Abstract
Self-assembled silver nanostructures on the industry-grade monocrystalline GaAs (100) wafer surface were obtained via physical vapor deposition and characterized by optical reflection spectroscopy, scanning electron microscopy, and current–voltage curve measurements. Reflection spectra of the samples with Ag equivalent thicknesses of 5, 7.5, and 10 nm demonstrated wide plasmonic bands in the visible range of spectra. Thermal annealing of the nanostructures led to narrowing of the plasmonic bands caused by major transformations of the film morphology. While the As prepared films predominantly had a small-scale labyrinth structure, after annealing well-separated silver nanoislands are formed on the gallium arsenide surface. A clear correlation between films morphology and their optical and electrical properties is elucidated. Annealing of the GaAs substrate with Ag nanostructures at 100 °C under control of the resistivity allowed us to obtain and fix the structure at the percolation threshold. It is established that the samples at the percolation threshold possess the properties of resistance switching and hysteresis.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bright optical properties of plasmonic nanostructures near the surface plasmon resonance have potential to be used for development of the plasmonic-based devices and for enhancement of light harvesting for a variety of applications. In recent years, a close coupling of plasmonic nanoparticles to absorbing semiconductor has been utilized to enhance absorption in ultrathin film solar cells (Schaadt et al. 2005; Atwater and Polman 2010; Hong et al. 2012; Yang et al. 2011). From the perspective of photovoltaic applications, plasmon resonant absorption represents an unwanted loss process, but off-resonant enhanced scattering can yield increases in absorption and photocurrent collection (Nakayama et al. 2008; Beck et al. 2011). Although theory gives an advantage to aluminum over gold and silver (Hylton et al. 2013), considerable improvement of solar cell performance was reported in recent experiments with nanoparticles of both noble metals spread over the solar cells surface (Shu et al. 2010; Wang et al. 2012; Liu et al. 2011). Since the optical properties of plasmonic nanostuctures strongly depend on nanoparticle size and shape, the study of the relations between morphologies, optical, and electrical characteristics of the nanoparticles is a requirement for achieving their high-potential applications in photovoltaics and electrooptics.
In this contribution, we demonstrate the physical basis of an alternative application of plasmonic metal nanostructures on the semiconductor surfaces. It is based on the controlled resistance switching of the metal nanostructures induced by the tiny changes of their morphology. We supposed that such structures may be used as an element of the memory devices. Due to compatibility with the advanced technology of integrated circuits, it can be quickly implemented in real devices manufacturing.
It is well-known that metal nanostructures on semiconductor substrates may be obtained by different techniques (Sun 2010). In particular, ordered arrays of silver nanoparticles were produced via template deposition (Liu et al. 2013; Vidoni et al. 2003). For our experiments, physical vapor deposition was used. It involves condensation of a vaporized material onto a substrate, and includes highly controllable vacuum technique such as thermal evaporation (Pandey et al. 2011). Among well-known techniques of metallic nanostructures synthesis on the semiconductor surface, thermal evaporation in a vacuum chamber is well-developed technologically, possess the growth rate control, and purity of deposited materials. However, such technique does not enable obtaining of surface nanostructures with controlled parameters due to self-organization. Several possible ways for the modifications of growth processes were discussed in references (Shirakawa and Komiyama 1999; Lee et al. 2011; Vartanyan et al. 2012; Yin et al. 2007). Nevertheless, more research is needed to explore the process of self-organization of metal deposits on the semiconductor surfaces in more detail. In particular, the interdependence between optical and electrical properties of Ag nanostructures on the GaAs surface is to be addressed.
In this work, we describe the results of experimental investigations of silver nanostructures deposited on the GaAs surface. First, we present the interdependence between the morphology of Ag self-organized nanostructures on GaAs and their reflection spectra and their changes under thermal annealing. Second, we present the results of investigation of the correlation between electrical properties, morphology, and optical properties of silver nanostructures. We also demonstrate the resistance switching phenomenon of the obtained nanostructures in an unusual planar configuration of the supply electrodes.
