Abstract
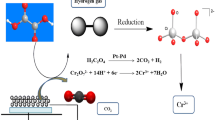
Nanosized nickel phosphide (Ni2P) has been synthesized via hydrothermal reaction with environmental-friendly red phosphorus and nickel chloride. The reaction mechanism has been studied by measurement techniques of IC, XRD ,TEM, EDS, and XPS. The results showed that the particle sizes of as-prepared Ni2P are in nanoscale ranging from 10 to 30 nm. In hydrothermal reaction, red phosphorus reacts with water to its oxyacids, especially its hypophosphorous acid (or hypophosphite) which can reduce nickel chloride to nickel, and then metallic nickel will penetrate into the rest of red phosphorus to generate nano-Ni2P. Furthermore, the catalytic performance of as-synthesized Ni2P for the hydrodesulfurization of dibenzothiophene has been tested. It has been shown that the HDS reaction process over Ni2P catalyst agrees well with the pseudo-first order kinetic equation, and the HDS conversion can reach up to 43.83% in 5 h with a stable increasing catalytic activity during the whole examination process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nickel phosphide has a variety of phases, in which Ni2P is a kind of material with excellent corrosion resistance, wear resistance, and oxidation resistance. The nano-crystal of Ni2P has good plasticity, toughness, and higher specific heat, making it adaptable for a variety of carriers as the catalytic center (Wu et al. 2013; Song et al. 2010; Koranyi et al. 2009). At the same time, because of its special intrinsic high catalytic activity and selective hydrogenation such as high desulfurization, carbon deposition resistance, resistance to poisoning, and so on (Zhang et al. 2011), Ni2P also overcomes the weakness of low stability of the traditional catalyst (Savithra et al. 2013), which makes it to have higher HDS activity than a sulfide catalyst (Duan et al. 2009). The Ni2P catalyst is possibly a new catalytic material which can replace the traditional sulfide catalysts and becomes the hotspot of current research on hydrodesulfurization (Song et al. 2012a, b).
At present, the main preparation methods of Ni2P used as a catalyst are as follows (Song et al. 2007; Wang et al. 2009; Muthuswamy and Savithra 2011; Cecilia et al. 2009; Chen et al. 2010): (1) direct combination of metal nickel and red phosphorus under high temperature and protective atmosphere, (2) solid phase reaction of halogenated nickel and phosphorus, (3) reaction of nickel salt and phosphine, (4) decomposition of organic nickel compounds, (5) molten electrolysis of nickel salt, (6) reduction of phosphate containing nickel, etc. But most of these preparation methods need high temperature and high pressure, and some of them still need very expensive or dangerous materials; many reactions even use highly toxic substances such as phosphine as phosphorus source.
Therefore, hydrothermal (solvothermal) synthesis methods of phosphides developed in recent years (Kirill et al. 2006; Jennifer et al. 2005; Liu et al. 2007; Huang et al. 2012; Wang et al. 2013; Huang et al. 2013; Huang et al. 2014; Liu et al. 2010), utilizing the disproportionation reaction of elemental phosphorus (especially non-toxic red phosphorus) in the water (solvent) (Jiang and Shen 2000), is both simple in preparation as well as causing less environmental concerns. With the rising conflict between petroleum deterioration and environmental protection, the extensive and in-depth exploration of the synthesis process, phase structure, surface properties, and catalytic performance of Ni2P prepared by hydrothermal method will become the hot focus. The most basic synthesis reaction process will fully demonstrate the important significance of theoretical research and potential application prospects of this new catalytic material. But up till now, little work has been done on the reaction mechanism and HDS performance of Ni2P nanoparticle prepared by hydrothermal method (Christos et al. 2016; Sophie et al. 2013).
In this study, we reported a synthesis mechanism of Ni2P nanoparticle via hydrothermal route using nontoxic red phosphorus and nickel chloride. The process was concise and environmental. Furthermore, the catalytic performance of the as-synthesized Ni2P in dibenzothiophene hydrodesulfurization (HDS) was examined.
Experimental
Chemicals
All chemicals involved in the experiments were analytical reagents: Red phosphorus (P), nickel chloride (NiCl2·6H2O), potassium hydroxide (KOH),ZSM-5, dibenzothiophene (C12H8S) (Aladdin, AR standard) , and decahydronaphthalene (C10H18) (Sinopharm, AR standard).
