Abstract
Nanosized Ag2CrO4/few layer boron nitride composites were prepared via in situ precipitation method. The crystal structure, morphology, optical properties, and charge carrier behavior were investigated by X-ray diffraction, transmission electrical microscopy, UV-vis diffuse reflectance spectroscopy, and electrochemical impedance spectroscopy, respectively. The photocatalytic activities of the as-prepared hybrids were discussed by degradation of rhodamine B under visible-light irradiation. Experimental results showed that the average size of pure Ag2CrO4 particles was about 20 nm. Moreover, the degradation efficiency of the as-prepared hybrids was first increased and then decreased with increasing the usage amount of few layer boron nitride nanosheets. When it was 10 wt%, in 120 min, the degradation efficiency of the as-prepared hybrids had reached the maximum of 96.7%. It was much higher than 75% of pure Ag2CrO4 nanoparticles. After 3 cycles of the degradation, the efficiency of the as-prepared composites was decreased from 96.7 to 91.8%. Trapping experiment results revealed that holes played a major role during the photocatalysis process. In addition, electrochemical impedance spectroscopy results indicated that few layer boron nitride nanosheets could enhance the separation and transfer of photogenerated electrons and holes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, environmental problem in many countries becomes more and more serious due to the industrialization, population growth, emission of poisonous pollutants, and so on (Tong et al. 2012; Pirhashemi and Habibi-Yangjeh 2015). Recently, many semiconductors, such as TiO2 (Wandre et al. 2015), ZnO (Peng et al. 2013), and Zn2SnO4 (Mali et al. 2015), can be activated under the UV irradiation. However, those visible-light-driven photocatalysts, such as AgBr (Zhao et al. 2016), Ag3VO4 (Pirhashemi and Habibi-Yangjeh 2016a, b), Ag2CrO4 (Pirhashemi and Habibi-Yangjeh 2016a, b), and Ag2SO4 (Cao et al. 2015), have drawn much attentions due to their wide absorption range under the visible light. They can be used as the efficient photocatalysts. Among them, Ag2CrO4 is an important one for its strong optical absorption coefficient in visible-light region as well as narrow band gap. However, the photocatalytic activity of Ag2CrO4 is usually weakened by photocorrosion (Yu et al. 2014a, b, 2016; Putri et al. 2015; Komtchou et al. 2016) and the recombining of photogenerated electrons and holes. Researchers find that it can be improved by using some two-dimensional materials. For example, Shang et al. have reported that g-C3N4 could increase the photocatalytic efficiency of Ag2CrO4 (Shang et al. 2017). Moreover, Xu et al. have showed that graphene could also improve the organic pollutant degradation of Ag2CrO4 (Xu et al. 2015).
Moreover, recent reports show that boron nitride (BN) can enhance the photocatalytic properties of many Ag-based photocatalysts, such as Ag3VO4 (Lv et al. 2016), AgI (Choi et al. 2015), and Ag2PO4 (Song et al. 2014). As is well-known, few layer boron nitride nanosheets (FBNNS), which can be prepared from BN, possess excellent optical properties, thermal, chemical stability, and high specific surface area (Marsh et al. 2015; Li et al. 2013). However, as far as we have known, there were no reports for using FBNNS to improve the photocatalytic properties of pure Ag2CrO4 nanoparticles.
In this work, the Ag2CrO4/FBNNS hybrids with various usage amounts of FBNNS were prepared via in situ precipitation method. The photocatalytic activities of the as-prepared hybrids were evaluated by degradation of rhodamine B (RhB) solution under the visible light. Experimental results indicated that FBNNS could obviously improve the photocatalytic activities of pure Ag2CrO4 and the possible photocatalytic mechanism was also proposed.
Experimental section
Materials
FBNNS were prepared basing on our previous report (Wu et al. 2017). Potassium chromate (K2CrO4), silver nitrate (AgNO3), and RhB were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All the chemicals were analytical grade.
Preparation of the Ag2CrO4/FBNNS hybrids
Firstly, a certain amount of FBNNS (0, 2, 6, 10, or 14 wt% basing on the weight of K2CrO4) was stirred with 40 mL H2O for 30 min. Subsequently, 20 mL 0.05 mmol/mL AgNO3 solution and 40 mL 0.0125 mmol/mL K2CrO4 solution were slowly added into the above mixture, respectively. Finally, the obtained precipitate was fully washed by using distilled water and dried at 50 °C for 6 h. According to the usage amount of FBNNS, the as-prepared Ag2CrO4/FBNNS hybrids were labeled as Ag2CrO4/FBNNS-2 wt%, Ag2CrO4/FBNNS-6 wt%, Ag2CrO4/FBNNS-10 wt%, or Ag2CrO4/FBNNS-14 wt%, respectively.
