd 509(8):3541–3546. https://doi.org/10.1016/j.jallcom.2010.10.212
Jia XH, Tian MG, Dai RR, Lian DD, Han S, Wu XY, Song HJ (2017) One-pot template-free synthesis and highly gas sensing properties of ZnSnO3 hollow microspheres. Sens Actuators B Chem 240:376–385. https://doi.org/10.1016/j.snb.2016.08.146
Jin WX, Ma SY, Tie ZZ, Jiang XH, Li WQ, Luo J, Xu XL, Wang TT (2015) Hydrothermal synthesis of monodisperse porous cube, cake and spheroid-like α-Fe2O3 particles and their high gas-sensing properties. Sensors Actuators B Chem 220:243–254. https://doi.org/10.1016/j.snb.2015.05.098
Khan F, Baek S, Kim JH (2017) Influence of oxygen vacancies on surface charge potential and transportation properties of Al-doped ZnO nanostructures produced via atomic layer deposition. J Alloys Compd 709:819–828. https://doi.org/10.1016/j.jallcom.2017.03.133
Kida T, Fujiyama S, Suematsu K, Yuasa M, Shimanoe K (2013) Pore and particle size control of gas sensing films using SnO2 nanoparticles synthesized by seed-mediated growth: design of highly sensitive gas sensors. J Phys Chem C 117(34):17574–17582. https://doi.org/10.1021/jp4045226
Kim BK, Choi SD (2004) SnO2 thin film gas sensor fabricated by ion beam deposition. Sensors Actuators B Chem 98:239–246
Korotchenkov G (2005) Gas response control through structural and chemical modification of metal oxide films: state of the art and approaches. Sensors Actuators B Chem 107(1):209–232. https://doi.org/10.1016/j.snb.2004.10.006
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem Mater 13(10):3169–3183. https://doi.org/10.1021/cm0101069
Kuang XL, Liu TM, Zhang YY, Wang WX, Yang MP, Zeng W, Hussain S, Peng XH (2015) Urchin-like SnO2 nanoflowers via hydrothermal synthesis and their gas sensing properties. Mater Lett 161:153–156. https://doi.org/10.1016/j.matlet.2015.08.090
Lee J (2009) Gas sensors using hierarchical and hollow oxide nanostructures: overview. Sensors Actuators B Chem 140(1):319–336. https://doi.org/10.1016/j.snb.2009.04.026
Lemraski MS, Nadimi E (2017) Acetone gas sensing mechanism on zinc oxide surfaces: a first principles calculation. Surf Sci 657:96–103. https://doi.org/10.1016/j.susc.2016.11.013
Li TM, Zeng W (2017) New insight into the gas sensing performance of SnO2 nanorod-assembled urchins based on their assembly density. Ceram Int 43(1):728–735. https://doi.org/10.1016/j.ceramint.2016.10.001
Li ZJ, Lin ZJ, Wang NN, Huang YW, Wang JQ, Liu W, Fu YQ, Wang ZG, Wang NN (2016) Facile synthesis of α-Fe2O3 micro-ellipsoids by surfactant-free hydrothermal method for sub-ppm level H2S detection. Mater Des 110:532–539. doi:https://doi.org/10.1016/j.matdes.2016.08.035
Lian XX, Li Y, Tong XQ, Zou YL, Liu XL, An DM, Wang Q (2017) Synthesis of Ce-doped SnO2 nanoparticles and their acetone gas sensing properties. Appl Surf Sci 407:447–455. https://doi.org/10.1016/j.apsusc.2017.02.228
Likhodii SS, Musa K, Cunnane SC (2002) Breath acetone as a measure of systemic ketosis assessed in a rat model of the ketogenic die. Clin Chem 48(1):115–120
Liu C, Zhao LP, Wang BQ, Sun P, Wang QJ, Gao Y, Liang XS, Zhang T, Lu GY (2017) Acetone gas sensor based on NiO/ZnO hollow spheres: fast response and recovery, and low (ppb) detection limit. J Colloid Interface Sci 495:207–215. doi:https://doi.org/10.1016/j.jcis.2017.01.106
Nasution TI, Nainggolan I, Hutagalung SD, Ahmad KR, Ahmad ZA (2013) The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sensors Actuators B Chem 177:522–528. https://doi.org/10.1016/j.snb.2012.11.063
Ngo YT, Hur SH (2016) Low-temperature NO2 gas sensor fabricated with NiO and reduced graphene oxide hybrid structure. Mater Res Bull 84:168–176. https://doi.org/10.1016/j.materresbull.2016.08.004
Park S (2017) Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J Alloys Compd 696:655–662. https://doi.org/10.1016/j.jallcom.2016.11.298
Ren F, Gao LP, Yuan YW, Zhang Y, Alqrni A, Al-Dossary OM, Xu JQ (2016) Enhanced BTEX gas-sensing performance of CuO/SnO2 composite. Sens Actuators B Chem 223:914–920. https://doi.org/10.1016/j.snb.2015.09.140
Sun P, Sun YF, Ma J, You L, Lu GY, Fu WY, Li MH, Yang HB (2011) Synthesis of novel SnO2/ZnSnO3 core–shell microspheres and their gas sensing properities. Sensors Actuators B Chem 155(2):606–611. https://doi.org/10.1016/j.snb.2011.01.017
Sun G, Chen HL, Li YW, Ma GZ, Zhang SS, Jia TK, Cao JL, Wang XD, Bala H, Zhang ZY (2016) Synthesis and triethylamin sensing properties of mesoporous α-Fe2O3 microrods. Mater Lett 178:213–216. https://doi.org/10.1016/j.matlet.2016.04.209