Synthesis of materials
Hydrothermal treatment of red phosphorus
The hydrothermal treatment process of red phosphorus was carried out as follows: First, red phosphorus (6.2 g) was ground for 20 min with 20 ml water in a mortar. Then, the mixture was transferred into a Teflon-lined stainless steel autoclave with 40 ml distilled water to make sure that the filling factor was 80% and heated at 200 °C for 10 h. After the autoclave cooled to room temperature, the filtrate L1 of hydrothermal red phosphorus was obtained; Finally, the filtrate L1 (60 ml) was heated again at 200 °C for 10 h to get the liquid L2.
Hydrothermal synthesis of nano-Ni2P
The hydrothermal synthesis of nano-Ni2P was carried out as follows: First, NiCl2▪6H2O (1.91 g) was mixed in the filtrate L1 (60 ml). The mixture was adjusted to pH = 13 with 1 mol/L KOH solution, then the green suspension liquid was transferred into a Teflon-lined stainless autoclave and heated at 200 °C for 5 h. Finally, when the autoclave cooled to room temperature, the powder A was separated by vacuum filtration, rinsed repeatedly with distilled water, and then dried at 60 °C for 3 h. To investigate the synthesis mechanism of Ni2P via hydrothermal route, red phosphorus (6.2 g) obtained by the first step was put to NiCl2▪6H2O (1.91 g) solution and adjusted to pH = 13; then the mixture was transferred into a Teflon-lined stainless steel autoclave and heated at 200 °C for 5 and 10 h, respectively. The as-prepared powder B and powder C were collected by vacuum filtration, rinsed repeatedly with distilled water and then dried at 60 °C for 3 h.
Hydrothermal synthesis of supported Ni2P/ZSM-5
The hydrothermal synthesis of supported Ni2P/ZSM-5 was carried out as follows: First, red phosphorus (6.2 g) of hydrothermal treatment was mixed with NiCl2•6H2O (1.91 g) in a beaker with 60 ml distilled water, then ZSM-5 (2.4 g) were added to the mixed aqueous suspension under vigorous electromagnetic stirring, and the mixture was adjusted to pH = 13 with 1 mol/L KOH solution. Finally, the mixture was transferred into a 80 ml Teflon-lined stainless steel autoclave (filling ratio:80%) and heated at 200 °C for 10 h. The as-prepared powder D was collected by vacuum filtration, rinsed repeatedly with distilled water, and then dried at 60 °C for 3 h. In a typical synthesis experiment, red phosphorus was excess, and the upper solution after hydrothermal process was clear and colorless, proving that the nickel elements were totally consumed and converted into nickel phosphide (Ni2P). Based on this fact, the weight of the Ni2P in Ni2P/ZSM-5 system was calculated according to the quality of the nickel element in nickel chloride (NiCl2•6H2O).
Characterization and test
Characterization analysis
The concentration of various radicals were tested by ion chromatography (IC; Model PIC-10A (120255), Puren Instrument Co., Qingdao) which used YSA (001) as chromatographic column. The XRD patterns were recorded on a Bruker AXS-D8 Advance powder diffractometer with a Cu Kα radiation source, and the XRD patterns were collected at 40 Kv and 40 mA with a scanning rate of 5o/min from 20 to 90o. A JEM-1200EX (JEOL Co, Japan) transmission electron microscope was operated at 100 kV to obtain TEM images, and the EDS spectrum was collected using Oxford Instruments’ INCA EDS system. The XPS on Ni2P catalyst was obtained with an ESCALAB 250Xi Termo spectrometer equipped with a monochromatic X-ray source Al Kα under ultra-high vacuum and a hemispherical analyzer.
