Abstract
Zinc oxide (ZnO) nanoparticles and ZnO/graphene (ZG) nanocomposite were synthesized via simple chemical route and its application as a photoanode for dye-sensitized solar cell (DSSC) was demonstrated. The prepared ZnO and ZG were structurally characterized by X-ray diffraction and micro-Raman techniques. The scanning electron micrograph of ZG revealed the spherical-shaped ZnO nanoparticles of particle size ~160 nm was anchored on the two-dimensional graphene sheets. UV–Vis absorption spectroscopy showed that the ZG nanocomposite has enriched visible light absorption. The DSSCs were fabricated using the synthesized ZnO and ZG nanocomposite as photoanode and the effect of low-cost organic dyes on the photovoltaic performances of the solar cells were investigated. Comprehensive performances of ZG are better than that of ZnO-based DSSCs. The ZG DSSCs show power conversion efficiency (PCE) of 1.5 and 0.98 % for RB and EY sensitized electrodes, respectively. Moreover, the ZG dominates in many aspects due to the presence of graphene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanocomposites composed of two or more materials with desired performance are recently one of the most researched areas, mainly due to the synergic effect between the materials that are essential for technological applications in various areas (Xiang et al. 2012). Metal oxides and carbon allotropes are two of the most attractive materials in the materials science community. ZnO is the most investigated functional material in the family of metal oxides (Wahab et al. 2007; Marlinda et al. 2012). It is a II–VI compound semiconductor with a direct bandgap of 3.37 eV at room temperature. The superior properties such as anti-oxidation, large excitation binding energy (60 meV), higher electron mobility (115–155 cm2 V−1 s−1), and chemical stability of ZnO have great potential in the applications for optical detector, gas sensor, solar cell, short-wavelength UV laser, photocatalysts, microsensors and blue or green optoelectronic devices, and so on (Anta et al. 2012; Rani et al. 2008). One of the carbon allotropes, graphene is a two dimensional, one-atom-thick layer made of carbon atoms in a hexagonal lattice which has high carrier mobility, high thermal conductivity, electrical conductivity, and high surface area 2,600 m2 g−1 (theoretical prediction) (Marlinda et al. 2012; Dhar et al. 2013; Sahoo et al. 2014; Zhao et al. 2014). Therefore, ZnO–graphene nanocomposite (ZG) is expected to exhibit remarkable properties in the applications like solar energy conversion, energy storage, optoelectronic, and photocatalytic activity (Lightcap et al. 2013; Xiang et al. 2012; Marlinda et al. 2012).
Currently, ZnO–graphene nanocomposites (ZG) are attracting a great deal of attention due to their potential applications in recent research areas (Akhavan 2010; Chen et al. 2011; Cuong et al. 2010; Hwang et al. 2011). The dye-sensitized solar cells (DSSCs) are considered as prospective tools for their versatility such as low cost, convenient fabrication, industrial applications, and high power conversion efficiencies (>12 %) (Gratzel and Hagfeldt 2000; Gratzel 2001, 2003, 2005). It has been shown that TiO2 combined with graphene shows enhanced photovoltaic performance. According to the reported literatures (Tang et al. 2010; Lightcap et al. 2013; Chen et al. 2013; Yang et al. 2010; Lee et al. 2012), the highly improved performance of the TiO2/graphene nanocomposite can be attributed to the higher transfer rate of photo-generated electrons and suppressed recombination of the photo-induced electron–hole pairs. Therefore, we expect the same behavior for ZG nanocomposite DSSCs.
In this paper, a simple one-step chemical reduction route was proposed to prepare a ZG nanocomposite using graphene oxide (GO) and zinc acetate Zn(Ac)2·2H2O as source materials, and N2H4 was used as reducing agent. The synthesized ZG nanocomposite was subjected to structural and optical characterizations. We have used the prepared ZG nanocomposite as a working electrode in DSSC. The DSSCs were made using low-cost organic dyes such as rose bengal (RB) and eosin yellow (EY) as sensitizer, and their photovoltaic performances have been studied.
Experimental
Materials
Zinc acetate dihydrate Zn(Ac)2·2H2O (Merck), glycerol (Sigma), rose bengal (RB, Alfa), eosin yellow (EY, Alfa), sodium hydroxide pellets (NaOH, Nice), graphite plate (Alfa), graphite powder (Alfa), 37 % hydrochloric acid (HCl, Merck), 98 % sulfuric acid (H2SO4, Merck), hydrogen peroxide (H2O2, Merck), potassium permanganate (KMnO4, Merck), hydrazine hydrate (N2H4, Merck), ethylene glycol (EG, Merck), potassium iodide (KI, Merck), iodine (I, Merck), and absolute ethanol (Merck) were purchased and used as received without further purification.
