Abstract
Increased pollution of ground and surface water and emerging new micropollutants from a wide variety of industrial, municipal, and agricultural sources has increased demand on the development of innovative new technologies and materials whereby challenges associated with the provision of safe potable water can be addressed. Heterogeneous photocatalysis using visible-light sensitized TiO2 photocatalysts has attracted a lot of attention as it can effectively remove dissolved organic compound in water without generating harmful by-products. On this note, recent progress on visible-light sensitive TiO2 synthesis via wet chemical N-doping method is reviewed. In a typical visible-light sensitive TiO2 preparation via wet chemical methods, the chemical (e.g., N-doping content and states) and morphological properties (e.g., particle size, surface area, and crystal phase) of TiO2 in as-prepared resultants are sensitively dependent on many experimental variables during the synthesis. This has also made it very difficult to provide a universal guidance at this stage with a certainty for each variable of N-doping preparation. Instead of one-factor-at-a-time style investigation, a statistically valid parameter optimization investigation for general optima of photocatalytic activity will be certainly useful. Optimization of the preparation technique is envisaged to be beneficial to many environmental applications, i.e., dissolved organic compounds removal in wastewater treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
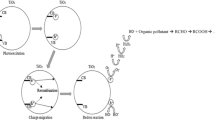
Increased pollution of ground and surface water and emerging new micropollutants from a wide variety of industrial, municipal, and agricultural sources has challenged the viability of current water treatment practices on meeting the regulation and increased demand of all water users. There is a clear need for the development of innovative new technologies and materials whereby challenges associated with the provision of safe potable water can be addressed. In recent years, heterogeneous photocatalysis technique by semiconductors has attracted a lot of attention in the water purification of dissolved-organic-compound contained water without generating harmful by-products (Dai and Yin 2014; Han et al. 2014; Yin et al. 2010; Zhang et al. 2012; Xia et al. 2014). Among the available, semiconductive TiO2 nanoparticles has proven to be the most promising (Graetzel 2001), with reported advantages of low cost and toxicity, greatly enhanced surface area, tunable properties which can be modified by size reduction, doping, or sensitizer, no substantial loss of photocatalytic activity after repeated process cycles, enhanced photo-induced charge transport (Hagfeldtt et al. 1992) and no depletion layer formation on the surface (Nazeruddin et al. 1993). Besides, due to the intensive research on the photocatalytic activity of TiO2, the mechanism of its degradation of dissolved organic compounds is well understood, which can be illustrated as follows (Zou and Zhu 2007; Gaya and Abdullah 2008):
-
1.
Absorption of efficient photons (hv ≥ EG = 3.2e) by TiO2
$$({\text{TiO}}_{2} ) + {\text{hv}} \to {\text{e}}^{ - }_{\text{CB}} + {\text{h}}^{ + }_{\text{VB}}$$(1) -
2.
Oxygen ionsorption (first step of oxygen reduction; oxygen’s oxidation degree passes from 0 to −1/2)
$$({\text{O}}_{2} )_{\text{ads}} + {\text{ e}}^{ - }_{\text{CB}} \to {\text{O}}_{2}^{ \bullet - }$$(2) -
3.
Neutralization of OH− groups by photoholes which produces OH• radicals
$$({\text{H}}_{2} {\text{O}} \Leftrightarrow {\text{H}}^{{^{ + } }} + {\text{OH}}^{ - } )_{\text{ads}} + {\text{h}}_{\text{VB}}^{ + } \to {\text{H}}^{ + } + {\text{OH}}^{ \bullet }$$(3) -
4.
Oxidation of the organic reactant via successive attacks by OH radicals
$${\text{R}} + {\text{OH}}^{ \bullet } \to {\text{R}}^{{^{\prime } \bullet }} + {\text{H}}_{ 2} {\text{O}}$$(4) -
5.
Direct oxidation by reaction with holes
$${\text{R}} + {\text{h}}^{ + } \to {\text{R}}^{ \bullet + } \to {\text{ degradation products}}$$(5)
However, it is well known that the band gap energy of TiO2 is intrinsically wide between 3.0 and 3.2 eV (3.0 for rutile and 3.2 for anatase). This means plain TiO2 semiconductors only absorb a small portion (3.6–5.2 %, depending the weather conditions) of solar spectrum in the UV region. It is of great interests that if TO2 photocatalysts could possess the ability of directly utilizing visible sunlight. Early attempts on visible-light sensitization of TiO2 mainly involved the doping of transition metal elements (Hoffmann et al. 1995; Choi et al. 1994a; Wang et al. 2000), which, however, has the drawbacks of require intensive energy and expensive ion implantation facilities, thermally unstable, increase free charge carrier trapping in bound electron-hole pairs, tend to form charge carrier recombination centers, possible photo-corrosion, and harmful nature of the dopants (Wang et al. 1999; Yamashita et al. 1998; Choi et al. 1994b). More importantly, these cationic dopants only result in limited band gap changes, and in some cases, do increase the light absorption, but not the photocatalytic activity. As a consequence, more significant advances in this field were achieved on doping TiO2 photocatalyst with non-metallic or anionic elements such as carbon, sulfur, halides, phosphor, and boron (Zhao et al. 2005; Chen et al. 2008a). While the valence band edge of TiO2 primarily derives from oxygen 2p orbitals and the conduction band (CB) edge from titanium 3d orbitals, non-metallic doping can result in electronic transitions from the dopant instead of oxygen 2p or 3p orbitals to the Ti 3d orbitals (Chen et al. 2008b). Asahi et al. first recognized the visible-light sensitivity of TiO2 with a certain amount of nitrogen dopants in 2001, and now nitrogen has proven to be one of the most effective non-metallic dopant due to its similar size to oxygen and low ionization energy (Chen et al. 2011; Park et al. 2002).