HDS reaction tests
The activity tests were carried out in a 100 ml stainless steel autoclave with mechanical stirring. The decahydronaphthalene solution containing 0.03 wt% (sulfur content) dibenzothiophene was selected as model reactant. In a typical experiment, 40.0 g of dibenzothiophene solution was charged into the reactor, together with 0.4 g of the Ni2P catalyst. Prior to the reaction, the reactor was purged three times with H2 to exchange the air inside. The reaction was carried out at 300 °C and 2.0 MPa H2 (initial pressure at room temperature) for different times (1 ~ 4 h) with a stirring rate of 500 r min−1. The liquid product was collected by centrifugation and analyzed on an Agilent 7890 GC-MS instrument equipped with a HP-5MS column. The desulfurization efficiency of DBT is calculated by the following formula:
Results and discussions
Hydrothermal treatment of red phosphorus
The ion chromatogram of filtrate L1 diluted 50-fold is shown in Fig. 1. Table 1 presents the concentration of various phosphorus radicals in filtrate L1. In hydrothermal reaction, red phosphorus reacts with water to form its oxyacids, including hypophosphorous acid, phosphorous acid, and phosphoric acid (Yao et al. 1998). The pH value of filtrate L1 is 1 ~ 3. Fig. 2 is the ion chromatograph of L2 diluted 1000-fold obtained by hydrothermal treatment of filtrate L1. The re-hydrothermal solution L2 is still clear. The concentration of different phosphorus ionic groups in liquid L2 are displayed in Table 2.The pH value of liquid L2 increases slightly to 2 ~ 4.
Comparing Table 1 and Table 2, it can be found that the hypophosphorous acid in the liquid L2 disappears, the concentration of phosphorous acid decreases, and the concentration of phosphoric acid increases, which indicates that the hypophosphorous acid will further convert to phosphorous acid and phosphoric acid under hydrothermal condition, and the reaction is quick (complete transformation of hypophosphorous acid); the phosphorous acid also can be converted to phosphoric acid, but the reaction is relatively slow (partial conversion of phosphorous acid). According to Hydride Transfer Theory proposed by Hersch (Jiang and Shen 2000), hypophosphorous acid can react with water to generate phosphorous acid in acidic medium; meanwhile, phosphorous acid also can react with water to generate phosphoric acid, i.e.,
The explanation of eutectoid phosphorus is as below:
The Eqs. (1) ~ (3) can explain that the hypophosphorous acid in liquid L2 disappears, the concentration of phosphorous acid decreases, and the concentration of phosphoric acid increases. While, the Eq. (3) can explain the reduce of the total phosphorus content in the solution.
Hydrothermal synthesis of nano-Ni2P
Fig. 3 shows the X-Ray diffraction patterns of the hydrothermal products. From Fig. 3a, the diffraction peaks of the powder A are readily indexed to a crystalline phase of Ni (PDF# = 70–1849) which is strongly magnetic. When the red phosphorus of hydrothermal treatment replaces the filtrate L1, the peak intensity of Ni is weakened and peaks become wider obviously (Fig. 3b. In addition, the diffraction peaks of Ni2P (PDF# = 74–1385) appear and the mixture is weakly magnetic. Nevertheless, with the duration rising up to 10 h, the diffraction peaks completely change to Ni2P (PDF# = 74–1385, Fig. 3c) in which there is no magnetism.
Combining Figs. 1 and 3a, it is known that the filtrate L1 (especially the hypophosphorous acid which has a strong reducibility) will reduce NiCl2 to metallic nickel in hydrothermal conditions. After the autoclave is cooled to room temperature, the inner liner still retains a great residual pressure and gives off a certain amount of colorless and tasteless gas H2 (Cl and P are basically excluded, the reaction system of the solution has a strong reducibility and does not readily generate O2, so the resulting gas is H2). In addition to a small amount of precipitated powder A (Ni) in the inner liner, there also remain many powders of A (Ni) tightly adhered to the inner wall and the bottom, which is difficult to remove. As the initial system is adjusted to alkalinity, the nickel source of the reaction is Ni (OH)2, which is in accordance with the theory of the distribution of hydroxyl and nickel ions in chemical plating (Hersch 1955; Meeradder 1981; Jiang and Shen 2000; Stojan 2002; Zhang et al. 2013). The reaction mechanism is as below:
For the reactions generating the powder B and C, after the autoclaves cooled to room temperature, both of them still remain a high pressure and give off a lot of colorless and tasteless gas. A layer of the powder B (or C) is closely adhered to the inner wall and the bottom, and a large number of white bubbles are attached at the same time. Compared to the powder A, there is a sufficient amount of red phosphorus to provide sufficient hypophosphorous acid (or hypophosphite) for the following reaction (5) ~ (6). Therefore, the gas production increases; the inner wall and the bottom are completely black (adhesive powder B or C) with many white bubbles attached to them. It can further indicate that in the process of preparing Ni2P with red phosphorus as the source of phosphorus via the hydrothermal method, red phosphorus will not directly react with NiCl2, but the hypophosphorous acid with strong reducibility generated by the reaction of red phosphorus and water reduces the nickel source to metallic nickel. The generated nickel reacts with the rest of red phosphorus to produce Ni2P:
Therefore, the Eqs. (4) ~ (7) and (8) are the main reactions to prepare Ni2P with hydrothermal red phosphorus as phosphorus material via hydrothermal synthesis.
