Abstract
Cu nanoparticles were synthesized using low-temperature aqueous reduction method at pH 3, 5, 7, 9 and 11 in presence of ascorbic acid and polyvinylpyrrolidone. The nanoparticles were characterized using transmission electron microscopy, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction techniques. Results demonstrated a strong dependence of synthesis pH on the size, shape, chemical composition and structure of Cu nanoparticles. While lower pH conditions of 3 and 5 produced Cu0, higher pH levels (more than 7) led to the formation of Cu2O/CuO nanoparticles. The reducing capacity of ascorbic acid, capping efficiency of PVP and the resulting particle sizes were strongly affected by solution pH. The results of in vitro disk diffusion tests showed excellent antimicrobial activity of Cu2O/CuO nanoparticles against a mixture of bacterial strains (Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa), indicating that the size as well as oxidation state of Cu contributes to the antibacterial efficacy. The results indicate that varying synthesis pH is a strategy to tailor the composition, structure and properties of Cu nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Noble metal nanoparticles continue to be the focus of research due to their distinct physicochemical properties which are dramatically different from the bulk equivalents. They are increasingly used in applications such as optoelectronics, advanced devices, sensors and catalysis (Murray et al. 2000; Salata 2004; Roco et al. 2011; Fakruddin et al. 2012). Many metal nanoparticles are also well known for antimicrobial activity (e.g., Ag, Au, Cu, Zn, etc.) due to their high surface-to-volume ratio, as well as the ability to release metal ions (Ramyadevi et al. 2012; Hashemipour et al. 2011). However, their crystal structure and shape also play a role. For example, the bactericidal activity of Ag nanoparticles decreases with increase in particle size, while truncated triangular shaped particles are found to have greater bactericidal effect compared to spherical or rod shaped particles (Panacek et al. 2006; Pal et al. 2007).
While Ag nanoparticles are the most studied and best known for offering strong antimicrobial activity against a wide variety of microbes, they are also expensive. The investigation of Cu nanoparticles as an alternative is thus receiving much attention nowadays. This interest has opened a wide range of possibilities for Cu nanoparticles in antimicrobial (Kobayashi et al. 2013), antifungal, bacteriostatic (Cioffi et al. 2005) and antigerm applications and surface coatings (Muller 2009).
Cu nanoparticles can be prepared by different well-known methods, such as thermal reduction (Dhas et al. 1998), vapor deposition (Liu and Bondo 2003), microwave irradiation (Zhao et al. 2004), sono-chemical reduction (Kumar et al. 2001), metal vapor synthesis (Vitulli et al. 2002) as well as chemical reduction (Yang and Zhu 2003). Due to its simple process, suitability for small-scale sample preparation and the ease of control of reaction parameters, chemical reduction is the most widely used technique for Cu nanoparticle synthesis. However, alterations in synthesis conditions such as water content, surfactant concentration, reactant concentration, anions, pH and temperature have significant effect on the reaction kinetics, and thus the size and shape of the nanoparticles formed, which in turn affect their antimicrobial properties (Yang and Zhu 2003). Another challenge in the chemical synthesis of Cu nanoparticles is the tendency of the particles to oxidize. These oxide phases are thermodynamically more stable (Mott et al. 2007), with the Cupric oxide (Cu+2) oxidation state being the most prevalent. In aqueous solutions, this can be explained by the fact that the Cu2+ ion is smaller than cuprous oxide (Cu+) and, having twice the charge, interacts much more strongly with solvent water. This outweighs the second ionization energy of copper and renders the Cu2+ more stable. Cuprous oxide (Cu2O) is unstable in an aqueous solution; thus, it can be expected to rather form a mixture of Cu2+ and metallic copper (Cu0). However, with the addition of a complex forming agent, the stability of the copper oxides will improve; thus, a higher percentage of Cu+ is expected (Jeong et al. 2008). The antimicrobial activity of zero valent Cu nanoparticles has been widely reported (Gunawan et al. 2011; Godymchuk et al. 2015; Valodkara et al. 2012; Bagchia et al. 2013). Usman et al. (2013) reported a high level of antimicrobial activity of Cu nanoparticles against several microorganisms which includes S. aureus, B. subtilis, P. aeruginosa, Salmonella choleraesuis and C. albicans. On the other hand, oxide forms of Cu such as Cu2O, CuO also have been shown to exhibit antimicrobial effects (Katwal et al. 2015; Ahamed et al. 2014; Shaffiey et al. 2014; Abboud et al. 2014). Ren et al. reported antimicrobial efficacy of CuO nanoparticles generated by thermal plasma technology that contain traces of pure Cu and Cu2O nanoparticles toward a range of bacterial pathogens (Rena et al. 2009). These suggest that changes in chemical composition and surface charges and shape of Cu nanoparticles play a crucial role in determining their antimicrobial activity, leading to the objective of this work: to synthesize Cu nanoparticles by the chemical reduction method and to study the effect of pH on their shape, size and chemical composition and antibacterial efficacy. To the best of our knowledge, such investigation has not been concluded to date.
