Abstract
Density functional theory based calculations were performed to study vibrational properties of ceria, titania, and ceria-titania hybrid clusters. The findings revealed the dominance of vibrations related to oxygen when compared to those of metallic atoms in the clusters. In case of hybrid cluster, the softening of normal modes related to exterior oxygen atoms in ceria and softening/hardening of high/low frequency modes related to titania dimmers are observed. The results calculated for monomers conform to symmetry predictions according to which three IR and three Raman active modes were detected for TiO2, whereas two IR active and one Raman active modes were observed for CeO2. The comparative analysis indicates that the hybrid cluster CeTiO4 contains simultaneous vibrational fingerprints of the component dimmers. The symmetry, nature of vibrations, IR and Raman activity, intensities, and atomic involvement in different modes of the clusters are described in detail. The study points to engineering of CeTiO4 to tailor its properties for technological visible region applications in photocatalytic and electrochemical devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The study of small clusters has been an important research area, since it provides an interface and preliminary probe to reach the new phase of solids (de Jongh 1994). It provides an insight to understand the initial stages of growth process, nucleation, and formation of embryo to achieve nanomaterial, thin film, or bulk material (Barry et al. 2014). The entire properties and performance of resulting applications depend upon material properties which have direct connection with its crystalline structure and geometry. The diffraction studies are usually employed to experimentally access the structural properties of materials. On the other hand, by theoretical point of view, vibrational properties are well-known fingerprint of the structure and geometry of the materials (Jedidi et al. 2015). Despite availability of current state-of-art fabrication facilities of bulk materials, the controlled growth of small clusters is a challenging task. Therefore, the study of vibrational properties of small clusters of heterometallic nature is expected to provide a framework for the experimentalists to synthesis functional materials based on these building blocks.
Metallic oxides are renowned materials for applications in hydrogen production, energy storage, degradation treatment, batteries, supercapacitors, and several other applications in semiconducting/electronic/optical/optoelectronic devices (Henrich and Cox 1994). Keeping in view, the future demands of multifunctional materials for application in ultrafast, miniature, low-power, and low-cost devices there have been efforts to modify the properties of the existing metallic compounds (Salonitis et al. 2010; Tedstone et al. 2016). In order to boost up the performance of metallic oxides and introduce novel functionalities, combination of different materials achieved by different processes including doping, alloying, coating, dye sensitization, coupling, etc. is used (Park et al. 2010). Titania (TiO2) and ceria (CeO2) are two versatile metallic oxides containing metallic constituents from transition metal and rare earth metals, respectively.
Ceria has been extensively studied due to its outstanding physical and chemical properties for applications in sensors, oxygen storage/release, and thermochemical and electrochemical devices (Arurault et al. 2008; Tiziano et al. 2016). It is reported to show mixed ionic/electronic conductivity enabling its usage as mixed conductor (Tuller and Nowick (1975). Despite considerably high ionic conductivity than that of zirconia, ceria due to its mixed conductivity could not earn repute as electrolyte in fuel cells. It is generally believed that electronic part of total conductivity should not be higher than 1% for solid oxide to be used in fuel cell. Ceria-based materials due to oxygen partial pressure dependent thermal expansion rates offer their usage at temperatures less than 800 °C which makes these materials potential candidates for usage as electrolytes offering reduced operating temperatures (Blomen and Mugerwa 1993). Achieving ceria-based electrode, electrolyte, and interconnector materials having optimum electronic/ionic conductivity is highly desirable for improving the performance of electrochemical devices. The other uses consist of polishing the surfaces, filters, and catalytic convertors.
Titania on the other hand, besides conventional applications in UV resistance, coating, ceramics, printing ink, cosmetic, anti-degradation, paper industry, etc., has been found a potential material for electronic and optoelectronic usages (Gupta and Tripathi 2011). After demonstration of titania’s potential for water splitting from Fujishima and Honda in 1972, a new field of semiconductor photocatalysis emerged (Fujishima and Honda 1972). Titania is optically active in UV region due to which significant research efforts have been carried out to develop strategies to modify its properties for usage in visible region of electromagnetic spectrum (Pelaez et al. 2012). Achieving visibly active titania-based material is expected to provide breakthrough in ongoing research efforts for searching efficient materials for water purification, anti-fogging, self-cleaning, dye-sensitized solar cell, and photoelectrochemical applications (Wang et al. 1997; O’Regan and Gratzel 1991).
There have been few efforts to prepare ceria-titania compounds for different applications (Park et al. 2009; Lin and Xiaoming 2008; Shah et al. 2016). Park et al. reported preparation of architecture of ceria-titania catalysts synthesized through wet chemical methods (Park et al. 2009). They observed notably high catalytic activity for structures comprising of layers of metals/ceria/titania when compared with individual metal oxide counterparts. The synthesis of CeO2/TiO2 nanoparticles through colloid seeded deposition process and strong interaction between ceria and titania has been reported (Lin and Xiaoming 2008). An increase in catalytic activity of ceria upon ultrasound-assisted coating with titania has also been reported (Shah et al. 2016). However, these and several other reported strategies appeared to complicate the structure which introduce issues related to reproducibility of the properties of the architectures.