XPS spectra of Ni 2p and P 2p region of as-synthesized Ni2P were obtained for the analyzing the surface elemental composition and valence state of the Ni2P sample (Fig. 4). The Ni 2p3/2 spectrum (Fig. 4a) shows three peaks at 853.8, 856.7, and 862.1 eV which can be assigned to NiO, Ni2+ and the satellite of the Ni 2p3/2 peak (Ai et al. 2008; Ma et al. 2009; Yang et al. 2010), respectively. The P 2p spectrum (Fig. 4b) shows two peaks at 129.7 and 130.8 eV corresponding to the P 2p3/2 and P 2p1/2 in Ni2P, respectively (Li et al. 2015). Furthermore, the spectrum of P 2p also exhibits a peak at 133.7 eV, which can be attributed to oxidized P species formed on the surface of Ni2P due to air contact (Chen et al. 2009).
Fig. 5 shows the transmission electron microscopy (TEM) images of the hydrothermal products. It can be observed that the particle sizes of the powders A (Ni), B (Ni and Ni2P), C (Ni2P), and the red phosphorus (P) of hydrothermal treatment are all below 50 nm. Furthermore, the sizes of the powder A, B, and C (10 ~ 30 nm) calculated by Scherrer equation and the TEM results are in close agreement with each other. Namely, the synthesis technique of Ni2P via hydrothermal method with red phosphorus of hydrothermal treatment as phosphate source is simple and easy to control, the raw materials are cheap, and the particle size of Ni2P is at the nanometer level (Fig. 6).
HDS activity tests
The HDS activity tests are conducted in an autoclave reactor by using dibenzothiophene as a model S-containing compound over the as-prepared Ni2P catalyst. Table 3 is the HDS results of Ni2P catalysts with different reaction times.
Generally, the HDS of DBT undergoes two parallel routes involving direct desulfurization (DDS) and hydrodesulfurization (HYD) (Al-Rashidy et al. 2015). The DDS route mainly leads to the formation of biphenyl because of direct C–S bond rupture, while the HYD route mainly leads to the formation of cyclohexylbenzene through a hydrogenation–desulfurization process. The selectivity results (Table 3) show that the selectivity of Cyclohexylbenzene is increasing while the selectivity of Biphenyl is declining with the prolonging of reaction time. This may be because there are two types of sites in Ni2P, Ni(1) site with quasi tetrahedral coordination and Ni(2) sites with square pyramidal coordination. Ni(1) site is likely involved in the DDS while Ni(2) site is responsible for the high catalytic activity in the HYD pathway in the HDS reaction (Oyama and Lee 2008; Song et al. 2012a, b). Hence, the acceleration of the HYD pathway must be associated with the increasing amount of Ni(2) sites while the number of Ni(1) sites remains unchanged (Song et al. 2014). As shown in Table 3, the conversion of Ni2P placed in air for 2 months is 31.25% which is almost unchanged compared with fresh Ni2P. However, the conversion of supported Ni2P/ZSM-5 is 31.25% similar to unsupported Ni2P. Indeed, though supported catalysts should have a greater catalytic efficiency for a greater specific surface area and more active centers are exposed, the load rate of supported Ni2P/ZSM-5 is only 20% in this paper. Besides, the quality of the catalysts (Ni2P catalyst or supported Ni2P/ZSM-5 catalyst) used are equal in the HDS test process. Therefore, the conversion of supported Ni2P/ZSM-5 we obtained is not ideal. This may be due to an increase in specific surface area, but the total content of catalyst decreased.
According to Table 3, the first order fitting diagram of different HDS test times can be drawn. We can obtain an inclined straight line nearly through the origin with time (t) as the abscissa and –ln(1−x) as the ordinate as shown in Fig. 5, which according to pseudo-first order kinetic equation,
The rate constant (k) of this reaction is about 0.1. (x, desulfurization conversion; t, HDS test time; k, rate constant).