The research question is thus: how are the stability and oxidation probability of Cu nanoparticles affected by the presence of ascorbic acid (antioxidant) and polyvinylpyrrolidone (dispersant used to prevent colloidal aggregation) under different pH conditions. The antimicrobial properties of Cu synthesized nanoparticles were investigated against a mixture of bacterial strains consisting of S. aureus, P. aeruginosa and E. coli in order to provide a realistic and more challenging environment to the material. An attempt was also made to correlate the chemical and structural properties of the Cu nanoparticles with the observed bactericidal activities.
Materials and methods
Materials
All reagents used in the experiment were of analytical grade and obtained from Minema Chemicals, Randpark Ridge, Johannesburg, South Africa (CuSO4 and ascorbic acid) and Sigma-Aldrich, Centurion, South Africa (polyvinylpyrrolidone (PVP), H2SO4 and NaOH). All materials were used as received.
Synthesis of the Cu nanoparticles
CuSO4 (0.2 mol/L) and ascorbic acid (1.0 mol/L) solutions were prepared and bubbled with Argon gas for 30 min. 3 g of Polyvinylpyrrolidone (PVP) was added to 100 ml of the CuSO4 solution. The pH levels of individual solutions (PVP/CuSO4 and ascorbic acid) were adjusted to the required levels using either H2SO4 or NaOH. 100 ml of PVP/CuSO4 and 40 ml of ascorbic acid were mixed and stirred for 4 h. The final pH of the reaction mixture was noted. The precipitate was collected through centrifugation, rinsed with distilled water and ethanol, and finally dried in an oven for 24 h at 70 °C (Qing-ming et al. 2012).
Characterization
The crystallinity and phase composition of the Cu nanoparticles were investigated using a PANalytical XPERT-PRO diffractometer using Ni filtered CuKα radiation (λ = 1.5406 Å) with a fixed slit at 45 kV (voltage) and 40 mA (current) in the 2θ diffraction angle range of 4.5°–90°. The diffraction peaks were also used for the semiquantitative determination of the different oxidation states of copper; Cu0, Cu+ and Cu2+ in the samples using Rietveld analysis (High-score 3 +) indirectly. The X-ray diffraction patterns were also compared and interpreted with JCPDS (Joint Committee for Powder Diffraction Data) standard data. Morphological analysis of the samples was carried out using a Zeiss Auriba field emission scanning electron microscope (SEM) at an operating voltage of 5 kV. Samples were sputter-coated with carbon to avoid charging prior to imaging. The morphological details of the particles were investigated by a Jeol Jem 2100 transmission electron microscope (TEM). For TEM analysis, the powder samples were sonicated in ethanol for 2 min and dropped on a carbon-coated Cu-grid and dried.
Antibacterial properties
Antimicrobial properties of the nanoparticles were tested on a mixed culture of bacteria using the disk diffusion method. A suspension containing Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa was made up to a concentration of around 200 000 cfu/mL. A sterilized pour plate was prepared using the inoculum and allowed to stand for about 15 min. A well of 8 mm diameter was aseptically made at the center of each agar plate and 0.1 g of powder placed in the well. All treated plates including a control with only bacterial inoculation were incubated in an upright position for 48 h at 35 °C. After incubation, the diameter of the inhibition zones was measured, and it was confirmed that a bacterial lawn did form on the control plates. The screening tests were performed in triplicate, and the average results are reported here.