Characterization
The X-ray diffraction (XRD) patterns of the as-prepared samples were measured by X-ray diffraction (XRD, Model: D8ADVANCE, Bruker Co., Germany) with Cu-Kâ radiation. The UV-visible absorbance spectra of RhB were measured by a UV-visible spectrophotometer (Model: UV-722N, Shanghai Precision Instrument Co., Ltd., China). The solid state UV-vis diffuse reflectance spectra of the samples were obtained by using a UV/VIS/NIR spectrometer (DRS, Model: U-4100, Shimadzu Co., Japan) equipped with a diffuse reflectance accessory. The morphologies of the products were characterized by a transmission electrical microscope (TEM, Model: JEM-2100, JEOL Co., Japan) with an accelerating voltage of 120 kV.
Measurements of photocatalytic activity and electrochemical performances
The photocatalytic activities of the as-prepared Ag2CrO4/FBNNS hybrids were evaluated by degradation of RhB under visible light (λ > 420 nm). A 300-W Xe lamp was adopted as the light source with a 420-nm cutoff filter to provide visible-light irradiation. For degradation of RhB, 50 mg of the as-prepared hybrids were added into 150 mL RhB solution with an initial concentration of 10 mg/L. Before the illumination, the solution was stirred for 1 h in the dark to reach the adsorption-desorption equilibrium between the photocatalyst and RhB. Moreover, at 30 min interval, 4 mL of the suspension was collected and centrifuged to remove the photocatalyst. Then, the concentration of RhB was detected by a UV-vis spectrometer at the characteristic band of 553 nm. The photocatalytic decomposition kinetics was also investigated, and the Langmuir-Hinshelwood model was arranged as the following:
where C 0 and C are the adsorption equilibrium and RhB concentration at reaction time t, respectively. Moreover, k is the apparent reaction rate constant.
Electrochemical measurements were performed on an electrochemical workstation (Model: CHI660E, Shanghai Chenhua Co., China) by using a standard three-electrode cell with a working electrode, a platinum wire as counter electrode, and a standard Ag/AgCl in saturated KCl as reference electrode, respectively. The working electrodes were prepared by dip-coating as follows: 4 mg of the as-prepared photocatalyst was dispersed in 760 μL deionized water, 40 μL ethyl alcohol, and 20 μL nafion membrane solution to produce a suspension, which was then dip-coated onto the glass carbon electrode. The electrochemical impedance spectroscopies (EIS) were carried out at the open circuit potential within a frequency range from 0.01 to 105 Hz. In addition, 40 mL 0.5 M KNO3 solution was used in the experiments containing 0.1 M KCl as an electrolyte.
Results and discussion
XRD analysis
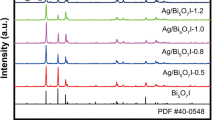
XRD patterns of pure Ag2CrO4 and the as-prepared Ag2CrO4/FBNNS hybrids with various mass fractions of FBNNS are shown in Fig. 1. It is seen in Fig. 1a that the diffraction peak at 26.4° was corresponded to (002) plane of FBNNS according to JCPDS No. 34-0421 (Fan et al. 2016). Moreover, in Fig. 1b–f, other peaks, especially that typical one at 32.4°, of the as-prepared Ag2CrO4/FBNNS samples could be attributed to Ag2CrO4 according to JCPDS No. 26-0952. These peaks were also in accord with previous report (Silva et al. 2016). It indicated that FBNNS did not change the structure of pure Ag2CrO4 nanoparticles.
RhB was chosen as the target pollutant to characterize the photocatalytic activity of pure Ag2CrO4 and the as-prepared Ag2CrO4/FBNNS hybrids under the visible light. It is seen in Fig. 2a that pure FBNNS had no effects on degradation of RhB solution. However, the as-prepared Ag2CrO4/FBNNS samples possessed better photocatalytic active than that of pure Ag2CrO4. Moreover, it could be seen that the usage amount of FBNNS obviously affected the photocatalytic properties of the as-prepared hybrids. It was first increased and then decreased with increasing the usage amount of the as-prepared samples. When the usage amount of FBNNS was 10 wt%, in 120 min, the as-prepared composites exhibited the highest photocatalytic activity of 96.7%. This was obviously higher than 75% of pure Ag2CrO4. These results might be caused by fewer usage amount of FBNNS that could improve the photocatalytic properties of pure Ag2CrO4 particles. However, excess amount of FBNNS might be impurities and hindered the light absorption of Ag2CrO4, restricting the improvement of photocatalytic activity.