Fig. 7 shows the X-Ray diffraction patterns of Ni2P before and after the HDS test. With the different HDS reaction time, all the diffraction peaks can be indexed to the crystalline phase of Ni2P (PDF# = 74–1385) before and after HDS test (Fig. 7 (a) ~ (f)), which shows that the Ni2P catalyst is stable in the HDS reaction.
The EDS spectrum of Ni2P-old, fresh Ni2P powders, and Ni2P after HDS test in Fig. 8a–c shows that the presence of Ni and P in the products, and the Ni/P ratio is equal to about 2.3:1, 2.7:1, and 2.5:1, respectively. The results once again indicate that the Ni2P catalyst is very stable before and after HDS reaction.
Conclusions
In this work, the reaction mechanism of Ni2P via hydrothermal synthesis is studied, and the as-synthesized products’ catalytic performances are examined in dibenzothiophene hydrodesulfurization reaction. Some conclusions from this study are summarized below:
-
1)
The reaction mechanism of Ni2P via hydrothermal synthesis is as follows: Firstly, red phosphorus (P) reacts with water to its oxyacids. Then strong reducibility of hypophosphorous acid (or hypophosphite) can reduce nickel chloride to metal nickel accompanying the generation of H2 gas. Finally, the metallic nickel reacts with the rest of the red phosphorus to produce Ni2P.
-
2)
The particle sizes of red phosphorus (P) after hydrothermal treatment are in nano-level (10 ~ 30 nm), and the particle sizes of Ni2P with the as-obtained nano red phosphorus as raw material are also in nanoscale.
-
3)
The Ni2P catalyst in the process of HDS test meets to the pseudo-first order kinetic equation.
-
4)
The stability of Ni2P active center during HDS performance test is excellent.
References
Ai L, Fang GJ, Yuan LY, Liu NS (2008) Influence of substrate temperature on electrical and optical properties of p-type semitransparent conductive nickel oxide thin films deposited by radio frequency sputtering. Appl Surf Sci 254:2401–2405
Al-Rashidy AH, Ali SA, Ahmed S, Razzak SA, Hossain MM (2015) Phenomenological kinetics modeling of simultaneous HDS of dibenzothiophene and substituted dibenzothiophene over CoMoP/Al2O3 catalysts. Chem Eng Res Des 104:819–827
Cecilia JA, Infantes-Molina A, Rodríguez-Castellón E (2009) A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene. J Catal 263(1):4–15
Chen YZ, She HD, Luo XH, Yue GH (2009) Solution-phase synthesis of nickel phosphide single-crystalline nanowires. Joural of Crystal Growth 311:1229–1233
Chen JX, Chen Y, Yang Q (2010) An approach to preparing highly dispersed Ni2P/SiO2 catalyst. Catal Commun 11(6):571–575
Christos K, Kyriakos B, Mantha G (2016) Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: a critical review. Appl Catal B Environ 181:156–196
Duan XP, Teng Y, Wang AJ (2009) Role of sulfur in hydrotreating catalysis over nickel phosphide. J Catal 261(2):232–240
Hersch P. (1955-1956) Trans Inst Met Finish 33:417–422
Huang H, Huang X, Zhu ZB, Dai JH (2012) Hydrothermal synthesis of cobalt phosphide nanoparticles. Ceram Int 38:1713–1715
Huang X, Sun JJ, Wang B, Huang H (2013) A novel solvothermal route to nanocrystalline Sn4P3 with red phosphorous as raw material. Adv Mater Res 704:241–245
Huang X, Dong Q, Huang H, Yue L, Zhu ZB, Dai JH (2014) Facile synthesis of iron phosphide Fe2P nanoparticle and its catalytic performance in thiophene hydrodesulfurization. J Nanopart Res 16:2785
Jennifer AA, Valentina GH, Stephanie LB (2005) Solvothermal syntheses of Cu3P via reactions of amorphous red phosphorus with a variety of copper sources. J Solid State Chem 178:970–975
Jiang XX, Shen W (2000) The fundamental and practice of electroless plating. National Defence Industry Press, Beijing, pp 12–20
Kirill AK, Yury VK, Sugata R (2006) A facile high-yield solvothermal route to tin phosphide Sn4P3. J Solid State Chem 179:3756–3762