Synthesis of zinc oxide (ZnO)
ZnO nanoparticles were synthesized by directly mixing 1 g of Zn(Ac)2·2H2O in 100 ml of deionized water under stirring condition. Then, a corresponding amount of 1 M NaOH aqueous solution was added to the above solution and stirred for 3 h. The resultant precipitate was centrifuged and washed with deionized water. Later, the final product was dried at 100 °C and the dried powder was annealed at 450 °C for 4 h.
Synthesis of ZG nanocomposite
GO was synthesized from graphite powder according to the improved Hummer’s method (Hummers and Offeman 1958). Initially, 900 mg of Zn(Ac)2·2H2O was added to 90 ml of GO (10 mg ml−1) solution and stirred for 3 h. Then, appropriate amount of N2H4 was added drop by drop to the above solution and the resulting mixture was stirred at 80 °C. After 3 h, the brown color solution turned to black indicating the reduction of GO to graphene. At this stage, the stirring is stopped and the precipitate was collected by centrifugation and then washed using water and alcohol. Further, the obtained powder was dried at 100 °C and then annealed at 450 °C for 4 h in a muffle furnace. Schematic illustration of the formation of ZG nanocomposite from graphene oxide is shown in Scheme 1.
Preparation of electrodes and cell assembly
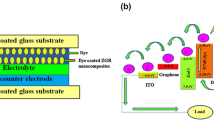
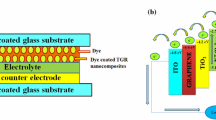
In the solar cell fabrication, synthesized ZnO and ZG powder were made into a paste by grinding powder in a mortar for about 30 min using glycerol as dispersing agent. Then, the paste was coated on the indium tin oxide (ITO) by doctor blade technique. In the electrode preparation, ITO substrate was covered with scotch tape 1 cm apart to control the thickness and area of the electrodes (1 cm × 1 cm). Then the prepared electrodes were annealed at 300 °C for 1 h to evaporate the solvent. The photoanode was prepared by immersing the slightly warmed ZnO and ZG electrodes into the 0.5 mM of RB and EY dye solutions for 12 h. The non-adsorbed dye was washed up with acetonitrile. For the preparation of counter electrode, a piece of graphite plate was scratched uniformly on the ITO plate. The graphite coated ITO electrodes and dye-coated ZnO and ZG electrodes were placed facing each other and held together using the binder clips. The electrolyte solution was prepared by mixing 0.127 g of iodine and 0.83 g of potassium iodide (KI) in 10 ml of ethylene glycol. The iodide electrolyte solution was injected between the anode and cathode prior to the I–V measurement.
Dye desorption study
In order to analyze the amount of dye loading, the dye was desorbed completely from the dye-loaded electrodes. In the desorption study, the dye-anchored ZnO and ZG electrodes were immersed into the 0.1 mM NaOH in ethanol for about 12 h. Further, the desorbed solutions were taken in a 1-cm path length cuvette as sample and NaOH solution as reference. A double beam UV–Vis spectrophotometer was used to measure the desorbed dye concentration.
Instrumentation
The structural analysis was carried out by powder PANalytical X-ray diffractometer with CuKα radiation (λ = 1.54 Å). The surface morphology and composition of the samples were examined by field emission scanning electron microscope (FE-SEM, FEI Nova 400 Nano SEM) equipped with the energy dispersive X-ray spectrometry (Brucker Quantax 200 AS). Raman-scattering measurements were performed in 180° backscattering geometry using a LabRamHR800 spectrometer from Horiba Jobin–Yvon equipped with a CCD detector. The sample was excited by 633 nm emission from a He–Ne laser and the accuracy of the wavenumber was about 0.3 cm−1. The absorbance spectra were measured using Shimadzu (UV2450) UV–Vis spectrophotometer. Oriel class-A solar simulator (91195A, Newport) was used as a light source, and a computer-controlled Autolab PGSTAT302N electrochemical workstation was employed for current–voltage (I–V) measurements.