RhB concentration versus visible-light irradiation time of the as-prepared samples (a). Kinetic linear simulation curves of the as-prepared samples (b). Rate constants κ of as-prepared samples under the irradiation of visible light (λ > 420 nm) (c). Cycling runs of pure Ag2CrO4 and as-prepared Ag2CrO4/FBNNS-10 wt% samples (d)
The reaction rate k was also investigated by fitting the first-order kinetic (Ahmed et al. 2016). It is seen in Fig. 2b, c that the k of the as-prepared Ag2CrO4/FBNNS-10 wt% was 1.5 times than that of pure Ag2CrO4. In addition, the k of the as-prepared Ag2CrO4/FBNNS composites was first increased and then decreased with increasing the usage amount of FBNNS. This result is similar with that of Fig. 2a.
In Fig. 2d, the degradation efficiency of pure Ag2CrO4 nanoparticles was decreased by 10.6% from 96.4 to 85.8% after it was circulated for three times. However, for the as-prepared Ag2CrO4/FBNNS-10 wt% sample, it was decreased by 4.9% from 96.7 to 91.8%. This indicated that the as-prepared hybrids were more stable than that of pure Ag2CrO4 during the photocatalytic process. The possible reason might be that the photoexcited electrons of Ag2CrO4 were incorporated into the FBNNS nanosheets, which was in favor of existing of the photogenerated electron-hole pairs.
TEM analysis
The morphologies of Ag2CrO4 nanoparticles and the as-prepared Ag2CrO4/FBNNS-10 wt% hybrids are shown in Fig. 3. It could be observed that the average size of Ag2CrO4 particles was about 20 nm, as is shown in Fig. 3a. They were well dispersed on the surfaces of the FBNNS, as is shown in Fig. 3b. This result revealed that the Ag2CrO4 nanoparticles might couple with FBNNS through physisorption, electrostatic binding, or charge transfer interactions.
UV-vis diffuse reflectance spectra analysis
Figure 4a shows that all the samples displayed a sharp absorption in the range of 600–750 nm. Moreover, the absorption plots of the as-prepared Ag2CrO4/FBNNS-10 wt% samples exhibited a slight red-shift compared to pure Ag2CrO4, as shown as the illustration in Fig. 4a. This result indicated that the band gap of the as-prepared Ag2CrO4/FBNNS hybrids was slightly narrow than that of pure Ag2CrO4 sample.
Figure 4b shows the plot of (αhν)1/2 versus photon energy (hν) for pure Ag2CrO4 and the as-prepared composites. An enlarged image of the as-prepared Ag2CrO4/FBNNS-10 wt% nanocomposites, pure Ag2CrO4, and the band gap energy was shown in the top right corner. It could be seen that the band gap energy of the as-prepared Ag2CrO4/FBNNS-10 wt% was 1.60 eV, which was lower than 1.64 eV of pure Ag2CrO4. This result indicated that FBNNS could slightly reduce the band gap energy of pure Ag2CrO4.
EIS analyses
EIS is usually adopted to discuss the charge transfer phenomena at the interfaces of semiconductor photoelectrodes (You et al. 2016). It could be observed in Fig. 5 that the impedance semicircle size of the pure Ag2CrO4 sample and the as-prepared Ag2CrO4/FBNNS-10 wt% hybrids was the biggest and smallest, respectively. This phenomenon indicated that the as-prepared Ag2CrO4/FBNNS hybrids, especially the sample of Ag2CrO4/FBNNS-10 wt%, could promote the separation efficiency of electron and hole compared with pure Ag2CrO4. That was to say, the as-prepared Ag2CrO4/FBNNS hybrids, especially the sample of Ag2CrO4/FBNNS-10 wt%, possessed faster charge transfer and better photocatalytic performance than that of pure Ag2CrO4. This result was in keeping with that of Fig. 2a.
The possible mechanism of photocatalytic activity
In order to further explore the photocatalytic mechanism of the as-prepared Ag2CrO4/FBNNS-10 wt% hybrids. The main active species (holes and hydroxyl cavenger) for the degradation of RhB were assessed by the trapping experiments. As is shown in Fig. 6, the degradation efficiency of RhB was slightly decreased by addition of tertiary butanol (t-BuOH, a hydroxyl radical scavenger) (Xu et al. 2016), while it was remarkably decreased by addition of ethylene diamine tetraacetic acid (EDTA, hole scavenger) (Xing et al. 2015). This result revealed that holes were the main active species during the photocatalytic process.