Koranyi TI, Coumans AE, Hensen EJM (2009) The influence of metal loading and activation on mesoporous materials supported nickel phosphide hydrotreating catalysts. Appl Catal A Gen 365(1):48–54
Li JY, Zhou XM, Xia ZM, Zhang ZY (2015) Facile synthesis of CoX(X=S, P) as an efficient electrocatalyst for hydrogen ecolution reaction. J Mater Chem A 3:13066–13071
Liu SL, Liu XZ, Xu LQ (2007) Controlled synthesis and characterization of nickel phosphide nanocrystal. J Cryst Growth 304(2):430–434
Liu ZY, Huang X, Zhu ZB (2010) A simple mild hydrothermal route for the synthesis of nickel phosphide powders. Ceram Int 36(3):1155–1158
Ma Z, Wang JB, Liu QF, Yuan J (2009) Microwave absorption of electroless Ni–Co–P-coated SiO2 powder. Appl Surf Sci 255:6629–6623
Meeradder JEAMVD (1981) On the mechanism of electroless plating. II. One mechanism for different reductants. J Appl Electrochem 11:395–400
Muthuswamy E, Savithra GHL (2011) Brock Stephanie L, synthetic levers enabling independent control of phase, size, and morphology in nickel phosphide nanoparticles. ACS Nano 5(3):2402–2411
Oyama ST, Lee YK (2008) The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-DMDBT. Catalysis 258:393–400
Savithra GHL, Bowker RH, Carrillo Bo A (2013) Rational design of nickel phosphide hydrodesulfurization catalysts: controlling particle size and preventing sintering. Chem Mater 25(6):825–833
Song LM, Li W, Zhang MH (2007) Preparation and characterization of Ni2P/SiO2-Al2O3 and its catalytic performance for hydrodesulfurization of 4, 6- Dimethyldibenzothiophene. Chin J Catal 28(2):143–147
Song H, Guo YT, Li F (2010) Preparation, hydrodesulfurization and hydrodenitrogenation performance of a Ni2P/TiO2-Al2O3 catalyst. Acta Phys -Chim Sin 26(9):2461–2467
Song H, Dai M, Guo YT (2012a) Preparation of composite TiO2-Al2O3 supported nickel phosphide hydrotreating catalysis and catalytic activity for hydrodesulfurization of dibenzothiophene. Fuel Process Technol 96:228–236
Song H, Dai M, Song HL (2012b) Ni2P catalyst for hydrodesulfurization. Progress In Chemistry 24:757–767
Song H, Wang J, Wang ZD, Song HL (2014) Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst. J Catal 311:257–265
Sophie C, David P, Cédric B (2013) Nanoscaled metal borides and phosphides: recent developments and perspectives. Chem Rev 113(10):7981–8065
Stojan SD (2002) Electroless deposition of metals and alloys. Modern Aspects of Electrochemistry 35:51–133
Wang JW, Johnston-Peck AC, Tracy JB (2009) Nickel phosphide nanoparticles with hollow, solid, and amorphous structures. Chem Mater 21(19):4462–4467
Wang B, Huang X, Zhu ZB, Huang H, Dai JH (2013) Hydrothermal synthesis of nano nickel phosphides and investigation of their thermal stability. Int J Mater Res 104:507–510
Wu SK, Lai PC, Lin YC (2013) Atmospheric hydrodeoxygenation of guaiacol over alumina-, zirconia-, and silica-supported nickel phosphide catalysts. ACS Sustain Chem Eng 1(3):349–358
Yang RC, Wu JS, Li XG (2010) Hydrotreating of crude 2-ethylhexanol over Ni/Al2O3 catalysts: influence of the Ni oxide dispersion on the active sites. Applied Catalysis A 383:112–118
Yao SZ, Zhu YB, He SE, Nie LH (1998) Handbook of chemical reactions of elements. Hunan Education Press, Changsha, pp 383–386
Zhang YJ, Song H, Jiang SR (2011) Progress of research on factors affecting HDS catalyst activity. Advances In Fine Petrochemical 12(10):23–30
Zhang BW, Liao SZ, Xie HW, Zhang H (2013) Progress of electroless amorphous and nanoalloy deposition: a review—part 1. Transactions of the IMF 91:310–318
Acknowledgements
Financial support from the Natural Science Foundation of Shandong Province of China (No.ZR2014BM022) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, Q., Han, Y., Huang, X. et al. Hydrothermal synthesis of Ni2P nanoparticle and its hydrodesulfurization of dibenzothiophene. J Nanopart Res 19, 123 (2017). https://doi.org/10.1007/s11051-017-3781-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3781-2