Results and discussion
The X-ray diffraction pattern of ZnO and ZG is shown in Fig. 1. The diffraction peaks at 31.8°, 34.5°, 36.3°, 47.6°, 56.6°, 62.8°, 68.1°, and 69.2° correspond to the (100), (002), (101), (102), (110), (103), (112), and (201) planes, respectively, and can be indexed to wurtzite structure of ZnO. The observed planes are also consistent with the standard JCPDS data file (Card No. 89-0510). On the other hand, the ZG nanocomposite also exhibits the similar planes corresponding to the ZnO wurtzite structure. In addition to the ZnO diffraction peaks, the appearance of weak broad peak at 25° is attributed to the characteristic peak of graphene. The absence of peaks from graphene oxide indicated that the reduction of graphene oxide is complete in the synthesis process (Xu et al. 2008; Li et al. 2011) and confirms the formation of ZG nanocomposite.
The room temperature Raman spectra of ZnO and ZG excited at 633 nm are presented in Fig. 2. The ZnO exhibits hexagonal wurtzite structure which belongs to the \({\text{C}}_{{6{\text{v}}}}^{4}\) space group having two formula units per primitive cell with all the atoms occupying the \({\text{C}}_{{ 3 {\text{v}}}}\) sites. According to group theoretical analysis, the phonons of wurtzite structure ZnO have irreducible representation (Zeferino et al. 2011; Cheng et al. 2010).
where B 1 modes are IR and Raman inactive, i.e., silent mode and E 2 modes are Raman active. The Raman and IR active modes A 1 and E 1 are polar, and therefore, each splits into LO and TO modes. The foremost sharp peak at 437 cm−1, E 2 (high) mode, is the characteristic peak of hexagonal wurtzite phase ZnO and the peaks at ~98, 380, 407, and 583 cm−1 correspond to E 2 (low), A 1 (LO), E 1 (TO), and E 1 (LO) modes, respectively. The second-order Raman scattering originating from the zone boundary phonons E 2(H)–E 2(L) give rise to a peak at ~338 cm−1. The experimentally observed modes at 328, 378, 432, and 578 cm−1 for the ZnO sample show that there is no major shift from the typical vibrational modes of wurtzite phase, which confirms the existing phase was purely of wurtzite type. Furthermore, the ZG sample shows the main characteristic peak of hexagonal wurtzite ZnO at 436 cm−1 corresponding to the vibration mode of E2 phonon was observed, and low-intensity peak at 332 cm−1 was caused by the multiple-phonon scattering processes of ZnO (Cheng et al. 2010). In addition, the peaks at 1,330, 1,592, and 2,674 cm−1 are also observed, which indicate that the graphene was preserved. The Raman active modes at 1,330 (D band) and 1,592 cm−1 (G band) correspond to breathing mode of κ-point phonons of A 1g symmetry and the E 2g phonon of sp 2 C atoms, respectively, and the second-order of zone boundary phonons gives rise to 2D (2,674 cm−1) band. The D band of graphene is more intense than the G band, where the higher intensity of D band reflects the prepared sample have more defects due to the incorporation of ZnO in graphene (Omar et al. 2014; Xu et al. 2011) and also the G band of ZG nanocomposite was shifted about 10 cm−1 toward the higher wavenumber region compared with graphene indicating the chemical interaction between graphene and ZnO (Fig. 2 (inset)) (Lv et al. 2014; Xu et al. 2011; Kamat 2010). The Raman spectrum showed ZnO vibrations as well as D, G, and 2D bands of graphene, suggesting that the structure of ZnO and graphene is retained in the ZG nanocomposite (Chen et al. 2011; Luo et al. 2012; Nayak et al. 2013) and the observed Raman results are in accordance with the XRD results.
Figure 3 depicts the FE-SEM images of ZnO and ZG nanocomposite. From the Fig. 3a, it is clear that the spherical-shaped ZnO nanoparticles are uniformly distributed and the particles are very closely packed and make the surface denser. The statistical average size of the particles is estimated as ~320 nm. In the case of Fig. 3b, there are two different morphologies, including nanoparticles from ZnO and wrinkled sheet-like morphology from graphene indicates ZnO nanoparticles are well anchored on the surface of the graphene sheets. The size of ZnO particles in ZG nanocomposite is ~160 nm which was distributed randomly on the graphene layer and the existence of ZnO is favorable to prevent the graphene from aggregation. Hence, the SEM image of ZG adds evidence for the formation of nanocomposite.