Results and discussion
It was observed that the pH of PVP/CuSO4 and ascorbic acid for each experiment decreased after the reaction had taken place. The individual solutions of pH 11, 9, 7, 5 and 3 changed to 6.5, 5.1, 4.4, 3.7 and 1.3, respectively, after the reaction. This could be due to the reduction of CuSO4 hydrate in an aqueous medium. While the Cu2+ ions form [Cu (OH)]+ which is acidic, the sulfate ions form bisulfate when in contact with water. This leads to the formation of an excess of H+ in the solution as the reaction proceeds, which could lower the pH of the mixture (Waller and Pickering 1992).
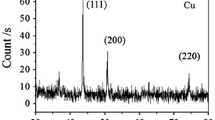
Figure 1 presents the XRD patterns of nanoparticles synthesized at different pH conditions. It can be seen that the diffraction patterns of nanoparticles prepared at pH 3 and 5 have narrow and well-defined peaks which shows that all particles are predominantly crystalline in nature with very little or no amorphous phase. The sharp peaks at about 43.4° and 50.6° correspond to the (111) and (200) planes of crystalline metallic Cu with bigger crystallite size. (ref. pattern Cu-00-003-1018, cubic).
XRD patterns of samples synthesized at pH 7 or above show broader peaks at 29.9°, 36.4°, 42.3° and 74.1° which can be assigned to (110), (111) and (311) planes of Cu2O (ref. pattern Cuprite-04-004-7864, cubic). The additional peaks in these samples seen at 38.8° and 61.8° match well with the (111) and (−113) planes of crystalline CuO (ref. pattern Tenorite -04-004-7864, monoclinic). Broadening of peaks is observed for samples prepared above 7 suggesting smaller crystallite sizes.
Using Rietveld semiquantitative analysis (High-score 3+), the percentage composition of Cu phases was determined per sample, and the results are presented in Fig. 2. It is observed that the crystalline phase composition of the samples vary with changes in pH condition and different Cu phases namely Cu, Cu2O and CuO correspond to Cu0, Cu+ and Cu2+ oxidation states, respectively. Samples prepared at a pH of 3 and 5 contain more of Cu, whereas oxidized phases of Cu are observed from pH 5 onwards. From the data, it is evident that the sample synthesized at pH 7 contains highest concentration of Cu2O which decreases thereafter with further increase in pH. No zero-valent Cu is detected at pH 11; however, increasing the pH from 7 to 11 is found to favor the formation of CuO. From Fig. 2, it is further apparent that particles produced at lower pH levels of between 3 and 5 are constituted of Cu0, while those produced at an initial pH of 7 or above are made up Cu+, Cu2+ predominantly. This result also indicates that ascorbic acid effectively reduces Cu ions to single phase Cu at pH 3 and 5 although minor quantities of Cu2O and CuO are formed at pH 5. However, its reduction capacity seems to be diminished due to its instability and autoxidation in neutral alkaline conditions (Bode et al. 1990) which can explain the decreasing Cu concentration from pH 7 or above.
Taking these findings into consideration, it can be deducted that reactions that involve oxidation reduction and exchange of H+ and OH− ions are dependent of the oxidation/reduction potential of the species and the pH. Under similar preparation conditions, in acidic conditions (pH 3 and 5), direct reduction of Cu2+ to Cu0 is thermodynamically favored due to the higher reduction potential of Cu2+ (E0 = +0.34) in comparison with H+ (E0 = 0) ions. Increase in pH to 7, however, favors first and second hydrolysis of Cu which eventually forms Cu2O (Cu+) and CuO (Cu2+) as major forms. It is also noticeable that in highly alkaline solution of pH 11, the second hydrolysis is predominant resulting in higher concentration of thermodynamically more stable CuO (Cu2+).
Zayyoun et al. (2016) recently demonstrated that by sol–gel method, both stable Cu2O (pH below 6) and CuO nanoparticles (pH above 12) can be prepared by adjusting the acidity–basicity of the precursor solution. Nikam et al. (2014) also reported that pH dependent formation of Cu2O and CuO could be obtained from Cu (II) acetate as the metal precursor and using benzyl alcohol as the solvent, under the microwave condition. CuO nanoparticles were formed under basic conditions, while phase pure Cu2O was formed at a pH below 7. These results support our observation. Granata et al. (2016) observed that mildly reducing d-glucose was unable to reduce Cu ions in alkaline pH of 9 and 11 during chemical reduction where they obtained mixed oxidized phases of Cu/Cu2O.