In this review, recent literature on N-doped TiO2 nanoparticles prepared by wet chemical methods, i.e., hydrolysis of a TiO2 precursor with N-containing solution, are reviewed. Other doping methods reported in the literature include the sputtering and implantation (Batzil et al. 2006; Diwald et al. 2004a; Kitano et al. 2006), high-temperature-sintering TiO2 under N-containing atmosphere (Diwald et al. 2004b; Nakamura et al. 2004; Irie et al. 2003a), etc. Compared to these methods, wet chemical method can not only avoid the high-temperature-induced surface property change but also have the fine control of the nitrogen-doping level with the possibility of high N-content (reportedly up to 8 %). Other reported advantages include low temperature and energy costs, the simplicity in controlling crystal structure/morphology and particle size by simple variations in experimental conditions, such as hydrolysis rate, solution pH, and solvents. Up to date, the reported studies on N-doped TiO2 nanoparticles preparation via wet chemical methods are flourished but still lacking of parametric investigations on experimental preparation variables, and more importantly their influence on the visible-light photoactivity of as-synthesized products. It is evident to speculate that the chemical (e.g., N-doping content and states) and morphological properties (e.g., particle size, crystal phase) of as-prepared resultants are sensitively dependent on the experimental conditions during the synthesis.
Effects of N-doping on TiO2 visible-light sensitivity
Despite the viability of TiO2 visible-light sensitization through wet chemical N-doping, there are still several discrepancies in the literature regarding the promoting effect of N-doping on the visible-light sensitivity of TiO2:
Firstly, N-dopants in the TiO2 resultants can exist as either substitutional in form of Ti–N–Ti bond or interstitial in form of Ti–O–N or Ti–N–O bond as illustrated in Fig. 1. For the former, the literature agreed that the binding energy (BE) of N 1 s peaks is fingerprinted at 396–398 eV; for the later, that is 400–406 eV. The discovery of this variance can be dated back to the heated debate over the originality of Asahi’s work, as Sato challenged the first discovery of N-doped TiO2 and its visible-light sensitivity to his credit (Sato 2002). This was later cleared up by the fact that the N-doped TiO2 obtained by Asahi belonged to substitutional group, whereas the one reported by Sato et al. 15 years earlier was more interstitial like. Based on some theoretical densities-of-states (DOS) calculations of different substitutional doping (i.e., C, N, F, S for O), Asahi maintained the critical role of Ti–N bond on visible-light sensitivity of N–TiO2 in his work, although their samples in the absence of any Ti–N bond were indicative of visible-light activities as well. Almost at the same time, Sakatani and Koike suggested that the nitrogen atoms were doped at the interstitial sites and that these interstitial nitrogen atoms were responsible for visible-light response (Sakatani et al. 2001). Later on, Peng et al. explained that both interstitial and substitutional N-dopants can enable the visible-light sensitivity of TiO2 nanoparticles, whereas only the former is higher than the later in terms of visible-light activity (Peng et al. 2008). The nitrogen content in the N-doped TiO2 reported in the literature, which could be one of major indicators in terms of the doping quality, varies vastly from 0.08 to 8 % along with different degree red-shift towards the visible-light range. Low concentrations of nitrogen have been generally observed to be all interstitial, while higher levels of nitrogen incorporation in titania lattice encourage substitutional doping (Di Valentin et al. 2005a).
Schematic models for a substitutional N-doping, b interstitial N-doping, c substitutional NO-doping, d substitutional NO2-doping, and e interstitial NO-doping. Each atom of the supercell (16 TiO2 units) is fully relaxed to stabilize the system (Reprinted with permission from Elsevier.) (Asahi and Morikawa 2007)
It is estimated that the existence of these two N-doping states would also vary according to the different preparation techniques, in many cases, both coexisted (see Fig. 2). However, larger proportion of N-dopants in TiO2 nanoparticles prepared by wet chemical methods should more likely belong to the interstitial doping group (see Table 1), as it is a consensus that Ti-O bond is quite stable at the mild conditions, which make it very difficult for nitrogen to replace the oxygen sites during the diffusion-controlled wet chemical preparation. Plus, N-containing amino substances would undergo serious oxidation during the calcinations treatment. This could result in the existence of various NOx species, such as NO, NO2, NO2−, NO2 −, NO2 2−, NO3 −, and N2O2 2−, etc. in N-doped TiO2 resultants, which has been observed widely in the literature (Chen et al. 2005; Sakatani et al. 2003; Joung et al. 2006; Livraghi et al. 2006). Finally, it is noteworthy that the radius of these N-containing species are usually too large to dope into the TiO2 bulk, so they more likely exist at the surface of the catalysts particles through formed chemical bonds or be adsorbed at the meso-pores. This, however, should barely hinder its application of water treatment at all, as the photoactive surface of an N-doped TiO2 photocatalyst is generally considered to be sufficient during photodegradation of dissolved organic compounds. In addition, N-doping was observed to have promoting effect on the surface area and the number of surface hydroxyl groups (Wang et al. 2005), of which the later would in turn increase the concentration of beneficial free hydroxyl radicals for the TiO2 photodecomposition in the application of water treatment (Yang et al. 2009).