As the results of XRD, Raman and FE-SEM confirmed the formation of ZnO nanoparticles and ZG nanocomposite and clarified the structural characteristics of ZnO and graphene is not affected during the synthesis of ZG nanocomposite. The ZnO nanoparticle and ZG nanocomposite electrodes were prepared as discussed in experimental part. The UV–Vis absorption spectra of ZnO and ZG films are shown in Fig. 4. The absorption band at 370 nm for ZnO film, which originates from the band gap transitions confirmed the existence of highly crystalline ZnO (Zhang et al. 2009a). In the case of ZG nanocomposite, the absorbance has increased in the entire region and the absorption edge shifted toward higher wavelength region (red shift) due to the incorporation of graphene. The bandgap energy of graphene incorporated ZnO is lower (2.78 eV) than bare ZnO (3.19 eV). Therefore, this result indicated that narrowing of the band gap of ZnO occurred with the addition of graphene. The reduction in bandgap of ZnO with the addition of graphene might be attributed to the chemical interaction between ZnO and graphene (Zhang et al. 2010a, b). The analysis of the obtained results suggests that the presence of graphene can tune the optical properties of ZnO (Zhang et al. 2009b; Liu et al. 2011).
Figure 5 shows UV–Vis absorption spectra of (a) EY, EY sensitized ZnO and ZG electrodes and (b) RB, RB sensitized ZnO and ZG electrodes. The RB and EY dyes are xanthene sensitizers with high absorption coefficient in the visible region and have absorption in the visible region with maximum absorbance at 556 and 535 nm, respectively, and satisfy the major condition for their use as sensitizers in DSSCs (Chen et al. 2007). The dye-anchored ZnO electrodes show an absorption band in the UV region corresponding to the band to band transition of ZnO, whereas other bands observed at 558 and 537 nm originate from the dye molecules adsorbed on the ZnO surface. Therefore, it is clearly evident that, the dye molecules are loaded on ZnO electrodes and they enable ZnO to absorb visible light. On the other hand, ZG electrodes also show UV absorption around 365 nm as well as a broad visible absorption band. The absorption bands of ZG and dye-anchored ZG get broadened with higher absorption intensities in the visible region compared with bare ZnO due to the addition of graphene. Hence, the improvement of absorbed photon energy toward the visible region is expected to enhance the photoconductivity of ZG compared with bare ZnO. Further, it can be observed that the absorption peaks of dyes anchored on the ZnO and ZG electrodes are broadened and blue-shifted compared to the spectra of neat dye solutions. This is hence an indication that the anchoring of dyes is via chemical interactions to the ZnO and ZG surface (Moser and Gratzel 1984; Hagfeldt et al. 2010).
To support the effective adsorption of dyes used in our study, we also investigated the absorption spectra of desorbed dyes from ZnO and ZG electrodes, and the spectra are presented in Fig. 6. From the absorbance spectra, the dye desorbed from the ZG electrodes show higher absorption intensity than ZnO electrodes, which confirms the higher dye anchoring capability of ZG electrodes. Also, the naked eye inspection (photographs) provides a clear view of better dye adsorption of ZG electrodes than ZnO electrodes, which is shown in Fig. 6 (inset). It is found that more number of dye molecules are attached to the surface of the ZG electrodes, which might be due to the high surface area of graphene and chemical interaction between the dye molecules and graphene (Shahzad et al. 2013). It is suggested that the enhancement of dye adsorption in ZG electrodes is not only due to simple physisorption but also attributed to the π–π stacking interaction between the dye molecule and the aromatic domains of graphene (Grätzel 2005; Shahzad et al. 2013). The amount of adsorbed dye is calculated using Beer’s law and is presented in Table 1.
The photovoltaic examination of DSSCs made with ZnO and ZG electrodes was carried out by measuring the current–voltage (I–V) characteristic curves using an electrochemical workstation under irradiation of artificial sunlight from 100 W xenon arc lamp with incident light intensity of 40 mW cm−2 (AM 1.5). The photovoltaic performances such as fill factor (FF) and PCE (η) of DSSCs were calculated according to the following equations:
where V oc is the open-circuit voltage (V), J sc the short-circuit current density (mA cm−2), and V m (V) and J m (mA cm−2) the voltage and the current density at the point of maximum power output in the current density–voltage (J–V) plot, respectively, and P in is the incident light power (Nazeeruddin et al. 2011). The obtained parameters of DSSCs such as J sc, V oc, FF, and PCE calculated from the J–V curves are summarized in Table 1.