The analysis of vibrational properties gives a direct insight into nature of chemical bonding and structure of the materials upon which major properties depend. Therefore, vibrational spectroscopy provides highly useful and quick technique to investigate the materials for exploration of their potentials. IR (infrared) and Raman are two fundamental and supplementary techniques for vibrational characterization of materials which explore respective periodic changes of dipole moments and polarizabilities produced by atomic/molecular vibrations (Wartewig 2003). Investigation of Raman scattering provides vibrational fingerprint of the molecule on the basis of inelastic scattering of light from it (Valley et al. 2013). These techniques explore vibrational modes which are ways in which molecule or cluster can vibrate and any molecule offer a number of such modes in accordance with group theory. In case of simple molecules having center of symmetry, the vibrations are IR active/Raman inactive and vice versa. However, in other molecules which do not have inversion symmetry, the modes may be simultaneously IR and Raman active or simultaneously inactive besides individual characters. On the other hand, all normal modes are concurrently IR and Raman active for larger and complex molecules having low symmetry or no symmetry. The complex vibrational modes are superposition of simple and basic vibrations. The entire theoretically allowed vibrational modes in case of complex structures are sometimes observed, and sometimes these are masked by neighboring stronger modes.
When a vibrational mode becomes aligned with same symmetry component of polarizability, it becomes Raman active. Therefore, higher intensity Raman mode points to higher polarizability which indicates preferential ease to distort the electronic cloud (Parker 1983). It can also be said that more intense Raman mode points to availability of preferentially dense electronic cloud. IR spectroscopy is sensitive to change in dipole moment, whereas Raman spectroscopy provides information about change in polarizibility of the molecules under study. Though both techniques differ in several aspects, their simultaneous use has been a reliable tool to provide thorough molecular information (Nakamoto 2006). We report here a study on possibilities to produce ceria-titania hybrid material on the basis of the first principles investigations. This work was carried out with motivation to predict a material for energy applications having high catalytic activity and good chemical as well as physical stability.
Computational details
The entire work was performed using linear combination of atomic orbitals (LCAO) scheme within local density approximation (LDA) and hybrid functional B3LYP (Becke, three-parameter, Lee-Yang-Parr) schemes implemented in Amsterdam Density Functional (ADF) molecule package (te Velde 2001). The anatase geometry of TiO2 was taken as starting structure for simulating TiO2 molecule in which Ti was replaced by Ce to produce CeO2 molecule. The structure of TiO2 was assembled in the form of neutral clusters Ti2O4 using graphical user interface (GUI) of ADF. The relaxed structure and shape of Ti2O4 was found in agreement with the literature (Kim 2014). The clusters Ce2O4 and CeTiO4 were also produce accordingly. The structures were fully relaxed and geometrically optimized using valance quadruple zeta plus polarization functions for all atoms/electrons (QZ4P) slater-type orbital (STO) basis sets available in ADF library (Van Lenthe and Baerends 2003). The calculations were non-spin-polarized and non-relativistic. The slater-type orbitals of QZ4P quality available in ADF library suitably deal with 4f states and yield right order of orbital energies (Van Lenthe and Baerends 2003). All electrons calculations were carried out without freezing any orbital in case of B3LYP. The convergence criterion was taken as 10−6 eV/molecule, and gradient of 0.001 au/Å was taken for all studied structures. The value of relative error in numerical integration grid was found to be of the order of 10−7 according to test of precision. For numerical integrations, Becke grid quality “very good” was employed, whereas the density fitting (ZlmFit) quality was also chosen as very good. This tight convergence criterion appears to provide precise integrations and energy convergence which are needed for accurate numerical differentiations to avoid predictions of imaginary frequencies in ADF (Bridgeman and Cavigliasso 2002). Hessian update method of Broyden-Fletcher-Goldfarb-Shanno was used. The computational error in energy and bond length was estimated to be 0.001 Hartree and 0.001 pm, respectively. A mix parameter of 0.2 was used when direct inversion in iteration space does not apply. The nuclei were treated as point charges and frozen core approximation was adopted to treat electronic energy levels. For oxygen, 5 valance basis sets were used with 1s2 as frozen leaving 2s22p4 as valance states, whereas for Ti, 11 valance basis sets were used with 3d24s24p as valance states. In case of Ce, the number of valance basis sets was 21 with frozen subshells up to 4d and 4f1 5d1 6s2 as valance shells. Hirshfeld charge analysis is given which is a robust way to produce meaningful charges (Guerra et al. 2004).