Experimental section
GaAs was chosen as the substrates material due to the spectral position of its absorption band relative to the plasmonic absorption band of silver nanoparticles. The 400 μm thick, double-side polished undoped gallium arsenide (100) wafers (from Wafertech) were used in all experiments. Thin silver films were vacuum-deposited on the substrates in the vacuum chamber PVD-75 (Kurt J. Lesker) at residual pressure of ~10−7 Torr. As a source of material, the tungsten boat with high-pure silver (99.99 %) was used. The amount and rate of silver deposition were controlled during the evaporation process with the quartz crystal oscillator.
Morphology of the obtained silver structures was studied using the scanning electron microscope merlin (Carl Zeiss) at the accelerating voltage of 5–15 kV.
Taking into account the spectral range of the silver nanoparticles plasmonic absorption, which matches partially with the GaAs bandgap, the reflection spectra were measured for the investigation of optical properties. For this purpose, the multichannel photonic analyzer PMA-12 (Hamamatsu) was used in the spectral range of 350–1100 nm and at different incident angles. The reflection spectra of the samples were measured at ambient conditions.
To study the electrical properties of silver nanoparticles on the GaAs substrates, conductive electrodes were used. The width of electrodes was 7 mm, and the distance between them was of 2 mm. Current–voltage curve measurements were performed using picoammeter/voltage source, keithley 6487.
Results and discussion
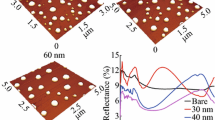
Reflection spectra of silver nanostructures on the GaAs substrate are presented in Fig. 1 as a difference between the reflectivity of GaAs substrate with deposited Ag nanostructure and without it. Figure 1 shows such contributions of silver to the reflectivity of the hybrid material measured at the incidence angle of 30°. The mass thicknesses of the silver deposits were 5, 7.5, and 10 nm. Silver nanostructures demonstrate the wide spectrum with maximum near 850 nm (Fig. 1 a). It is due to the collective effects of the reflection on nanoparticles described theoretically by authors Kachan and Ponyavina (2002). Sharp features in the reflection spectra near 870 nm are due to the edge of the fundamental absorption in GaAs (Kudykina 1993). Clearly, the reflectivity increases with the amount of the deposited silver. In the spectral range of 900–1000 nm, the samples with thickness of 7.5 and 10 nm demonstrate an increase of the reflectivity while the 5 nm silver film does not contribute to the reflectivity in this spectral range due to the absence of silver nanostructures with corresponding resonance frequencies.
Figure 1b demonstrates the influence of thermal annealing on the reflectivity of silver nanostructures on gallium arsenide substrates. The samples were heated in ambient conditions to 200 °C. After annealing, the reflection spectra narrowed as compared to non-annealed samples. This narrowing is due to the modification of the silver nanostructure morphology by surface diffusion of silver atoms on which the optical properties of metallic island films depends strongly (Kelly et al. 2003; Toropov et al. 2012, 2014; Gladskikh et al. 2014).
At the wavelengths shorter than 500 nm, the reflectivity differences acquire negative values, i.e., silver nanoparticles function as an antireflection coating.
Examples of the obtained SEM images of silver nanoparticles on the GaAs substrates are presented in Fig. 2. Equivalent mass thickness of Ag was 5 nm. Morphology of the Ag film deposited on the GaAs substrate has a strongly irregular labyrinth structure. Samples with equivalent thickness of 7.5 and 10 nm have the same structure and differ only in the larger filling factor.
Thermal annealing leads to dramatic changes in the morphology of nanostructures. Due to thermally activated surface diffusion, the labyrinth structure segregates into well-separated islands of different sizes. SEM image presented in Fig. 2b substantiates the conclusions already made in view of aforementioned narrowing and shift of plasmonic band in the reflection spectrum caused by annealing. For gold deposited on GaAs substrates, similar behavior was reported in (Leem et al. 2011). This is not surprising as the cohesive energy of gold (3.82 eV) is larger than that of silver (2.95 eV) (Kambe 1955). More detailed analysis of the dewetting process is difficult because the reliable data on the surface and interface energies for the Ag/GaAs (100) pair are still lacking (Lazar and Otyepka 2015). Nevertheless some conclusions may be done based on the similarity between gold and silver. The observed Volmer–Weber growth (Lüth 2010) and formation of self-assembled Au droplets on GaAs surfaces can be described based on the estimated stronger binding between Au adatoms (3.01 eV) than binding between Au adatom and GaAs substrate (2.96 eV) (Lee et al. 2015).