Secondly, it is still subject to a debate that whether doped nitrogen or oxygen vacancies are responsible for the photocatalytic activity shift towards visible-light range. While most of works in this area agreed upon the necessity of N presence in N-doped TiO2, Ihara et al. claimed that the visible-light sensitivity of N–TiO2 nanoparticles arise from the oxygen-deficient sites, as the existence of N-dopants was only necessary to retard the reoxidation of oxygen-deficient TiO2, which authors considered to be essential for visible-light sensitization (Ihara et al. 2003). The recent development on this subject provided by Lin et al. further suggested that N-dopants can only affect the absorption below 500 nm wavelength, whereas the O vacancies are responsible for the visible-light sensitization at wavelength above 500 nm (Lin et al. 2005). Generally, low concentrations of nitrogen within TiO2 could promote interstitial doping and cause a large decrease in the formation energy of oxygen vacancies in the anatase from 4.2 to 0.6 eV, as a result of the excess electrons created in the oxygen vacancy being trapped on the nitrogen site (Zhao et al. 2008; Dunnill and Parkin 2011). While some authors attributed resulted color centers and enhanced photocatalytic activity to these oxygen vacancies (Zhao and Liu 2008; Pan et al. 2013), others believe that the presence of oxygen vacancies instead acts as recombination sites for electrons and holes leading to poor performance (Prabakar et al. 2007). It is safe to speculate that an optimal point between visible-light band gap enhancement and increased electron–hole recombination effects in N-doped TiO2 may exist by tightly control the N-dopant incorporation (i.e., level and types) (Irie et al. 2003b; Yang et al. 2010).
Thirdly, Asahi ascribed the visible-light absorbance in N-doped TiO2 to the band gap narrowing effect, which was resulted from the interaction between N2p and O2p states (Asahi et al. 2001). Later on, several other researcher in their follow-up studies on N-doped TiO2 suggested that the creation of new inter-bands localized within the band gap of TiO2 is the origin of visible-light activity based on density-functional-theory (DFT) calculations (Di Valentin et al. 2004, 2005a, b; Wang et al. 2006; Lee et al. 2005; Yang et al. 2006). For example, Di Valentin et al. suggested that the single-atom nitrogen impurities form either diamagnetic (N −b ) or paramagnetic (N •b ) bulk centers. Both types of Nb centers give rise to localized N2p states within the band gap of the oxide, as illustrated in Fig. 3, rather than mixing with O2p states for both rutile and anatase TiO2. To clarify, Payne and his co-workers further claimed that the hybridization between N2p and O2p states is only possible when the N-doping level exceeds 20 % in anatase TiO2 (Lin et al. 2005); Yang et al. found that the interaction between N2p and O2p states is less pronounced when N-doping is the substitutional type (Yang et al. 2006). Later on, Sun et al. attempted to correlate the concentration of NO2 2− with the visible-light activity of as-prepared N-doped TiO2 photocatalysts, as their DFT calculations supported a NO2 2−-induced formation of new mid-band energy levels near CB (Sun et al. 2009). Now it is generally agreed that the substitutional incorporation of nitrogen would result in an increase of ~0.14 eV in energy in the valance band of anatase due to the overlap between N2p and O2p states; In the case of interstitial doping, the incorporation of nitrogen creates an inter-band state 0.74 eV above the valence band, as illustrated in Fig. 4 (Di Valentin et al. 2005a, 2007).
Sketch of the proposed mechanism for the processes induced by vis-light irradiation of the N-doped sample in O2 atmosphere (Reprinted with permission from the American Chemical Society.) (Livraghi et al. 2006)
Schematic showing the valance and conduction bands of the three different forms of N-doped anatase (Reprinted with permission from the Royal Society of Chemistry.) (Dunnill and Parkin 2011)
To sum up, photocatalytic enhancement and nitrogen content are more likely a more complex indirect correlation of doping types (i.e., substitutional or interstitial), existence of oxygen vacancies and band structure types (i.e., hybridization of N2p and O2p states or creation of a new inter-band). Other factors to the uncertainty could arise from the content of other visible-light-induced dopants (such as C, S, etc.) as impurities from organic precursors or simply the chemisorbed N-containing molecules on as-prepared TiO2 nanoparticles surface that are non-active, such as NH3.
Experimental parameters of wet chemical N-doping method and their effects
An attempt to summarize the recent literature on visible-light sensitive N-doped TiO2 nanoparticles synthesized by wet chemical methods is undertaken (Table 1), of which key experimental variables are listed for comparison. Optimal values of the experimental parameters in each study are also underlined where possible. The following sections will deal with each variable and its influences on the resulting photocatalytic activity of N-doped TiO2 nanoparticles.