SEM images showing surface morphology of samples prepared under different pH conditions are presented in Fig. 3. The image analysis shows that synthesis pH greatly influences the surface morphology and size of Cu nanoparticles. It is apparent that particles produced in acidic conditions (pH 3 and 5) are relatively bigger (biggest being pH 3) and clustered, whereas the formation of smaller and more isolated particles is observed at pH 7 and above. The degree of aggregation is higher for particles produced at pH 7 in comparison with the ones produced at alkaline conditions (pH 9 and 11). The reduction in particle size from pH 7 onwards is also in line with the corresponding XRD peak broadening proposing smaller crystallites. The slow nucleation of Cu nanoparticles in acidic pH leads to the formation of larger particles, whereas at high pH, due to the presence of accessible –OH ions, faster nucleation occurs, and hence, smaller size particles are formed (Granata et al. 2016). Kethirabalan and Gurusamy ( 2014) also reported a similar correlation between pH and size of biosynthesized Ag nanoparticles.
The size and shape of particles obtained under the different experimental conditions were analyzed by TEM, and Fig. 4 illustrates the representative TEM images (low- and high- resolution) of Cu nanoparticles produced at different pH levels. At highly acidic condition (pH 3), monodisperse polyhedral Cu particles between 200 and 500 nm size are formed. As the pH increases to 5, the particle sizes reduce to a 100–200 nm range. Some smaller particles are also observed in this case. According to XRD results, at pH 7 the predominant phase is Cu2O along with CuO and small concentration of Cu. Correspondingly, the sample appears to be aggregates of particles with relatively bigger cubic and smaller spherical particles (refer Fig. 4b’) in the TEM image. The cube morphology can be ascribed to the cubic crystal structure of Cu2O, whereas the spherical particles can be those of CuO (Nikam et al. 2014; Pande et al. 2008). However, the aggregates consisting of smaller particles are formed at pH 7 and above although the aggregate size reduces as the pH rises to 9 and 11. The particles at higher pH are not isolated but rather fused together and seem capped by surfactant. The extent of capping is found to increase as the pH increases. Although it is difficult to accurately determine the particle size due the presence of capping agent in these cases, it can be concluded that the average size is reduced to below 100 nm.
PVP, a polymeric surfactant, is used as a dispersing agent during synthesis. Zhang et al. (1996) observed more aggregated Ag nanoparticles due to the lack of steric protection from PVP when used in lower concentration in a chemical reduction process. According to them, if the PVP either adsorbed on the surface or chemically bonded to the nanoparticle, it is crucial in preventing particle aggregation. Granata et al. (2016) on the other hand reported the high capping efficiency of PVP when used with mild (d-glucose) reducing agent in the chemical reduction process to produce Cu nanoparticles. In this work, the effect of pH was not studied in detail.
In our case, the formation of significantly large particles of Cu at lower pH indicates the inefficiency of PVP to be absorbed on Cu0 particles to prevent particle growth. This may be due to the fact the PVP forms positively charged complexes under acidic conditions which reduces the binding capacity to cations (Kaul and Amiji 2004). However, at relatively higher pH (7 or above), PVP stabilizes Cu+ and Cu2+ ions through coordinated (nitrogen and oxygen polar groups donates lone-pair electrons) interactions. Cu+–PVP in the presence of sufficient concentration of –OH ions forms Cu2O, whereas at high pH, an excess of –OH ions Cu2+–PVP results in more stable CuO (Kaul and Amiji 2004; Yu et al. 2009).
The presence of stabilizers on nanoparticle surface might affect its antimicrobial properties. Burkowska-But et al. (2014) studied the influence of PVP and citrate stabilizers on antimicrobial activity of Ag nanoparticles in lake water bacteria. The results of the study indicated that PVP protected nanoparticles had significantly stronger bactericidal effect than its citrate counterpart. According to the authors, nanoparticles stabilized by PVP did not aggregate easily and, hence, strengthened their activity. In another study, Mathews and co-workers (Mathews et al. 2013) found that by modifying the surface of Cu nanoparticles with an inert polymer did not affect the release of ionic Cu from the surface although attenuation of the contact between bacteria and Cu surface was observed.