Based on the above results, the possible charge transfer schematic of the degradation of RhB under visible light for the as-prepared Ag2CrO4/FBNNS-10 wt% hybrids is proposed in Fig. 7. It could be seen that the electrons of Ag2CrO4 nanoparticles at Valence Band (VB) were excited and transferred to Conduction Band (CB) under visible-light irradiation. The holes were leaved in the VB of Ag2CrO4. The excited electrons in the CB of Ag2CrO4 could transfer to the surfaces of FBNNS due to the interfacial interaction between Ag2CrO4 and FBNNS. This effort could suppress the recombination of photogenerated electrons and holes and enhance the photocatalytic activity. At the same time, the excited electrons on the surfaces of FBNNS could be generated to form ·O2 − with oxygen. Moreover, the excited holes in the Ag2CrO4 could form OH· with H2O. Finally, the superoxide radical (·O2 −) and holes (h+) could oxidize RhB into H2O and CO2 during the photocatalytic reaction.
Conclusions
In summary, the Ag2CrO4/FBNNS hybrids were prepared via in situ precipitation method. The experimental results showed that the average size of Ag2CrO4 particles was about 20 nm and the as-prepared particles were well dispersed on the surfaces of FBNNS. Moreover, the usage amount of FBNNS played an important role on degradation efficiency of the samples. It was first increased and then decreased with increasing the usage amount of the as-prepared samples. When the usage amount of FBNNS was 10 wt%, in 120 min, the composites exhibited highest photocatalytic activity of 96.7%, which was higher than 75% of pure Ag2CrO4. FBNNS could decrease the band gap energy of pure Ag2CrO4, and that of the as-prepared Ag2CrO4/FBNNS-10 wt% samples was 1.61 eV. In addition, the holes played mainly active effects during the photocatalytic reaction process. This work could provide potential application in solving the organic pollutant and possess reference values for related works.
References
Ahmed G, Hanif M, Zhao L, Hussain M, Khan J, Liu Z (2016) Defect engineering of ZnO nanoparticles by graphene oxide leading to enhanced visible light photocatalysis. J Mol Catal A-Chem 425:310–321
Cao W, Chen L, Qi Z (2015) Microwave-assisted synthesis of Ag/Ag2SO4/ZnO nanostructures for efficient visible-light-driven photocatalysis. J Mol Catal A-Chem 401:81–89
Choi J, Reddy DA, Kim TK (2015) Enhanced photocatalytic activity and anti-photocorrosion of AgI nanostructures by coupling with graphene-analogue boron nitride nanosheets. Ceram Int 41:13793–13803
Fan D, Feng J, Liu J, Gao T, Ye ZM (2016) Chen, X. Lv, hexagonal boron nitride nanosheets exfoliated by sodium hypochlorite ball mill and their potential application in catalysis. Ceram Int 42:7155–7163
Komtchou S, Dirany A, Drogui P, Delegan N, El Khakani MA, Robert D, Lafrance P (2016) Degradation of atrazine in aqueous solution with electrophotocatalytic process using TiO2-x photoanode. Chemosphere 157:79
Li X, Hao X, Zhao M, Wu Y, Yang J, Tian Y, Qian G (2013) Exfoliation of hexagonal boron nitride by molten hydroxides. Adv Mater 25:2200–2204
Lv X, Wang J, Yan Z, Jiang D, Liu J (2016) Design of 3D h-BN architecture as Ag3VO4 enhanced photocatalysis stabilizer and promoter. J Mol Catal A-Chem 418-419:46–153
Mali SS, Shim CS, Hong CK (2015) Highly porous zinc stannate (Zn2SnO4) nanofibers scaffold photoelectrodes for efficient methyl ammonium halide perovskite solar cells. Sci Rep 5:11424
Marsh KL, Souliman M, Kaner RB (2015) Co-solvent exfoliation and suspension of hexagonal boron nitride. Chem Commun 51:187–190
Peng Y, Qin S, Wang WS, Xu AW (2013) Fabrication of porous Cd-doped ZnO nanorods with enhanced photocatalytic activity and stability. Cryst Eng Comm 15:6518–6525
Pirhashemi M, Habibi-Yangjeh (2016a) A novel ZnO/Ag2CrO4 nanocomposites with n-n heterojunctions as excellent photocatalysts for degradation of different pollutants under visible light. J Mater Sci-Mater El 27:4098–4108