Influences of TiO2 precursors
In a typical wet chemical preparation of TiO2 photocatalysts, TiO2 precursors were mostly either titanium alkoxides or inorganic titanium, such as TiCl3, TiCl4, Ti(SO4)2, and Ti(OH)4. When using inorganic-type TiO2 precursor, special attention needed to be paid on the effective removal of some ion impurities, as they are potentially harmful to the photoactivity of TiO2 formation. In their study, Harada et al. observed that the presence of Cl- ions on the surface of AC promotes the aggregation of titanium oxides species, and the formation of rutile TiO2 phase instead of anatase form. As one of two common TiO2 species, rutile generates fewer charge carriers with lower lifetimes than that of an anatase-type TiO2, when in the same nano-sized particles form with the same crystalline quality and reactive surface properties (Colbeau-Justin et al. 2003). As a result, rinsing with water or acids, such as HNO3, is essential. On the other hand, some of the ion presence appeared to be beneficial. For example, the SO 2-4 presence on TiO2 surface has been reported to show higher photocatalytic activity than pure TiO2 (Yamazaki et al. 2001; Kim et al. 2003; Huang et al. 1998).
Ti species in these TiO2 precursors exist as either Ti3+ or Ti4+, which could affect the preparation process as well. Wang et al. claimed that using Ti3+-containing precursors is advantageous for substitutional N-doping, as transformation of Ti3+ to Ti4+ during the hydrolysis would create an oxygen vacancy, making it easier for nitrogen atoms to occupy the oxygen sites during the crystal growth (Wang and Lewis 2006). Conversely, this process did not enhance N-doping results greatly (the highest sample with 1.7 % N-content) and suggested a majority of interstitial N-doping other than substitutional type. Another interesting TiO2 precursor seen in the literature is N-containing TiO2 precursors, such as Ti4+-bipyridine complex. Sano et al claimed its advantages to be the readily existed Ti–N bonds in the precursors, which could ease up the N-doping content increasing at room temperature (Sano et al. 2004). Nevertheless, their XPS results showed that N 1 s peaks at 396 eV (Ti–N) were only recognizable inside the bulk, whereas N 1 s peaks at 400 eV (Ti–O–N or Ti–N–O) were clearly spotted on the N-doped TiO2 surface. Results in this study confirmed that Ti–N bond does not necessarily related to the quality of N-doping directly in terms of visible-light sensitivity as they originally thought.
Influences of nitrogen precursors
In the literature, a number of studies used ammonia solution as nitrogen precursors during the hydrolysis preparation of N-doped TiO2. Compared with other amino group precursors, one possible drawback using ammonia as N precursors is the chemisorbed NH3 molecules on TiO2 surface, which might cover the photoactive sites that served as Lewis bases to capture photo-induced holes; this is detrimental to the oxidative degradation (Young and Desai 1989). This might diminish the overall photoactive promoting effect of the N-doping. Burda and his co-workers published a series of papers on hydrolysis methods using triethylamine as N precursor (Burda et al. 2003; Chen and Burda 2004). They claimed the superiority of their method by achieving up to 8 % N-content in their TiO2 nanoparticles resultants. Their preparation involved in the excess usage of N precursors and prolonged stirring after its addition into the TiO2 precursors. Another interesting N precursor reported in the literature is sulfur-containing solvent such as thiourea (Sakthivel et al. 2004), which was originally used to obtain S-doped TiO2. Similar to using the Ti(SO4)2 as TiO2 precursor, it is essential to use ethanol as solvent and use water or acid to wash the particles to remove the sulfur species, i.e., SO4 2−.
Influences of solvents and pH values
Adding some alcohol solvents (such as 2-isopropanol, ethanol, etc.) to the hydrolytic system can make it more volatile than using water alone. This could facilitate the evaporation and the following drying treatments. Nevertheless, excessive use of alcohol or high alcohol-to-TiO2 precursor volume ratio should be avoided, as it could shrink the body of colloidal formation and make it denser (Montoya et al. 1992). This could lead to less porosity and poor crystallinity of TiO2 nanoparticles formation at the later stage. As for using water alone, addition of a certain amount of acid is crucial to obtain a stable colloidal solution and to preserve the particles in their nano-sized form. This catalytic effect is brought about through the electrical charging of the hydroxide particles by proton adsorption, which hinders the gel agglomeration.
Acid addition is also likely to have a potential linkage to the TiO2 phase transition during the hydrolysis. Zhu et al. reported that high acid concentration (2.0 M) favored the formation of rutile phase TiO2 during the hydrolysis of TiOSO4·xH2O (see Fig. 5) (Zhu et al. 2005). The follow-up investigations on this effect in the presence of N-dopants are still needed. In terms of the acid type, HNO3 is preferable to HCl due to avoiding of the possible implication of an unfavorable Cl- ion effect on TiO2 crystal formation. In addition, the pH value of the hydrolytic system is also an important factor on the N-doping quality of nano-sized TiO2 resultants. In general, high pH values are unfavorable to nitrogen-doping efficiency, as Ti-bound amine groups are more easily substituted by anion OH− in the solution during the hydrolysis process. Therefore, usage of base in the solvents needs to be controlled in order to avoid any adverse effect on the N-doping efficiency. On the other hand, this might made the inorganic TiO2 precursors like TiCl3, TiCl4, Ti(SO4)2 somewhat desirable, as their hydrolysis usually results in an acid environment.