The disk diffusion method was used to evaluate the antimicrobial efficiency of our Cu particles produced under different pH conditions, and Fig. 5 shows the average of triplicate tests performed on the two batches of nanoparticles prepared under similar conditions. Three bacterial strains namely S. aureus, E. coli and P. aeruginosa were selected for the mixed bacterial culture. A culture with more than one strain of bacterial is one of the strategies to increase bacterial diversity to mimic a realistic situation where a synergistic interaction can impose a more challenging microbial environment (Schink 2002).
From the results, it is apparent that there is a relatively linear increase in bacterial killing efficacy as the synthesis pH increases. However, particles produced at pH 7 show relatively higher efficiency, very close to those produced at pH 11. For particles at pH lower than 7, the lower antimicrobial activity of the Cu particles correlates with an increase in their size as supported by SEM and TEM results. According to the reported literature (Veerasamy et al. 2011; Azam et al. 2012), larger nanoparticles due to their bigger size cannot easily penetrate through the cell walls of microorganisms and cause cell destruction. The particles prepared at pH 7 or above show smaller particle sizes which explains their higher antimicrobial efficiency in general. Other factors that can influence the antimicrobial efficiency of nanoparticles are the possibility of metal ion leaching and generation of reactive oxygen species from nanoparticle surface (Besinis et al. 2014; Dizaj et al. 2014). In this case, it is seen that cumulative content of oxidized phases of Cu increases with increase in synthesis pH. This can lead to increased Cu+ and Cu2+ release in the bacterial medium which facilitates their efficient binding to the negatively charged surface of the bacteria and eventually result in enhancement of bactericidal efficiency (Seil and Webster 2012). Conversely, the oxidative stress through reactive oxygen species induced by Cu2O and CuO can also result in cell damage and apoptosis (Sondi and Salopek-Sondi 2004). A combination of these effects of oxides along with smallest size of particles explains the significant contact killing efficiency of particles produced at pH 7 in comparison with those prepared at 9 and 11. The fact that the nanoparticles are less aggregated through stabilization by PVP at pH values above 7 and PVP does not affect the release of Cu ions from the nanoparticle surface also contributes positively to the observed higher bactericidal activities.
Azam et al. (2012) as well as Ahamed et al. (2014) revealed significant size dependent antimicrobial activity of CuO nanoparticles against various bacterial strains (E. coli, P. aeruginosa, K. pneumoniae, E. faecalis, S. Flexneri, S. typhimurium, P. vulgaris and S. aureus). Lee et al. (2011) reported superior antimicrobial properties of cubic Cu2O in comparison with octahedral Cu2O, and in recent work by Meghana et al. (2015), the mechanism of antimicrobial activity in Cu oxide nanoparticles in E. Coli was investigated. According to them, after the initial cell damage caused on the cell wall, further damage is caused by two different pathways; Cu2O forms Cu+-peptide complex, while CuO forms free radicals to cause cell destruction. They also suggest that the oxidation state has a major role deciding the activity, where Cu2O showed efficient activity and cell affinity. These reports are in agreement with our findings on size and oxidation state depended antimicrobial activity of Cu nanoparticles which in turn is directly dependent on the synthesis pH conditions.
Conclusions
Cu nanoparticles were synthesized at different pH levels using the aqueous reduction method and ascorbic acid and PVP. The nanoparticles produced vary in size, morphology and are found at different levels of oxidation. Ascorbic acid, being a mild reducing agent, could not reduce Cu ions in alkaline conditions due to its instability. The particle size decreased with increasing synthesis pH which could be correlated to the efficiency of PVP in dispersing and capping the Cu ions in alkaline conditions. The bactericidal efficiency of the prepared samples was tested against a mixed bacterial culture consisting of S. aureus, E. coli and P. aeruginosa. The results indicated that the contact killing efficacy of particles is dependent on size as well as the oxidation sate. Cu particles produced at pH 7 showed higher killing efficiency compared to those produced at below pH 7 and close to that of pH 11. The significant contact killing efficiency of samples prepared at pH 7 and above could be due to the combined effect of size, leaching of ions and reactive oxygen generation. The nanoparticles synthesized in alkaline conditions thus offer good potential in antimicrobial packaging and coatings applications.