Pirhashemi M, Habibi-Yangjeh (2016b) A ultrasonic-assisted preparation of novel ternary ZnO/Ag3VO4/Ag2CrO4 nanocomposites and their enhanced visible-light activities in degradation of different pollutants. Solid State Sci 55:58–68
Pirhashemi M, Habibi-Yangjeh A (2015) Ternary ZnO/AgBr/Ag2CrO4 nanocomposites with tandem n-n heterojunctions as novel visible-light-driven photocatalysts with excellent activity. Ceram Int 41:14383–14393
Putri LK, Ong WJ, Wei SC, Chai SP (2015) Heteroatom doped graphene in photocatalysis: a review. Appl Surf Sci 358:2–14
Shang Y, Chen X, Liu W, Tan P, Chen H, Wu L, Ma C, Xiong X, Pan J (2017) Photocorrosion inhibition and high-efficiency photoactivity of porous g-C3N4/Ag2CrO4 composites by simple microemulsion-assisted co-precipitation method. Appl Catal B-Environ 204:8–88
Silva GS, Gracia L, Fabbro MT, Serejo Dos Santos LP, Beltran-Mir H, Cordoncillo E, Longo E, Andres J (2016) Theoretical and experimental insight on Ag2CrO4 microcrystals: synthesis, characterization, and photoluminescence properties. Inorg Chem 55:8961–8970
Song Y, Xu H, Wang C, Chen J, Yan J, Xu Y, Li Y, Liu C, Li H, Lei Y (2014) Graphene-analogue boron nitride/Ag3PO4composite for efficient visible-light-driven photocatalysis. RSC Adv 4:56853–56862
Tong H, Ouyang S, Bi Y, Umezawa N, Oshikiri M, Ye J (2012) Nano-photocatalytic materials: nano-photocatalytic materials: possibilities and challenges. Adv Mater 24:229–251
Wandre TM, Gaikwad PN, Tapase AS, Garadkar KM, Vanalakar SA, Lokhande PD, Sasikala R, Hankare PP (2015) Sol-gel synthesized TiO2-CeO2 nanocomposite: an efficient photocatalyst for degradation of methyl orange under sunlight. J Mater Sci-Mater El 27:825–833
Wu X, Zhao ZH, Sun Y, Li H, Zhang C, Wang Y, Zeng SS, Zhang H (2017) Few-layer boron nitride nanosheets: preparation, characterization and application in epoxy resin. Ceram Int 43:2274–2278
Xing Y, Que W, Yin X, Liu X, Javed HMA, Yang Y, Kong LB (2015) Fabrication of Bi2Sn2O7-ZnO heterostructures with enhanced photocatalytic activity. RSC Adv 5:27576–27583
Xu D, Cheng B, Cao S, Yu J (2015) Enhanced photocatalytic activity and stability of Z-scheme Ag2CrO4-GO composite photocatalysts for organic pollutant degradation. Appl Catal B-Environ 164:380–388
Xu H, Liu L, Song Y, Huang L, Li Y, Chen Z, Zhang Q, Li J (2016) BN nanosheets modified WO3 photocatalysts for enhancing photocatalytic properties under visible light irradiation. J Alloy Compd 660:48–54
You D, Pan B, Jiang F, Zhou Y, Su W (2016) CdS nanoparticles/CeO2 nanorods composite with high-efficiency visible-light-driven photocatalytic activity. Appl Surf Sci 363:154–160
Yu C, Li G, Kumar S, Yang K, Jin R (2014b) Phase transformation synthesis of novel Ag2O/Ag2CO3 heterostructures with high visible light efficiency in photocatalytic degradation of pollutants. Adv Mater 26:892–898
Yu C, Wei L, Zhou W, Chen J, Fan Q, Liu H (2014a) Enhancement of the visible light activity and stability of Ag2CO3, by formation of Ag2O/Ag2CO3, heterojunction. Appl Surf Sci 319:312–318
Yu C, Zhou W, Hong L, Yuan L, Dionysiou DD (2016) Design and fabrication of microsphere photocatalysts for environmental purification and energy conversion. Chem Eng J 287:117–129
Zhao Z, Wang M, Yang T, Fang M, Zhang L, Zhu H, Tang C, Huang Z (2016) In situ co-precipitation for the synthesis of an Ag/AgBr/Bi5O7I heterojunction for enhanced visible-light photocatalysis. J Mol Catal A-Chem 424:8–16
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Youth Science and Technology Foundation of Higher Education Institutions of Hebei Province, China (grant number Q2012111), and Natural Science Foundation of Hebei Province, China (grant number E2013210011).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, Xf., Zhao, Zh., Sun, Y. et al. Preparation and characterization of Ag2CrO4/few layer boron nitride hybrids for visible-light-driven photocatalysis. J Nanopart Res 19, 193 (2017). https://doi.org/10.1007/s11051-017-3892-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3892-9