Structural features of hydrogen-titanate (top left), anatase (top right), and rutile (bottom right), as well as the phase transitions from the hydrogen-titanate to anatase and rutile, in dilute and concentrate acid solutions, respectively (Reprinted with permission from the American Chemical society.) (Zhu et al. 2005)
Other than acidity and basicity, addition of certain electrolytes in the solvents could have influences on the formation of Ti, N-containing organic complex. Some particular benefits arising from a certain electrolyte may vary from case to case. Sathish et al. reported the positive effects of Na2S presence during their N-doped TiO2 preparation through the hydrolysis of TiCl3 with aqueous ammonia; it induced the in situ formation of transient (NH4)x−TiSx complex and a reductive solution environment, of which the latter is beneficial for the removal of any dissolved oxygen (Sathish et al. 2005). Gole et al. discovered that the addition of Pd(NO3)2 in the hydrolysis of Ti[OCH(CH3)2]4 with triethylamine could increase the N-doping level from 5.1 % to a high level of 17.6 % (Gole et al. 2004), though the detailed explanation of the effect was lacking in the study.
Influences of post N-doping treatments
General wet chemical photocatalysts preparation requires minimum calcinations temperature (around 200 °C) to enable the proper crystallinity of the TiO2 nanoparticles, which is deeply related to its electronic properties, i.e., charger carrier lifetimes. However, unnecessary high temperature (>500 °C) is to avoid for two reasons. Firstly, high temperatures induce the shrinkage of N-doped TiO2 surface area due to the grain (i.e., crystallite size) growth or nanoparticles agglomerating effect throughout the treatment process, of which typical results are shown in Fig. 6a. Secondly, high temperatures also favor the TiO2 phase transition from anatase to rutile. In extreme cases, a complete transition is expected when calcinations temperature is >700 °C in air. They are both detrimental to the resulting photocatalytic activity. Reportedly, some of the ambient gases are likely to affect the calcination temperature on TiO2 crystal phase transition. For example, using protective hydrogen gas alone reportedly confined the complete anatase-to-rutile phase transition temperature of a TiO2 between 525 and 550 °C, which is much lower than that (700 °C) in the presence of air (Hamasaki et al. 1994). Furthermore, it is also of great interests to know if there are possible N-dopants-related high-temperature effects on the TiO2 phase transition. For interstitial N-doped TiO2, Wang et al. observed that the phase transition temperature remains un-affected, which started at around 400 °C) (Wang et al. 2005). Most recently, Xu et al. reported that the substitutional doping of nitrogen can inhibit the anatase-to-rutile transformation and confined the transition temperature to around 800 °C under their experimental conditions (Xu et al. 2008).
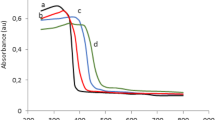
a Surface area of typical N-doped TiO2 under different calcinations temperatures; b the corresponding UV–Vis DRS spectra, where bare TiO2 was synthesized without any N-dopants (Reprinted with permission from Taylor & Francis,.) (Zhang et al. 2015)
On the other hand, in terms of retaining sufficiently high N-doping levels in N-doped TiO2 resultants, literature studies reported that increasing temperature, especially when above 400 °C, could diminish the visible-light absorbance due to the thermal release of the doped nitrogen and its re-substitution by oxygen atoms (see Fig. 6b). This was usually accompanied by the fading of the color intensity of the resultants. Microscopically, high calcinations temperatures (above 600 °C) are unfavorable to the formation of both interstitial and substitutional doped N, while Nosaka et al. reported the calcinations temperatures for maximum amount of two kinds of doped N atoms are 400 and 500 °C, respectively (Nosaka et al. 2005).
Conclusions
It should be noted that there is not yet a clear and direct correlation between N-doping level and overall photocatalytic activity, since the latter can also be affected by many other factors, such particle size, surface area, and functionalization. This has also made it very difficult to provide a universal guidance at this stage with a certainty for each variable of N-doping preparation. Instead of one-factor-at-a-time style investigation, a statistically valid parameter optimization investigation for general optima of photocatalytic activity will be certainly useful. Optimization of the wet chemical N-doping technique is envisaged to be beneficial to the near-term development of TiO2 photocatalysts or its nanocomposites for solar-driven environmental clean-up, i.e., dissolved organic compounds removal in wastewater treatment.
To test N-doped TiO2 photocatalytic activity, some lab-scale investigations on the degradations of dissolved organic compounds under simulated environmental conditions were carried out. It seemed that these efforts firstly aimed at decomposition of organic dyes or colored compounds, such as methylene blue (MB), azo dyes acid, including acid orange 7 (AO7), procion red MX-5B (MX-5B), and reactive black 5 (RB5), etc. However, due to the possibility of TiO2 visible-light sensitization by dyes, this might give rise to an uncertainty that minor part of photodecomposition may proceed via non-N-doping-induced visible-light photocatalysis (Kisch and Macyk 2002). Accordingly, the latest attempts in this nature have shifted their interests to non-colored dissolved organic compounds, such as 4-chlorophenol, phenol, 2-propanol, benzoic acid, trichloroethylene (TCE), etc. Various decomposition degree and rates were reported, of which most claimed to be satisfactory, and yet not directly comparable between studies. It should also be noted that photocatalytic decomposition of large molecular weight organics in solution by N-doped TiO2 nanoparticles has not yet been reported. This is mainly due to the fact that depending on different dissolved organic compounds, the degree and rate of total mineralization, i.e., decomposition of organic pollutants into harmless products, varies (Li et al. 2007). Supposedly, this could be greatly relieved by developing TiO2/adsorbent composites, as it has a large capability of adsorbing degradation intermediates (Zhang et al. 2009, 2010, 2011). Further investigations in this aspect are certainly of great interests, especially in terms of water treatment applications. At the current stage, the feasibility of their applications is still restricted to dilute solutions.