Deposition of silver nanostructures on the gallium arsenide surface exerts influence not only on its optical properties. Electrical properties undergo major changes as well. Again, the nature of these changes depends on the film morphology. The current–voltage curve of silver nanoparticles on the GaAs surface has typical linear form inherent for GaAs, however the resistivity of an undopped GaAs wafer was of 4 × 109 Ω while the resistivity of GaAs with silver film was only 341 Ω. This is due to the labyrinth structure of the deposited thin metal film formed under these conditions on the gallium arsenide surface in the same way as it takes place on the dielectric substrates (Vartanyan et al. 2014). Therefore, such large changes of the resistivity can be attributed to the formation of the conduction channels. Destruction of these channels leads to the sharp increase of the resistivity in the course of annealing (Fig. 3a). If the annealing process is stopped right after the resistivity jump, the obtained structures acquire very peculiar nonlinear electrical properties that are described below. Annealing of the GaAs substrate with Ag nanostructures at 100 °C under control of the resistivity allows us to obtain and fix the structure at the percolation threshold.
The current–voltage curve of the structures at the percolation threshold is presented in Fig. 3b. Conductivity of the Ag film that is constant at the applied voltages below 7 V undergoes at this voltage a sharp, an order of magnitude rise. If after that the voltage is dropped, the film remains in the state of high conductivity.
We propose the following explanation for the observed hysteresis of the film conductivity. At the percolation threshold, the connectivity of the metallic paths that lead from one electrode to another is only slightly disturbed. In particular, there are very narrow trenches to which almost full voltage of the source is applied. Hence, at the threshold voltage the electrostatic attraction of opposite edges becomes strong enough to deform them slightly, to narrow the gap and to enhance the tunneling current through the gap. Note that the silver film can become conductive and remain in such state, as it was observed in the experiments performed on dielectric substrates (Gladskikh et al. 2014; Vartanyan et al. 2014).
If the annealing process is not stopped at the percolation threshold, the obtained metallic nanostructure acquires very different properties. The resistance of the gallium arsenide with the silver film annealed at 180 °C for 1 h amounts to 4 × 109 Ω, barely different of that without the silver nanostructure. It is not surprising as the silver nanoparticles are completely separated. Hence, they cannot contribute considerably to the conductivity. In this case, the conductivity switching is also absent.
Conclusion
Physical vapor deposition of silver onto the gallium arsenide substrate leads to the formation of labyrinth nanostructures. Thermal annealing at moderate temperatures greatly modifies the film morphology and its optical and electrical properties. After annealing, well-separated silver nanoparticles are formed on the surface of gallium arsenide. Hence, physical vapor deposition may serve as an alternative way to obtain much demanded combination of semiconductor material with plasmonic metal nanoparticles (Ushanov et al. 2014).
The reflection difference spectra clearly demonstrate excitation of plasmon resonances localized in the silver nanoparticles formed on the gallium arsenide surface. The spectral position of the plasmon bands may be tuned by changing the amount of the deposited silver as well as by the subsequent thermal annealing. Of special interest is the antireflection action of the obtained silver nanostructures in the spectral range of 350–500 nm. As the plasmon absorption bands are expected to lie at longer wavelengths this may lead to enhanced absorption of light in the semiconductor substrate.
The silver nanostructures on the gallium arsenide surface possess also very peculiar electrical properties. At the sufficient mass density, silver deposits on the gallium arsenide surface are highly conductive and completely shunt the substrate conductivity. After annealing at elevated temperatures, the film separates into isolated nanoparticles with negligible conductivity as compared to the conductivity of the substrate. The island films annealed at moderate temperatures possess the most interesting properties, if the annealing process is stopped at the percolation threshold. The films obtained in this way are highly resistive at low voltages but transit into low resistance state at higher voltage, the transition voltage being 7 V. This state of low resistance is maintained when the voltage is reduced. Thus, the film preserves memory of the applied voltage.