A full scan of vibrational frequencies was performed. ADF performs numerical calculation of frequencies by employing differentiation of energy gradients (Fan and Ziegler 1992). The calculation of bond energy was performed by computing the difference of energies of entire molecule and constituent atoms which were taken as spherically symmetric and spin restricted (Bickelhaupt and Baerends 2000). Thermodynamic properties including heat capacity, internal energy, entropy, moment of interia, etc. were calculated at room temperature (at 298.15 K) and atmospheric pressure of 1 atm within ideal gas approximation, thereby ignoring the electronic contributions. The analysis of thermal properties was carried out using partition function depending upon temperature, and the contributions from normal modes having frequencies lower than 20 cm−1 are ignored. ADF calculates electronic internal energy by assuming spherically averaged neutral atoms. The values of gas phase PV/n = RT, enthalpy H = U + PV, and Gibbs free energy G = H−TS were also calculated.
Results and discussions
Prior to the study of the properties of hybrid cluster CeTiO4, the starting monomers TiO2 and CeO2 were investigated. The optimized geometry of TiO2 molecule revealed LDA (B3YLP) calculated Ti=O bond length of 167.2 pm (164.2 pm) and bond O=Ti=O angle of 108.4° (111.5°) which are consistent with the literature (Wu and Wang 1997). The LDA (B3YLP) calculated value of bond energy is −20.037 eV (−24.968 eV). The molecule is in C2V symmetry having closed shell configuration with irreducible representations including subspecies A1, A2, B1, and B2 (Chen et al. 2015; Bunker and Jensen 1998). The value of gross atomic charge (per Hirshfeld population analysis) is −0.4453 per O and 0.8905 per Ti. The calculated value of dipole moment per analytic integration is 7.064 Debye (6.755 Debye). The symmetry of the molecule indicates twofold rotation about axis of symmetry C2 in such a way that it has two Ϭv planes passing through C 2-axis of the molecule (Bunker and Jensen 1998). If C-axis is taken along z-axis then mirror plans will be denoted by Ϭxz and Ϭyz. Therefore, the molecule has four symmetry elements named as \( E,{\mathrm{C}}_2^{\mathrm{z}},{\upsigma}_{\mathrm{xz}},{\upsigma}_{\mathrm{yz}} \). Therefore, the current nonlinear molecule has three normal modes (3 N-6 = 3) grouped into irreducible representation mentioned by Γvib = 2A1 + B2. The calculated results conform to symmetry predictions according to which partial vibration density of states (PVDOS) indicates three modes of vibration. The PVDOS related to TiO2 revealed presence of three modes at 0.042 eV (327 cm−1) with A1 symmetry, 0.118 eV (943 cm−1) having B2 symmetry, and 0.120 eV (956 cm−1) with A1 symmetry. The analysis revealed dominance of vibrations related to O when compared to those of Ti in PVDOS of TiO2.
The calculations revealed the presence of three IR and three Raman active modes. In order to characterize the modes which are IR and/or Raman active, dipole moment and polarizability need to be monitored. If dipole moment changes then mode is IR active, whereas if polarizability changes then mode is Raman active. The mode at 327 cm−1 with A1 symmetry having respective IR and Raman intensities of 8.68 km/mol and 6.42 Å4/amu is identified as scissoring bending mode. The second mode observed at 943 cm−1 with A1 symmetry indicates respective IR and Raman intensities of 54.29 km/mol and 44.08 Å4/amu is of symmetric stretching nature. This mode is symmetrical mode whose dipole moment does not change in direction but in magnitude which makes this mode IR active. The highest frequency mode found at 956 cm−1 is of asymmetric stretching nature showing respective IR and Raman intensities of 236.43 km/mol and 6.93 Å4/amu.
The other monomer CeO2 starting from the geometry of TiO2 was also studied. The LDA (B3YLP) calculated value of Ce=O bond length as 181.7 pm (177.4 pm), whereas entire length of the molecule (O=Ce=O length) was 363.4 pm (354.6 pm). The value of B3LYP calculated Hirshfeld gross charge was calculated as 0.8901 on Ce and −0.4449 on each O atom. The LDA (B3YLP) calculated value of bond energy is −23.324 eV (−27.868 eV). The relaxed geometry of CeO2 molecule indicates it to be linear and centrosymmetric having center of inversion. Therefore, predictions of point group theory indicate that modes which are IR active will be Raman inactive and vice versa. The monomer being linear centrosymmetric has D∞h point group having C∞ principal axis of rotation passing through inversion center i. It has three normal modes with \( {\Sigma}_{\mathrm{u}}^{+},{\Pi}_{\mathrm{u}} \) symmetries as IR active and mode with \( {\Sigma}_{\mathrm{g}}^{+} \)symmetry as Raman active (Fan and Ziegler (1992). Being linear, CeO2 has four (i.e., 3 N-5=4) modes of vibrations. Dipole moment should be zero which is consistent with our negligibly small calculated value of 0.0819 D. The calculated PVDOS predicted presence of four vibrational modes at 114, 115, 726, and 818 cm−1. The analysis indicates hybrid nature of the lowest frequency degenerate mode (at 114 and 115 cm−1) and the highest frequency mode at 818 cm−1, whereas the mode at 726 cm−1 is solely related to oxygen vibrations. In accordance with mutual exclusion rule, CeO2 molecule has two IR active and one Raman active modes. The modes found at 114, 115, and 818 cm−1 are found IR active with respective intensities of 53.26, 53.26, and 430.85 km/mol. The doubly degenerate band (Ϭxz and Ϭyz) found at the lowest frequency has the least intensity is of scissoring bending nature. This degeneracy is available for linear molecule of CeO2 to produce an additional normal mode when compared with nonlinear molecule of TiO2. The most intense and the highest frequency IR active mode found at 818 cm−1 is of asymmetrical stretching nature. The energy of bending mode should be smaller than that of stretching mode which is in accordance with our observations. For a molecule to be IR active, a change in electrical dipole moment should be available. It means unlike symmetric vibrations, the asymmetric vibrations shall provide IR peaks. Therefore, the observed peaks in IR spectra represent polar bonds and/or nonsymmetric vibrations. The intensity of peak in IR spectrum is direct measure of dipole moment. Therefore, less intense peak related to scissoring mode at 114 and 115 cm−1 indicates less change in charge when compared with asymmetric mode appeared at 818 cm−1. Asymmetric vibrations often produce the highest energy mode.