References
Ananpattarachai J, Kajitvichyanukul P, Seraphin S (2009) Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J Hazard Mater 168:253–261
Asahi R, Morikawa T (2007) Nitrogen complex species and its chemical nature in TiO2 for visible light sensitized photocatalysis. Chem Phys 339:57–63
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293:269–271
Batzil M, Morales EH, Diebold U (2006) Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys Rev Lett 96:026103
Belver C, Bellod R, Fuerte A, Fernandez-Garcia M (2006) Nitrogen-containing TiO2 photocatalysts Part 1. Synthesis and solid characterization. Appl Catal B Environ 65:301–308
Burda C, Lou Y, Chen X, Samia ACS, Stout J, Gole JL (2003) Enhanced nitrogen doping in TiO nanoparticles. Nano Lett 3:1049–1051
Chen X, Burda C (2004) Photoelectron spectroscopic investigation of nitrogen-doped titania nanoparticles. J Phys Chem B 108:15446–15449
Chen X, Burda C (2008) Electronic Origin of the Visible-light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J Am Chem Soc 130:5018–5019
Chen X, Lou Y, Samia ACS, Burda C, Gole JL (2005) Formation of oxynitride as the photocatalytic enhancing site in nitrogen-doped titania nanocatalysts: comparison to a commercial nanopowder. Adv Funct Mater 15:41–49
Chen X, Glans P, Qiu X, Dayal S, Jennings WD, Smith KE, Burda CJ, Guo J (2008) X-ray spectroscopic study of the electronic structure of visible-light responsive N-C- and S-doped TiO2. Electron Spectrosc Relat Phenom 162:67–73
Chen X, Liu L, Yu PY, Mao SS (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331:746–751
Choi W, Termin A, Hoffmann MR (1994a) Einflüsse von Dotierungs-Metall-Ionen auf die photokatalytische Reaktivität von TiO2-Quantenteilchen. Angew Chem 106:1148–1149
Choi W, Termin A, Hoffmann MR (1994b) The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J Phys Chem 98:13669–13679
Colbeau-Justin C, Kunst M, Huguenin D (2003) Structural influence on charge-carrier lifetimes in TiO2 powders studied by microwave absorption. J Mater Sci 38:2429–2437
Dai YR, Yin LF (2014) Enhancement of photocatalytic activity for electrospun C@Ti/anatase fibers by lattice distortion under anisotropic stress. Catal Sci Technol 4:456–463
Di Valentin C, Pacchioni G, Selloni A (2004) Origin of the different photoactivity of N-doped anatase and rutile TiO2. Phys Rev B Solid State 70:085116
Di Valentin C, Pacchioni G, Selloni A, Livraghi S, Giamello E (2005a) Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J Phys Chem B 109:11414–11419
Di Valentin C, Pacchioni G, Selloni A (2005b) Theory of carbon doping of titanium dioxide. Chem Mater 17:6656–6665
Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini MC, Giamello E (2007) N-doped TiO2: theory and experiment. Chem Phys 339:44–56
Diwald O, Thompson TL, Grolaski EG, Walck SD, Yates JT (2004a) The effect of nitrogen ion implantation on the photoactivity of TiO2 rutile single crystals. J Phys Chem B 108:52–57
Diwald O, Thompson TL, Zubkov T, Goralski EG, Walck SD, Yates JT (2004b) Photochemical activity of nitrogen-doped rutile TiO2(110) in visible light. J Phys Chem B 108:6004–6008
Dunnill CW, Parkin IP (2011) Nitrogen-doped TiO2 thin films: photocatalytic applications for healthcare environments. Dalton Trans 40:1635–1640
Gaya I, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C Photochem Rev 9:1–12
Gole JL, Stout JD, Burda C, Lou Y, Chen X (2004) Highly efficient formation of visible light tunable TiON photocatalysts and their transformation at the nanoscale. J Phys Chem B 108:1230–1240
Graetzel M (2001) Photoelectrochemical cells. Nature 414:338–344
Hagfeldtt A, Bjorksten U, Lindquist SE (1992) Photoelectrochemical studies of colloidal TiO2-films: the change separation process studied by means of action spectra in the UV region. Sol Energy Mater Sol Cell 27:293–304
Hamasaki Y, Ohkubo S, Murakami K, Sei H, Nogami G (1994) Photoelectrochemical properties of anatase and rutile films prepared by the sol-gel method. J Electrochem Soc 141:660–663
Han C, Yang M, Weng B, Xu Y (2014) Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys Chem Chem Phys 16:16891–16903
Hoffmann MR, Martin ST, Choi W, Bahnemannt DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69
Huang Y, Zhao B, Xie Y (1998) A novel way to prepare silica supported sulfated titania. Appl Catal A Gen 171:65–73
Ihara T, Miyoshi M, Iriyama Y, Matsumoto O, Sugihara S (2003) Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl Catal B Environ 42:403–409
Irie H, Washizuka S, Yoshinob N, Hashimoto K (2003a) Visible-light induced hydrophilicity on nitrogen-substituted titanium dioxide films. Chem Commun 1298–1299
Irie H, Watanabe Y, Hashimoto K (2003b) Nitrogen-concentration dependence on photocatalytic activity of TiO2−xNx powders. J Phys Chem B 107:5483–5486