Wang LL, Dou HM, Lou Z, Zhang T (2013) Encapsuled nanoreactors (Au@SnO2): a new sensing material for chemical sensors. Nano 5:2686–2691
Wang LL, Deng JN, Lou Z, Zhang T (2014) Cross-linked p-type Co3O4 octahedral nanoparticles in 1D n-type TiO2 nanofibers for high-performance sensing devices. J Mater Chem A 2(26):10022–10028. https://doi.org/10.1039/c4ta00651h
Wang TT, Ma SY, Cheng L, Luo J, Jiang XH, Jin WX (2015a) Preparation of Yb-doped SnO2 hollow nanofibers with an enhanced ethanol-gas sensing performance by electrospinning. Sensors Actuators B Chem 216:212–220. https://doi.org/10.1016/j.snb.2015.04.040
Wang XB, Wang YY, Tian F, Liang HJ, Wang K, Zhao XH, ZS L, Jiang K, Yang L, Lou XD (2015b) From the surface reaction control to gas-diffusion control: the synthesis of hierarchical porous SnO2 microspheres and their gas-sensing mechanism. J Phys Chem C 119(28):15963–15976. https://doi.org/10.1021/acs.jpcc.5b01397
Wang YL, Liu C, Wang L, Liu J, Zhang B, Gao Y, Sun P, Sun YF, Zhang T, Lu GY (2016a) Horseshoe-shaped SnO2 with annulus-like mesoporous for ethanol gas sensing application. Sens Actuators B Chem 240:1321–1329
Wang Q, Yao N, An DM, Li Y, Zou YL, Lian XX, Tong XQ (2016b) Enhanced gas sensing properties of hierarchical SnO2 nanoflower assembled from nanorods via a one-pot template-free hydrothermal method. Ceram Int 42(14):15889–15896. https://doi.org/10.1016/j.ceramint.2016.07.062
Wang Y, Zhao ZT, Sun YJ, Li PW, Ji JL, Chen Y, Zhang WD, Hu J (2017) Fabrication and gas sensing properties of Au-loaded SnO2 composite nanoparticles for highly sensitive hydrogen detection. Sensors Actuators B Chem 240:664–673. https://doi.org/10.1016/j.snb.2016.09.024
Xiao T, Wang XY, Zhao ZH, Li L, Zhang L, Yao HC, Wang JS, Li ZJ (2014) Highly sensitive and selective acetone sensor based on C-doped WO3 for potential diagnosis of diabetes mellitus. Sens Actuators B Chem 199:210–219. https://doi.org/10.1016/j.snb.2014.04.015
Yamazoe N, Sakai G, Shimanoe K (2003) Oxide semiconductor gas sensors. Catal Surv Jpn 7(1):63–75. https://doi.org/10.1023/A:1023436725457
Yang HM, Ma SY, Jiao HY, Chen Q, Lu Y, Jin WX, Li WQ, Wang TT, Jiang XH, Qiang Z, Chen H (2017) Synthesis of Zn2SnO4 hollow spheres by a template route for high-performance acetone gas sensor. Sensors Actuators B Chem 245:493–506. https://doi.org/10.1016/j.snb.2017.01.205
Yin L, Chen DL, Cui X, Ge LF, Yang J, Yu LL, Zhang B, Zhang R, Shao GS (2014) Normal-pressure microwave rapid synthesis of hierarchical SnO2@rGO nanostructures with super high surface areas as high-quality gas-sensing and electrochemical active materials. Nano 6:13690–13700
Yin ML, Yu LM, Liu SZ (2017) Synthesis of thickness-controlled cuboid WO3 nanosheets and their exposed facets-dependent acetone sensing properties. J Alloys Compd 696:490–497. https://doi.org/10.1016/j.jallcom.2016.11.149
Zeng Y, Bing YF, Liu C, Zheng WT, Zou GT (2012a) Self-assembly of hierarchical ZnSnO3-SnO2 nanoflakes and their gas sensing properties. Trans Nonferrous Met Soc China 22(10):2451–2458. https://doi.org/10.1016/S1003-6326(11)61484-2
Zeng W, Liu TM, Lin LY (2012b) Ethanol gas sensing property and mechanism of ZnSnO3 doped with Ti ions. Mater Sci Semicond Process 15(3):319–325. https://doi.org/10.1016/j.mssp.2012.02.003
Zhang CL, Wang J, Hu RJ, Qiao Q, Li XG (2016) Synthesis and gas sensing properties of porous hierarchical SnO2 by grapefruit exocarp biotemplate. Sensors Actuators B Chem 222:1134–1143. https://doi.org/10.1016/j.snb.2015.08.016
Zhang HL, Hui ZZ, Tang XW, Wei RH, Yang J, Dai JM, Song WH, Luo HM, Zhu XB, Sun YP (2017a) Self-assembled c-axis oriented δ-MoN thin films on Si substrates by chemical solution deposition: growth, transport and superconducting properties. J Alloys Compd 704:453–458. doi:https://doi.org/10.1016/j.jallcom.2017.02.084
Zhang S, Song P, Zhang J, Yan HH, Li J, Yang ZX, Wang Q (2017b) Highly sensitive detection of acetone using mesoporous In2O3 nanospheres decorated with Au nanoparticles. Sens Actuators B Chem 242:983–993. https://doi.org/10.1016/j.snb.2016.09.155
Funding
This study was funded by the National Natural Science Foundation of China (No. 51372103 and 51202092).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lian, D., Shi, B., Dai, R. et al. Synthesis and enhanced acetone gas-sensing performance of ZnSnO3/SnO2 hollow urchin nanostructures. J Nanopart Res 19, 401 (2017). https://doi.org/10.1007/s11051-017-4094-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-4094-1