In case of linear CeO2 molecule, one oxygen atom moves away from Ce to generate a net charge and dipole moment. The mode at 726 cm−1 reveals symmetric stretching vibrations. This mode, solely related to oxygen vibrations per PVDOS, is IR inactive because both oxygen atoms move away/towards cerium atom in similar way to cancel out each other’s effect and net dipole moment becomes zero. Since linear CeO2 has inversion center, so according to “exclusion rule,” no mode related to this molecule should be simultaneously IR and Raman active. As per expectation, out of four observed modes, where three are IR active, the mode 726 cm−1 is only Raman active having intensity 35.26 Å4/amu.
Before calculating vibrational properties of the target cluster CeTiO4, similar calculations were made on dimmers Ti2O4 and Ce2O4 clusters. In case of Ti2O4, the LDA (B3YLP) calculated Ti=O and Ti–O bond lengths are found to be 162.1 pm (162.7 pm) and 182.2 pm (184.9 pm), respectively. The LDA (B3YLP) calculated O=Ti=Ti angle was found to be 119.6° (123.6°) where O–Ti–O angle was observed as 85.3° (85.5°). The intermetallic bond length Ti=Ti calculated using LDA (B3YLP) was found 268 pm (271.6 pm). The value of LDA (B3YLP) calculated bond energy is −48.543 eV or −1119.43 kcal/mol (−54.894 eV or −1265.89 kcal/mol). The dipole moment was found as 0.0214 D (0.000 D) as a result of LDA (B3YLP) calculations. The B3YLP calculated Hirshfeld values of gross charge per atom (by taking Z minus electrons) are found as 0.8136 (Ti), 0.8136 (Ti), −0.3977 (O), −0.4159 (O), −0.3977 (O), and −0.4159 (O). The cluster was observed to be in C(2H) symmetry with irreducible representations having subspecies Ag, Bg, Au, and Bu.
The observations point out that the cluster Ti2O4 has vibrational modes at 92, 188, 203, 295, 342, 420, 535, 707, 745, 1012, and 1028 cm−1. The Ti atoms contribute to the vibrational modes throughout, whereas major involvement of O atoms is limited to extreme and central modes only. The mode at 188 cm−1 is dominated by exterior oxygen atoms in O=Ti bond, whereas 203 cm−1 is dominated by Ti. The O atoms in single bonds and Ti atoms are responsible for producing vibrational mode observed at 745 cm−1. The highest frequency mode observed at 1028 cm−1 is dominated by Ti atoms and the exterior oxygen atoms making double bonds with Ti. Figure 1 shows sketches of Ti2O4 cluster exhibiting vibrations of the atoms observed at the calculated normal modes.
The lowest frequency modes 92 and 188 cm−1 are IR active and Raman inactive having respective IR intensities of 35.34 and 19.25 km/mol. These modes (Fig. 1a, b) indicate presence of twisting modes of Ti=O that reveals rocking type vibrations of the entire cluster. The modes observed at 203 and 295 cm−1 are both IR inactive and Raman active revealing twisting vibrations (Fig. 1c, d) with Raman intensities of 8.22 and 2.1 Å4/amu, respectively. The mode at 342 cm−1 gives O–Ti–O rocking and O=Ti twisting vibrations (Fig. 1e) is IR active and Raman inactive showing IR intensity of 43.61 Å4/amu. The mode 420 cm−1 reveals O–Ti–O scissoring mode and Ti–Ti stretching vibrations (Fig. 1f) showing IR inactive and Raman active mode with intensity of 8.13 cm−1. The modes at 535 and 707 cm−1 showing respective O–Ti–O stretching and rocking vibrations (Fig. 1g, h) are both IR inactive and Raman active with Raman intensities of 8.13 and 3.39 Å4/amu, respectively.