Joung S, Amemiya T, Murabayashi M, Itoh K (2006) Relation between photocatalytic activity and preparation conditions for nitrogen-doped visible light-driven TiO2 photocatalysts. Appl Catal A Gen 312:20–26
Kim HJ, Nam KH, Shul YG (2003) Preparation of TiO2 fiber and its photocatalytic properties. Mater Sci For 439:271
Kisch H, Macyk W (2002) Visible-light photocatalysis by modified titania. Chem Phys Chem 3:399
Kitano M, Funatsu K, Matsuoka M, Ueshima M, Anpo M (2006) Preparation of nitrogen-substituted TiO thin film photocatalysts by the radio frequency magnetron sputtering deposition method and their photocatalytic reactivity under visible light irradiation. J Phys Chem B 110:25266–25272
Kobayakawa K, Murakami Y, Sato Y (2005) Visible-light active N-doped TiO2 prepared by heating of titanium hydroxide and urea. J Photochem Photobiol A Chem 170:177–179
Lee J, Park J, Cho J (2005) Electronic properties of N- and C-doped TiO2. Appl Phys Lett 87:11904-1–11904-3
Li J, Ma W, Lei P, Zhao J (2007) Detection of intermediates in the TiO2-assisted photodegradation of Rhodamine B under visible light irradiation. J Environ Sci 19:892–896
Lin Z, Orlov A, Lambert RM, Payne MC (2005) New insights into the origin of visible light photocatalytic activity of nitrogen-doped and oxygen-deficient anatase TiO2. J Phys Chem B 109:20948–20952
Liu Y, Chen X, Li J, Burda C (2005) Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 61:11–18
Livraghi S, Votta A, Paganini MC, Giamello E (2005) The nature of paramagnetic species in nitrogen doped TiO2 active in visible light photocatalysis. Chem Commun 498-500
Livraghi S, Paganini MC, Giamello E, Selloni A, Di Valentin C, Pacchioni G (2006) Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J Am Chem Soc 128:15666–15671
Montoya JA, Viveros T, Dominguez JM, Canales LA, Schifter I (1992) On the effects of the sol-gel synthesis parameters on textural and structural characteristics of TiO2. Catal Lett 15:207–217
Nakamura R, Tanka T, Nakato Y (2004) Mechanism for visible light response in anodic photocurrents at N-doped TiO2 film electrodes. J Phys Chem B 108:0617–10620
Nazeruddin MK, Kay A, Rodicio I, Humphry-Baker R, Muller E, Liska P, Vlachopoulos N, Graetzel M (1993) Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc 115:6382–6390
Nosaka Y, Matsushita M, Nishino J, Nosaka A (2005) Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci Technol Adv Mater 6:143–148
Pan X, Yang M, Fu X, Zhang N, Xu Y (2013) Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 5:3601–3614
Park CH, Zhang SB, Wei SH (2002) Origin of p-type doping difficulty in ZnO: the impurity perspective. Phys Rev B 66:073202-1–073202-3
Peng F, Cai L, Yu H, Wang H, Yang J (2008) Synthesis and characterization of substitutional and interstitial nitrogen-doped titanium dioxides with visible light photocatalytic activity. J Solid State Chem 181:130–136
Prabakar K, Takahashi T, Nezuka T, Takahashi K, Nakashima T, Kubota Y, Fujishima A (2007) Annealing effect on structural, morphological, and optical properties of reactive sputtered films for mediated heterogeneous photocatalyst. J Vac Sci Technol A 25:1188–1192
Sakatani Y, Koike H (2001) Japanese Patent 2001072419
Sakatani Y, Nunoshige J, Ando H, Okusako K, Koike H, Takata T, Kondo JN, Hara M, Domen K (2003) Photocatalytic decomposition of acetaldehyde under visible light irradiation over La3+ and N co-doped TiO2. Chem Lett 32:1156–1157
Sakthivel S, Kisch H (2003) Photocatalytic and photoelectrochemical properties of nitrogen-doped titanium dioxide. ChemPhysChem 4:487
Sakthivel S, Janczarek M, Kisch H (2004) Visible light activity and photoelectrochemical properties of nitrogen-doped TiO. J Phys Chem B 108:19384–19387
Sano T, Negishi N, Koike K, Takeuchi K, Matsuzawa S (2004) Preparation of a visible light-responsive photocatalyst from a complex of Ti4+ with a nitrogen-containing ligand. J Mater Chem 14:380–384
Sathish M, Viswanathan B, Viswanath RP, Gopinath CS (2005) Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO nanocatalyst. Chem Mater 17:6349–6353
Sathish M, Viswanathan B, Viswanath RP (2007) Characterization and photocatalytic activity of N-doped TiO2 prepared by thermal decomposition of Ti-melamine complex. Appl Catal B Environ 74:307–312
Sato S (2002) Photocatalysts sensitive to visible light. Science 295:626–627
Sato S, Nakamura R, Abe S (2005) Visible-light sensitization of TiO2 photocatalysts by wet-method N doping. Appl Catal A Gen 294:131–137
Sun J, Qiao L, Sun S, Wang G (2008) Photocatalytic degradation of orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J Hazard Mater 155:312–319