References
Abboud Y, Saffaj T, Chagraoui A, Bouari AE, Brouzi K, Tanane O, Ihssane B (2014) Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl Nanosci 4:571–576
Ahamed M, Alhadlaq HA, Khan MAM, Karuppiah P, Al-Dhabi NA (2014a) Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J Nanomater. doi:10.1155/637858
Ahamed M, Alhadlaq HA, Khan MM, Karuppiah P, Aldhabi NA (2014b) Synthesis, characterization and antimicrobial activity of copper oxide nanoparticles. J Nanomater. doi:10.1155/2014/637858
Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A (2012) Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed 7:6003–6009
Bagchia B, Kara S, Deyb SK, Bhandarya S, Roya D, Mukhopadhyayc TK, Dasa S, Nandya P (2013) In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloid Surf B 108:358–365
Besinis A, De Peralta T, Handy RD (2014) The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 8:1–16
Bode AM, Cunningham L, Rose RC (1990) Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin Chem 36:1807–1809
Burkowska-But A, Sionkowski G, Walczak M (2014) Influence of stabilizers on the antimicrobial properties of silver nanoparticles introduced into natural water. J Environ Sci 26:542–549
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D’Alessio M, Zambonin PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 7:5255–5262
Dhas NA, Raj CP, Gedanken A (1998) Synthesis, characterization, and properties of metallic copper nanoparticles. Chem Mater 10:1446–1452
Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K (2014) Antimicrobial activity of the metals and metal oxide nanoparticles. Mat Sci Eng C 44:278–284
Fakruddin M, Hossain Z, Afroz H (2012) Prospects and applications of nanobiotechnology: a medical perspective. J Nanobiotech. doi:10.1186/1477-3155-10-31
Godymchuk A, Frolov G, Gusev A, Zakharova O, Yunda E, Kuznetsov D, Kolesnikov E (2015) Antibacterial properties of copper nanoparticle dispersions: influence of synthesis conditions and physicochemical characteristics. Mater Sci Eng 98:12033–12041
Granata G, Yamaoka T, Pagnanelli F, Fuwa A (2016) Study of the synthesis of copper nanoparticles: the role of capping and kinetic towards control of particle size and stability. J Nanopart Res. doi:10.1007/s11051-016-3438-6
Gunawan C, Teoh WY, Marquis CP, Amal R (2011) Induced adaptation of bacillus sp. to antimicrobial nanosilver. ACS Nano 5:7214–7225
Hashemipour H, Zadeh ME, Pourakbari R, Rahimi P (2011) Int J Phys Sci 6:4331–4336
Jeong EH, Woo JY, Cho YH, Jeong YK, Kima KH, Kimb BK (2008) Holographic polymer-dispersed liquid crystals using vinyloxytrimethylsilane. Int Sci. doi:10.1002/PI.2510
Katwal R, Kaur H, Sharma G, Naushad M, Pathania D (2015) Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J Ind Eng Chem 31:173–184
Kaul G, Amiji M (2004) Polymeric gene delivery systems. In: Wise DL, Hasirci V, Lewandrowski KU, Yaszemski MJ, Altobelli DW, Trantolo DJ (eds) Tissue engineering and novel delivery systems. Marcel Dekker, Inc, New York, pp 333–367
Kethirabalan C, Gurusamy A (2014) Antibacterial activity of pH-dependent biosynthesized silver nanoparticles against clinical pathogen. BioMed Res-Int. doi:10.1155/2014/725165
Kobayashi Y, Shirochi T, Yasunda Y, Morita T (2013) Preparation of metallic copper nanoparticles by reduction of copper ions in aqueous solution and their metal-metal bonding properties. Int J Chem Molec Nucl Mater Metall Eng 7:769–772
Kumar RV, Mastai Y, Diamant Y, Gedanken A (2001) Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J Mater Chem 11:1209–1213
Lee Y-J, Kim S, Park S-H, Park H, Huh Y-D (2011) Morphology-dependent antibacterial activities of Cu2O. Mater Lett 65:818–820
Liu Z, Bondo Y (2003) A novel method for preparing copper nanorods and nanowires. Adv Mater 15:303–305
Mathews S, Hans M, Mücklich F, Solioz M (2013) Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl Environ Microbiol 79:2605–2611
Meghana S, Kabra P, Chakraborty S, Padmavathy N (2015) Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv 5:12293–12299
Mott D, Galkowski J, Wang L, Lou J, Zhong CJ (2007) Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23:5740–5745