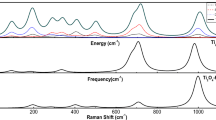
The vibrational mode of scissoring nature (Fig. 1i) observed at 745 cm−1 is IR inactive and Raman active having Raman intensity of 15.32 Å4/amu. The neighboring mode at 749 cm−1 shows strong stretching vibrations with Raman inactive and IR active having high IR intensity of 482.27 km/mol. The highest frequency modes at 1028 and 1012 cm−1 revealed Ti=O stretching vibrations (Fig. 1j, k). The mode at 1012 cm−1 is IR active with very high IR intensity of 500.94 km/mol, whereas it is Raman inactive. The highest frequency mode at 1028 cm−1 is IR inactive and Raman active with intensity of 97.95 Å4/amu. The Raman and IR spectra along with partial and integrated PVDOS calculated for Ti2O4 cluster are given in Fig. 2. The dominant Raman mode is related to vibrations of Ti and exterior O atoms in O=Ti bond. On the other hand, the dominant IR modes are related to vibrations of Ti in such a way that the mode at 745 cm−1 got contributions from interior O atoms.
Similar calculations were performed on Ce2O4 dimmer starting from geometry of Ti2O4. The values of LDA (B3YLP) calculated bond length Ce=O and Ce=Ce were found to be 176.6 pm (177 pm) and 344.6 pm (356.9 pm), respectively. The values of LDA (B3YLP) calculated Ce–O bond lengths at one arm (Ce–O–Ce) of the quadcopter-like structure are different and are found to be 238.8 pm (256.8 pm) and 190.1 pm (187.1 pm). The value of LDA (B3YLP) calculated angle O=Ce=Ce is found as 143.7° (140.9°), whereas the angle Ce–O–Ce was found as 106.3° (106.0°). The cluster Ce2O4 has point group symmetry C(2H) with irreducible representations having subspecies Ag, Bg, Au, and Bu. The value of LDA (B3YLP) calculated bond energy is −50.133 eV or −1156.10 kcal/mol (−56.793 eV or −1309.68 kcal/mol). The value of LDA (B3YLP) calculated dipole moment is found negligible as 0.000000000 Debye (0.00000427 Debye).
The calculations predicted availability of vibrational modes at 51 (Au), 99 (Bg), 105 (Ag), 174(Au), 307(Ag), 343 (Bu), 559(Ag), 638(Bu), 774(Bu), and 788(Ag) cm−1 which got major contribution from O atoms throughout with involvement of Ce atoms only at low frequencies. Figure 3 shows vibrations of Ce and O atoms of the cluster at different modes of vibrations.
The mode at 65 cm−1 is IR active and Raman inactive having IR intensity of 19.75 km/mol shows Ce=O asymmetric stretching vibrations (Fig. 3a). The next mode at 95 cm−1 is IR active and Raman inactive with IR intensity of 19.82 km/mol shows Ce=O twisting vibrations. The modes found at 99 cm−1 (O–Ce=O symmetric stretching shown in Fig. 3b), 105 cm−1 (Ce=O twisting shown in Fig. 3c), and 150 cm−1 (Ce–Ce, Ce–O stretching) are IR inactive and Raman active having respective Raman intensities of 1.05, 7.30, 2.80 km/mol. The mode at 174 cm−1 sketched in (Fig. 3d) is IR active and Raman inactive giving IR intensity of 60.60 km/mol. The mode at 307 cm−1 having Ag symmetry shows O–Ce–O symmetric stretching and Ce=O twisting vibrations (Fig. 3e). It is IR inactive and Raman active with Raman intensity of 61.46 Å4/amu. The mode at 343 cm−1 mode with Bu symmetry shows strong O–Ce–O rocking and Ce=O twisting vibrations (Fig. 3f) reveals symmetric stretching vibrations due to which it is IR active with IR intensity of 187.05 km/mol and Raman inactive.
The mode 559 cm−1 with Ag symmetry shows O=Ce–O asymmetric stretching and Ce–O–Ce bending vibrations (Fig. 3g) with intense variations of Ce–O bond length. It is IR inactive and Raman active showing Raman intensity of 67.52 Å4/amu. The mode at 638 cm−1 having Bu symmetry shows O=Ce–O asymmetric stretching and Ce–O–Ce bending vibrations (Fig. 3h). It is IR active with intensity 238.02 km/mol and Raman inactive. The mode at 774 cm−1 is of Bu symmetry showing O=Ce–O asymmetric stretching and Ce–O–Ce bending vibrations (Fig. 3i). It is therefore IR active with the strongest intensity of 858.08 km/mol and Raman inactive. The highest frequency mode at 788 cm−1 having Ag symmetry revealed symmetric stretching Ce=O vibrations (Fig. 3j) is IR inactive and Raman active with intensity of 88.08 Å4/amu. Figure 4 gives IR spectrum, Raman spectrum, and PVDOS calculated for Ce2O4 dimmer.