Sun H, Bai Y, Liu H, Jin W, Xu N (2009) Photocatalytic decomposition of 4-chlorophenol over an efficient N-doped TiO2 under sunlight irradiation. J Photochem Photobiol A Chem 201:15–22
Wang H, Lewis JP (2006) Second-generation photocatalytic materials: anion-doped TiO2. J Phys Condens Matter 18:421
Wang Y, Cheng H, Hao Y, Ma J, Li W, Cai S (1999) Photoelectrochemical properties of metal-ion-doped TiO2 nanocrystalline electrodes. Thin Solid Films 349:120–125
Wang C, Bahnemann DW, Dohrmann JK (2000) A novel preparation of iron-doped TiO2 nanoparticles with enhanced photocatalytic activity. Chem Commun 1539-1540
Wang Z, Cai W, Hong X, Zhao X, Xu F, Cai C (2005) Photocatalytic degradation of phenol in aqueous nitrogen-doped TiO2 suspensions with various light sources. Appl Catal B Environ 57:223–231
Wang Z, Zhang F, Yang Y, Cui J, Sun Q, Guan N (2006) One-pot synthesis of visible-light-responsive TiO2 in the presence of various amines. Chin J Catal 27:1091–1095
Wang Y, Yu X, Sun D (2007) Synthesis, characterisation, and photocatalytic activity of TiO2−xNx nanocatalyst. J Hazard Mater 144:328–333
Xia T, Zhang Y, Murowchick J, Chen X (2014) Vacuum-treated titanium dioxide nanocrystals: optical properties, surface disorder, oxygen vacancy, and photocatalytic activities. Catal Today 225:2–9
Xu J, Ao Y, Fu D, Yuan C (2008) A simple route to synthesize highly crystalline N-doped TiO2 particles under low temperature. J Cryst Growth 310:4319–4324
Yamashita H, Honda M, Harada M, Ichihashi Y, Anpo M, Hirao T, Itoh N, Iwamoto N (1998) Preparation of titanium oxide photocatalysts anchored on porous silica glass by a metal ion-implantation method and their photocatalytic reactivities for the degradation of 2-propanol diluted in water. J Phys Chem B 102:10707–10711
Yamazaki S, Fujinaga N, Araki K (2001) Effect of sulfate ions for sol-gel synthesis of titania photocatalyst. Appl Catal A Gen 210:97–102
Yang K, Dai Y, Huang B, Han S (2006) Theoretical study of N-doped TiO2 rutile crystals. J Phys Chem B 110:24011–24014
Yang J, Dai J, Chen C, Zhao J (2009) Effects of hydroxyl radicals and oxygen species on the 4-chlorophenol degradation by photoelectrocatalytic reactions with TiO2-film electrodes. J Photochem Photobiol A Chem 208:66–77
Yang G, Jiang Z, Shi H, Xiao T, Yan Z (2010) Preparation of highly visible-light active N-doped TiO2 photocatalyst. J Mater Chem 20:5301–5309
Yin L, Niu J, Shen Z, Chen J (2010) Mechanism of reductive decomposition of pentachlorophenol by Ti-doped β-Bi2O3 under visible light irradiation. Environ Sci Technol 44:5581–5586
Young RA, Desai P (1989) Crystallite size and microstrain indicators in rietveld refinement. Archiwum Nauki o Materialach (Arch Mater Sci) 10:71–90
Zhang W, Zou L, Dionysio D (2015) A parametric study of visible-light sensitive TiO2 photocatalysts synthesis via a facile sol-gel N-doping method J Exp Nanosci (in press)
Zhang W, Zou L, Wang L (2009) Photocatalytic TiO2/adsorbent nanocomposites prepared via wet chemical impregnation for wastewater treatment: a review. Appl Catal A Gen 371:1–9
Zhang W, Zou L, Wang L (2010) Visible-light assisted methylene blue (MB) removal by novel TiO2/adsorbent nanocomposites. Water Sci Technol 61:2863–2871
Zhang W, Zou L, Wang L (2011) A novel charge-driven self-assembly method to prepare visible-light sensitive TiO2/activated carbon nanocomposites for dissolved organic compound removal. Chem Eng J 168:485–492
Zhang N, Liu S, Xu Y (2012) Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 4:2227–2238
Zhao Z, Liu Q (2008) Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J Phys D Appl Phys 41:025105
Zhao J, Chen C, Ma W (2005) Photocatalytic degradation of organic pollutant under visible light irradiation. Top Catal 35:269–278
Zhao L, Jiang Q, Lian J (2008) Visible-light photocatalytic activity of nitrogen-doped TiO2 thin film prepared by pulsed laser deposition. Appl Surf Sci 254:4620
Zhu HY, lan Y, Gao XP, Ringer SP, Zheng ZF, Song DY, Zhao JC (2005) Phase transition between nanostructure of titanate and titanium dioxides via simple wet-chemical reactions. J Am Chem Soc 127:6730–6736
Zou L, Zhu B (2007) Enhancing the reuse of treated effluent by photocatalytic process. J Adv Oxid Technol 10:273–281
Acknowledgments
Dr Wei Zhang is grateful for the support from Australian Endeavour Fellowship Program and University of South Australia Early Career Researcher Travel Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Jia, B., Wang, Q. et al. Visible-light sensitization of TiO2 photocatalysts via wet chemical N-doping for the degradation of dissolved organic compounds in wastewater treatment: a review. J Nanopart Res 17, 221 (2015). https://doi.org/10.1007/s11051-015-3026-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3026-1