Muller TJ (2009) Copper based nanomaterials for oxidation catalysis. (Thesis for Magister Scintiae), University of the Free State
Murray CB, Kagan CR, Bawendi MG (2000) Review: synthesis and characterization of monodisperse nanocrystals and closepacked nanocrystal assemblies. Mater Sci 30:545–610
Nikam AV, Arulkashmir A, Krishnamoorthy K, Kulkarn AA, Prasad BLV (2014) pH-dependent single-step rapid synthesis of CuO and Cu2O nanoparticles from the same precursor. Cryst Growth Des 14:4329–4334
Pal S, Tak YK, Song JM (2007) Does the antimicrobial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720
Panacek A et al (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253
Pande S, Jana S, Sinha A, Datta A, Pal T (2008) Nanoparticle catalyzed clock reaction. J Phys Chem C 112:3619–3626
Qing-ming L, Yasunami T, Kuruda K, Okido M (2012) Preparation of Cu nanoparticles with ascorbic acid by aqueous solution reduction method. Trans Nonferr Met Soc 22:2198–2203
Ramyadevi J, Jeyasubramanian K, Marikani A, Rajakumar G, Rahuman AA (2012) Mater Lett 71:114–116
Rena G, Hub D, Chengb EWC, Vargas-Reusc MA, Reipd P, Allakerc RP (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob AG 33:587–590
Roco MC, Mirkin CA, Hersam MC (2011) Nanotechnology research directions for societal needs in 2020: summary of international study. J Nanopart Res 13:897–919
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotech. doi:10.1186/1477-3155-2-3
Schink B (2002) Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek 81:257–261
Seil JT, Webster TJ (2012) Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomed 7:2767–2781
Shaffiey SF, Shapoori M, Bozorgnia A, Ahmadi M (2014) Synthesis and evaluation of bactericidal properties of CuO nanoparticles against Aeromonas hydrophila. J Nanomed 1:198–204
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 275:177–182
Usman MS, El Zowalaty ME, Shameli K, Zainuddin N, Salama M, Ibrahim NA (2013) Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed 8:4467–4479
Valodkara M, Rathorea PS, Jadejab RN, Thounaojamb M, Devkarb RV, Thakorea S (2012) Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J Hazard Mater 201–202:244–249
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver, nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120
Vitulli G, Bernini M, Bertozzi S, Pitzalis E, Salvadori P, Coluccia S, Matra G (2002) Nanoscale copper particles derived from solvated Cu atoms in the activation of molecular oxygen. Chem Mater 14:1183–1186
Waller PA, Pickering WF (1992) Effect of time and pH on the lability of copper and zinc sorbed on humic acid particles. Chem Spec Bioavailab 4:29–41
Yang M, Zhu J (2003) Spherical hollow assembly composed of Cu2O nanoparticles. J Cryst Growth 256:134–138
Yu W, Xie H, Chen L, Li Y, Zhang C (2009) Synthesis and characterization of monodispersed copper colloids in polar solvents. Nanoscale Res Lett 4:465–470
Zayyoun N, Bahmad L, Laaˆnab L, Jaber B (2016) The effect of pH on the synthesis of stable Cu2O/CuO nanoparticles by sol–gel method in a glycolic medium. Appl Phys. doi:10.1007/s00339-016-0024-9
Zhang Z, Zhao B, Hu L (1996) PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J Solid State Chem 121:105–110
Zhao Y, Zhu J, Bian N, Chen H (2004) Microwave-induced polyol-process synthesis of copper and copper oxide nanocrystals with controllable morphology. Eur J Inorg Chem 20:4072–4080
Acknowledgments
The authors wish to thank Department of Science and Technology (DST) and Council for scientific and Industrial Research (CSIR), South Africa for the financial support (Project No. HGER20S). The NCNSM, CSIR characterization facility and staff are acknowledged for their support with characterization of materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Details on potential conflicts of interest are included in the ‘publishing ethics’ section.
Rights and permissions
About this article
Cite this article
Moshalagae Motlatle, A., Kesavan Pillai, S., Rudolf Scriba, M. et al. Chemical synthesis, characterization and evaluation of antimicrobial properties of Cu and its oxide nanoparticles. J Nanopart Res 18, 312 (2016). https://doi.org/10.1007/s11051-016-3614-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3614-8