The calculation performed on the hybrid cluster CeTiO4 shows bond energy calculated using LDA (B3YLP) is found as −49.293 eV or −1136.74 kcal/mol (−55.648 eV or −1283.28 kcal/mol). The value of bond energy calculated using LDA was 0.000033 eV higher for single metallic bonds as compared to double metallic bonds. The values of LDA (B3YLP) calculated bond lengths O=Ti, O=Ce, and Ti=Ce are 162.8 pm (163.8 pm), 177.2 pm (176.5 pm), and 302.9 pm (307.5 pm), respectively. Moreover, LDA (B3YLP) calculated O–Ti and O–Ce bond lengths are 163.9 pm (177.1 pm) and found as 208.4 pm (204.3 pm). The LDA (B3YLP) calculated O=Ti=Ce and O=Ce=Ti angles are found to be 114.6° (120.8°) and 157.6° (149.1°), respectively. The values of B3YLP calculated gross Hirshfeld atomic charges are 0.9053 (Ce), 0.7591 (Ti), −0.4146 (O), −0.4391 (O), −0.4305(O), and −0.3802(O). The value of B3YLP calculated of dipole moment is found as 5.468 Debye. The frequency calculations predicted PVDOS of the cluster CeTiO4 are found in range up to 1200 cm−1. Though all atoms in the material contribute to the vibrational modes observed at 51, 61, 103, 255, 281, 301, 342, 536, 643, 760, 769, and 1002 cm−1 but O presented leading involvement. The lowest frequency modes at 51 and 103 cm−1 are dominated by O atoms making double bonds with Ce and Ti, respectively; however, major contribution comes from the O atom linked with Ce. These modes also got a reasonable contribution from Ce. The mode at 301 cm−1 is mainly dominated by central O atoms laying between Ti and Ce. These central O atoms are also responsible for vibrational modes observed at 536, 643, and 760 cm−1. The highest frequency mode at 1002 cm−1 got contributions from central O atoms and Ti atom. The geometry of cluster due to low symmetry suggests simultaneous subsistence of IR and Raman active modes.
The lowest frequency mode at 51 cm−1 is IR active mode with strong Ce=O twisting, O–Ti–O, O–Ce–O, and Ti=O twisting vibrations (Fig. 5a). The mode at 61 cm−1 is IR active mode with strong Ce=O twisting, O–Ti–O and O–Ce–O twisting, and weak Ti=O twisting vibrations. The 103 cm−1 is IR active mode with O–Ti–O and O–Ce–O wagging, Ti=O twisting, and Ce=O weak twisting vibrations (Fig. 5b). The mode at 255 cm−1 shows negligibly weak IR and weak Raman band with O–Ti–O and O–Ce–O twisting modes, Ti=O twisting, and Ce=O very weak twisting vibrations (Fig. 5c). The mode at 281 cm−1 is IR active mode showing O–Ti–O and O–Ce–O rocking, Ti=O mixed stretching and twisting, and weak Ce = O mixed stretching and twisting vibrations (Fig. 5d). The mode at 301 cm−1 is IR active mode with O–Ti–O twisting, O–Ce–O twisting, and weak Ci=O, Ti=O twisting vibrations (Fig. 5e). The mode at 342 cm−1 is IR active mode with O–Ti–O and O–Ce–O wagging, Ti–Ce asymmetric stretching, Ti=O twisting, and Ce=O weak stretching vibrations (Fig. 5f). The mode at 536 cm−1 is IR active with O–Ce–O symmetric stretching, O–Ti–O scissoring (bending), and negligible Ti=O, Ce=O twisting vibrations (Fig. 5g). The mode at 643 cm−1 is IR active showing O–T–O asymmetric stretching, O–Ce–O rocking and comparatively weak Ce=O twisting, and Ti=O twisting mode (Fig. 5h). The mode at 760 cm−1 is both IR and Raman active but shows very intense IR preference. The vibrational analysis shows Ce=O asymmetric stretching, O–Ce–O scissoring bending, O–Ti–O symmetric stretching, and negligible Ti=O vibrations (Fig. 5i). The mode at 769 cm−1 is Raman active but IR inactive. The O–Ti–O bond shows symmetric stretching vibrations, Ce=O bond shows asymmetric stretching, whereas O–Ce–O vibrates in scissoring bending mode.
The vibrations of Ti=O and Ti–Ce are comparatively absent. The highest frequency normal mode at 1002 cm−1 is both IR and Raman active but IR intensity is significantly high. The vibrational analysis indicates that it is of asymmetric stretching nature with dominant vibration of O=Ti bond. All other bonds show negligible vibrations (Fig. 5j). The IR and Raman spectra along with PVDOS calculated for the hybrid cluster are shown in Fig. 6.