References
Atwater HA, Polman A (2010) Plasmonics for improved photovoltaic devices. Nat Mater 9:205–213. doi:10.1038/nmat2629
Beck FJ, Mokkapati S, Catchpole KR (2011) Light trapping with plasmonic particles: beyond the dipole model. Opt Express 19:25230–25241. doi:10.1364/OE.19.025230
Gladskikh IA, Leonov NB, Przhibel’skii SG, Vartanyan TA (2014) The optical and electrical properties and resistance switching of granular films of silver on sapphire. J Opt Technol 81:280–284. doi:10.1364/JOT.81.000280
Hong L, Rusli Wang X, Zheng H, He L, Xu X, Wang H, Yu YuH (2012) Design principles for plasmonic thin film GaAs solar cells with high absorption enhancement. J Appl Phys 112:054326. doi:10.1063/1.4749800
Hylton NP, Li XF, Giannini V, Lee K-H, Ekins-Daukes NJ, Loo J, Vercruysse D, Van Dorpe P, Sodabanlu H, Sugiyama M, Maier SA (2013) Loss mitigation in plasmonic solar cells: aluminium nanoparticles for broadband photocurrent enhancements in GaAs photodiodes. Sci Rep 3:2874. doi:10.1038/srep02874
Kachan SM, Ponyavina AN (2002) Spectral properties of close-packed monolayers consisting of metal nanospheres. J Phys Condens Matter 14:103–111. doi:10.1088/0953-8984/14/1/309
Kambe K (1955) Cohesive energy of noble metals. Phys Rev 99:419. doi:10.1103/PhysRev.99.419
Kelly KL, Coronado E, Zhao LL, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107:668–677. doi:10.1021/jp026731y
Kudykina TA (1993) Optical properties of semiconductors (Ge, Si, GaAs, InSb) in the region of fundamental absorption. Phys Status Solidi B 179:215–221. doi:10.1002/pssb.2221790123
Lazar P, Otyepka M (2015) Accurate surface energies from first principles. Phys Rev B 91:115402. doi:10.1103/PhysRevB.91.115402
Lee SC, Dawson LR, Huang SH, Brueck SRJ (2011) Lithography-free nanoscale patterned growth of GaAs on Si (001) with sub 100 nm silica nanoparticles by molecular beam epitaxy. Cryst Growth Des 11:3673–3676. doi:10.1021/cg101363q
Lee D, Li M-Y, Sui M, Zhang Q, Pandey P, Kim E-S, Lee J (2015) Observation of shape, configuration, and density of silver nanoparticles on various GaAs surfaces via deposition amount, annealing temperature, and dwelling time. Nanoscale Res Lett 10:240. doi:10.1186/s11671-015-0950-z
Leem JW, Yu JS, Song YM, Lee YT (2011) Antireflective characteristics of disordered GaAs subwavelength structures by thermally dewetted silver nanoparticles. Sol Energy Mater Sol Cells 95:669–676. doi:10.1016/j.solmat.2010.09.038
Liu W, Wang X, Li Y, Geng Z, Yang F, Li J (2011) Surface plasmon enhanced GaAs thin film solar cells. Sol Energy Mater Sol Cells 95:693–698. doi:10.1016/j.solmat.2010.10.004
Liu W, Wang X, Xu R, Wang X, Cheng K, Ma H, Yang F, Lic J (2013) Long-range ordered Ag nanodot arrays grown on GaAs substrate using nanoporous alumina mask. Mater Sci Semicond Process 16:160–164. doi:10.1016/j.mssp.2012.05.008
Lüth H (2010) Solid surfaces, interfaces and thin films. Springer-Verlag, Berlin Heidelberg
Nakayama K, Tanabe K, Atwater HA (2008) Plasmonic nanoparticle enhanced light absorption in GaAs solar cells. Appl Phys Lett 93:121904–1–121904-3. doi:10.1063/1.2988288
Pandey PA, Bell GR, Rourke JP, Sanchez AM, Elkin MD, Hickey BJ, Wilson NR (2011) Physical vapor deposition of metal nanoparticles on chemically modified graphene: observations on metal–graphene interactions. Small 7:3202–3210. doi:10.1002/smll.201101430