In order to understand the individual role of the atoms in vibrational modes of the clusters, an analysis of PVDOS of individual atoms of the structures was made. Figure 7 shows comparison of integrated (total) and atomic PVDOS calculated for Ti2O4, Ce2O4, and the hybrid cluster CeTiO4. The results indicate softening of modes related to exterior O atoms (in O=Ce bonds) for the hybrid cluster when compared to Ce2O4 cluster. The modes at frequency values of 97 and 781 cm−1 observed for Ce2O4 are found red shifted to 55 and 763 cm−1, respectively, for the hybrid cluster. However, in case of exterior O atoms connected to Ti (in O=Ti bonds), the softening of high frequency and hardening of low frequency modes is observed. The modes observed at 92 and 193 cm−1 are blue shifted to 101 and 254 cm−1 respectively, whereas the highest frequency mode is red shifted from 1020 to 1001 cm−1 for hybrid cluster when compared to Ti2O4.
The comparison indicates decrease in oscillator strength of all modes and suppression of Ti modes at 200 and 534 cm−1 takes place in case of hybrid cluster. Moreover, softening of 342, 420, and 1020 cm−1 modes is observed. In case of Ce, we observed decrease in oscillator strength and softening of normal modes of vibration due to red shift of modes 781 to 760 cm−1, 560 to 535 cm−1, 312 to 283 cm−1, 149 to 101 cm−1, and 105 to 58 cm−1. The comparison of vibrational modes-related central oxygen atoms making single bonds (in quadcopter-like structure) was also made. No notable reduction in oscillator strength of normal modes is observed except the highest frequency mode whose strength is the highest for Ti2O4, medium for hybrid, and the lowest for Ce2O4 cluster. It is observed that O-related low frequency modes are softened, whereas high frequency modes are hardened for the hybrid cluster.
The analysis of energy levels pointed out that HOMO-LUMO gap for Ce2O4 and Ti2O4 clusters lays in IR and UV regions which shifts to visible region in case of CeTiO4. This finding pointed out importance of the proposed hybrid cluster which is optically active in visible region and is expected to contain major properties of ceria and titania. The LDA calculated thermodynamical properties of the studied clusters are given in Table 1. The intermediate values observed for the hybrid cluster point to possible engineering of the structure suited to specific applications which can be obtained via selection of metallic concentration Ce:Ti in the hybrid material. This study reports the vibrational fingerprints of basic structural unit of CeTiO4 and predicts synthesis of a range of such materials in molecular/nano/bulk to obtain tunable chemical and physical properties for applications in electronic, optical, optoelectronic, thermochemical, and electrochemical devices.
Summary
This study reports detailed first principles calculations of ceria, titania, and ceria-titania hybrid clusters performed within LDA and B3LYP approximations. The analysis indicates softening of modes related to exterior O atoms in O=Ce bonds and softening/hardening of high/low frequency modes related to exterior O atoms connected to Ti (in O=Ti bonds). The softening/hardening of central O-related low/high frequency modes for the hybrid cluster is also noticed. The results calculated for monomers conform to symmetry predictions according to which three IR and three Raman active modes were detected for TiO2, whereas two IR active and one Raman active modes were observed for CeO2. In case of dimmer Ti2O4, 11 normal modes were detected in which Ti atoms contribute to the vibrational modes throughout, whereas major involvement of O atoms is limited to extreme and central modes only. On the other hand, for Ce2O4, ten normal modes were predicted which got major contribution from O atoms throughout with involvement of Ce atoms only at low frequencies. In case of the hybrid cluster, 12 vibrational modes are observed which are simultaneously IR and Raman active. The symmetry, nature of vibrations, IR and Raman activity, intensities, and atomic involvement in different modes of the clusters are described in detail. Thermodynamical properties of the clusters are also given. The entire results point to engineering of CeTiO4 to tune its properties for several technological applications.
References
Arurault L et al (2008) Nanocrystallized ceria-based coatings prepared by electrochemistry on TA6V titanium alloy. Mat Res Bull 43:796–805. doi:10.1016/j.materresbull.2007.07.019
Barry NPE, Pitto-Barry A, Sanchez AM et al (2014) Fabrication of crystals from single metal atoms. Nature Comm 5:3851. doi:10.1038/ncomms4851
Bickelhaupt FM, Baerends EJ (2000) Kohn-Sham density functional theory: predicting and understanding chemistry. Rev Comput Chem 15:1–86. doi:10.1002/9780470125922.ch1
Blomen LJMJ, Mugerwa MN (1993) Fuel cell systems. Plenum Press, NY
Bridgeman AJ, Cavigliasso G (2002) Density functional study of the vibrational frequencies of Lindqvist polyanions. Chem Phys 279:143–159. doi:10.1016/S0301-0104(02)00450-0
Bunker PR, Jensen P (1998) Molecular symmetry and spectroscopy, Second edn. NRC Research Press, Ottawa
Chen M, Straatsma TP, Dixon DA (2015) Molecular and dissociative adsorption of water on (TiO2)n clusters, n = 1–4. J Phys Chem A 119:11406–11421. doi:10.1021/acs.jpca.5b07697
L. J. de Jongh (1994). Physics and chemistry of metal cluster compounds: model systems for small metal particles, Kluwer Academic Publishers
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on main group molecules. J Chem Phys 96:9005. doi:10.1063/1.462258
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38. doi:10.1038/238037a0
Guerra F et al (2004) Voronoi deformation density (VDD) charges: assessment of the Mulliken, Bader, Hirshfeld, Weinhold, and VDD methods for charge analysis. J Comput Chem 25:189–210. doi:10.1002/jcc.10351
Gupta SM, Tripathi M (2011) A review of TiO2 nanoparticles. Chinese Sci Bull 56:1639–1657. doi:10.1007/s11434-011-4476-1
Henrich VE, Cox PA (1994) The surface science of metal oxides. Cambridge University Press, Cambridge
Jedidi A, Li R, Fornasiero P et al (2015) Vibrational fingerprints of low-lying Pt(n)P(2n) (n = 1–5) cluster structures from global optimization based on density functional theory potential energy surfaces. J Phys Chem A 119:11711–11718. doi:10.1021/acs.jpca.5b08495
Kim JB (2014) Structural isomers of Ti2O4 and Zr2O4 anions identified by slow photoelectron velocity-map imaging spectroscopy. J Am Chem Soc 136:7159–7168. doi:10.1021/ja502713v
Lin Y, Xiaoming Z (2008) Preparation of highly dispersed CeO2/TiO2 core-shell nanoparticles. Mat Lett 62:3764–3766. doi:10.1016/j.matlet.2008.04.059
Nakamoto K (2006) Infrared and Raman spectra of inorganic and coordination compounds. Handbook of Vibrational Spectroscopy DOI. doi:10.1002/0470027320.s4104
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740. doi:10.1038/353737a0
Park JB et al (2009) High catalytic activity of Au/CeOx/TiO2(110) controlled by the nature of the mixed-metal oxide at the nanometer level. Proc Natl Acad Sci U S A 106:4975–4980. doi:10.1073/pnas.0812604106
Park JB et al (2010) Gold, copper, and platinum nanoparticles dispersed on CeOx/TiO2 (110) surfaces: high water-gas shift activity and the nature of the mixed-metal oxide at the nanometer level. J Am Chem Soc 132:356–363. doi:10.1021/ja9087677
Parker FS (1983) Applications of infrared, Raman and resonance Raman spectroscopy in biochemistry Plenum Press, New York and London
Pelaez M et al (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Cat B: Env 125:331–349. doi:10.1016/j.apcatb.2012.05.036
Salonitis K, Pandremenos J, Paralikas J et al (2010) Multifunctional materials: engineering applications and processing challenges. Int J Adv Manuf Technol 49:803–826. doi:10.1007/s00170-009-2428-6
Shah NH et al (2016) Ultrasonically engineered ceria-titania nanostructure mediated photocatalytic and sonocatalytic degradation of organic dye. Austin Chem Eng 3:1032
te Velde G (2001) Chemistry with ADF. J Comp Chem 22:931–967. doi:10.1002/jcc.1056
Tedstone AA et al (2016) Synthesis, properties, and applications of transition metal-doped layered transition metal dichalcogenides. Chem Mat 28:1965–1974. doi:10.1021/acs.chemmater.6b00430
Tiziano M et al (2016) Fundamentals and catalytic applications of CeO2-based materials. Chem Rev 116:5987–6041. doi:10.1021/acs.chemrev.5b00603
Tuller HL, Nowick AS (1975) Doped ceria as a solid oxide electrolyte. J Electrochem Soc 122:255–259. doi:10.1149/1.2134190
Valley N et al (2013) A look at the origin and magnitude of the chemical contribution to the enhancement mechanism of surface-enhanced Raman spectroscopy (SERS): theory and experiment. J Phys Chem Lett 4:2599–2604. doi:10.1021/jz4012383
Van Lenthe E, Baerends EJ (2003) Optimized slater-type basis sets for the elements 1–118. J Comput Chem 24:1142–1156. doi:10.1002/jcc.10255
Wang R et al (1997) Light-induced amphiphilic surfaces. Nature 388:431–432. doi:10.1038/41233
Wartewig S (2003) Basic principles of vibrational spectroscopy, in IR and Raman spectroscopy: fundamental processing, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, doi: 10.1002/3527601635.ch4
Wu H, Wang L-S (1997) Electronic structure of titanium oxide clusters: TiOy (y=1–3) and (TiO2)n (n=1–4). J Chem Phys 107:8221–8228
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Majid, A., Bibi, M. First principles study of vibrational dynamics of ceria-titania hybrid clusters. J Nanopart Res 19, 122 (2017). https://doi.org/10.1007/s11051-017-3823-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3823-9