Schaadt DM, Feng B, Yu ET (2005) Enhanced semiconductor optical absorption via surface plasmon excitation in metal nanoparticles. Appl Phys Lett 86:063106–1–063106-3. doi:10.1063/1.1855423
Shirakawa H, Komiyama H (1999) Migration-coalescence of nanoparticles during deposition of Au, Ag, Cu, and GaAs on amorphous SiO2. J Nanopart Res 1:17–30. doi:10.1023/A:1010085430195
Shu G-W, Liao W-C, Hsu C-L, Lee J-Y, Hsu I-J, Shen J-L, Yang M-D, Wu C-H, Lee Y-C, Chou W-C (2010) Enhanced conversion efficiency of GaAs solar cells using Ag nanoparticles. Adv Sci Lett 3:368–372. doi:10.1166/asl.2010.1137
Sun Y (2010) Metal nanoplates on semiconductor substrates. Adv Funct Mater 20:3646–3657. doi:10.1002/adfm.201001336
Toropov NA, Kaliteevskaya EN, Leonov NB, Vartanyan TA (2012) Mutual modification of silver nanoparticle plasmon resonances and the absorptive properties of polymethine-dye molecular layers on a sapphire surface. Opt Spectrosc 113:616–620. doi:10.1134/S0030400X12120090
Toropov NA, Parfenov PS, Vartanyan TA (2014) Aggregation of cyanine dye molecules in the near fields of plasmonic nanoparticles excited by pulsed laser irradiation. J Phys Chem C 118:18010–18014. doi:10.1021/jp505234j
Ushanov VI, Chaldyshev VV, Il’inskaya ND, Lebedeva NM, Yagovkina MA, Preobrazhenskii VV, Putyato MA, Semyagin BR (2014) Fröhlich resonance in the AsSb/AlGaAs system. Phys Sol State 56:1952–1956. doi:10.1134/S106378341410031X
Vartanyan TA, Leonov NB, Khromov VV, Przhibel’skii SG, Toropov NA, Kaliteevskaya EN (2012) Granular metal films on the surfaces of transparent dielectric materials studied and modified via optical means. Proc SPIE 8414:841404. doi:10.1117/12.923123
Vartanyan TA, Gladskikh IA, Leonov NB, Przhibel’skii SG (2014) Fine structures and switching of electrical conductivity in labyrinth silver films on sapphire. Phys Sol State 56:816–822. doi:10.1134/S1063783414040349
Vidoni O, Neumeier S, Bardou N, Pelouard J-L, Schmid G (2003) Self-assembly of gold nanoclusters on molecularly modified GaAs. J Clust Sci 14:325–336. doi:10.1023/B:JOCL.0000005067.63818.39
Wang R, Pitzer M, Fruk L, Hu D, Schaadt DM (2012) Nanoparticles and efficiency enhancement in plasmonic solar cells. J Nanoelectron Optoelectron 7:322–327. doi:10.1166/jno.2012.1311
Yang M, Fu Zh, Lin F, Zhu X (2011) Incident angle dependence of absorption enhancement in plasmonic solar cells. Opt Express 19:A763–A771. doi:10.1364/OE.19.00A763
Yin Z, Tang X, Liu W, Deny S, Zhao J, Zhang D (2007) Study of InAs/GaAs quantum dots grown by MOVPE under the safer growth conditions. J Nanopart Res 9:877–884. doi:10.1007/s11051-006-9161-y
Acknowledgments
This work was partially supported by Ministry of Education and Science (Project No 2014/190) and the Government of Russia (Grant No. 074-U01), EU Project LIMACONA (IRSES-GA-2013-612600), the RFBR (14-02-31281), and the President’s Grant (MK 4694.2015.2). The authors are grateful to V.V. Chaldyshev for many useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gladskikh, P.V., Gladskikh, I.A., Toropov, N.A. et al. Correlation between structural, optical, and electrical properties of self-assembled plasmonic nanostructures on the GaAs surface. J Nanopart Res 17, 424 (2015). https://doi.org/10.1007/s11051-015-3236-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3236-6