Figure 7 depicts the J–V characteristic curves of ZnO and ZG-based DSSCs. From the observed results, it is evident that the photovoltaic performance of ZG is better than the bare ZnO-based DSSCs. The enhancement of parameters such as PCE, V oc, J sc, and FF was influenced by two factors such as higher dye loading of ZG electrodes and anti-recombination effect of graphene. In ZG nanocomposite, ZnO is a good electron donor material and graphene is a relatively good electron acceptor material, so the interaction between these two materials provides better electron transport. Briefly, the 2D graphene bridges behave as an electron transfer channel due to its higher electron conduction (Kongkanand et al. 2007). Under light irradiation, the conduction band of ZnO receives electrons from HOMO level of dye molecule. As the ZnO nanoparticles are anchored on graphene, the excited electrons are captured without any hindrance. The collected electrons can be transported from ZnO to the conductive substrate efficiently through graphene bridges and hence the recombination was suppressed (Chen et al. 2013; Omar et al. 2013). The scheme of the electron transfer process in (a) ZnO DSSC and (b) ZG DSSC is shown in Scheme 2. Furthermore, the high optical transparency of graphene allows more sunlight to the dye molecules and enhances the light harvesting in the DSSCs. Moreover, the results showed that the maximum part of the solar light is unused in the ZnO-based DSSCs conversion process because the dyes which we have used have sharp absorption bands in the visible region, so they convert photons only below 600 nm. Whereas in ZG-based DSSCs, the broad absorption behavior was observed due to the incorporation of graphene. This enhances the photoconductivity leading to higher PCE. Further investigations are in progress, to realize the importance of graphene incorporation with different proportions and to determine the enhancement in the photovoltaic efficiency and performance of ZG composites.
Conclusions
The ZnO nanoparticles and ZG nanocomposite were successfully prepared by chemical reduction route. The (002) plane along with ZnO diffraction peaks in the XRD pattern indicated the presence of both ZnO and graphene in the nanocomposite. Similarly, in the micro-Raman spectrum, the presence of ZnO as well as graphene vibrational modes confirmed the formation of nanocomposite. The morphology of the synthesized composite indicates that the ZnO nanoparticles were embedded on the wrinkled graphene sheets. The optical absorbance of the ZG nanocomposite was enhanced in the visible region compared to bare ZnO. This was caused by the chemical interaction between ZnO and graphene that might have led to the creation of electron–hole pairs during light radiation. The presence of graphene in ZnO suppressed the electron–hole recombination and increased the efficiency of the cells. The ZG nanocomposite possessed great dye adsorption behavior, extended visible light absorption, enhanced charge transport, and power conversion efficiencies simultaneously.
References
Akhavan O (2010) Graphene nanomesh by ZnO nanorod photocatalysts. ACS Nano 4:4174–4180
Anta JA, Guillen E, Zaera RT (2012) ZnO-based dye-sensitized solar cells. J Phys Chem C 116:11413–11425
Chen Z, Li F, Huang C (2007) Organic D-π-A dyes for dye sensitized solar cell. Curr Org Chem 11:1241–1258
Chen YL, Hu ZA, Chang YQ, Wang HW, Zhang ZY, Yang YY, Wu HY (2011) Zinc oxide/reduced graphene oxide composites and electrochemical capacitance enhanced by homogeneous incorporation of reduced graphene oxide sheets in zinc oxide matrix. J Phys Chem C 115:2563–2571
Chen L, Zhou Y, Tu W, Li Z, Bao C, Dai H, Yu T, Liu J, Zou Z (2013) Enhanced photovoltaic performance of a dye-sensitized solar cell using graphene–TiO2 photoanode prepared by a novel in situ simultaneous reduction–hydrolysis technique. Nanoscale 5:3481–3485
Cheng B, Sun W, Jiao J, Tian B, Xiao Y, Lei S (2010) Disorder-induced Raman scattering effects in one-dimensional ZnO nanostructures by incorporation and anisotropic distribution of Dy and Li codopants. J Raman Spectrosc 41:1221–1226
Cuong TV, Phama VH, Chung JS, Shina EW, Yoo DH, Hahn SH, Huh JS, Rue GH, Kima EJ, Hur SH, Kohl PA (2010) Solution-processed ZnO-chemically converted graphene gas sensor. Mater Lett 64:2479–2482
Dhar P, Ansari MHD, Gupta SS, Siva VM, Pradeep T, Pattamatta A, Das SK (2013) Percolation network dynamicity and sheet dynamics governed viscous behavior of polydispersed graphene nanosheet suspensions. J Nanopart Res 15:2095/1–2095/12
Gratzel M (2001) Molecular photovoltaics that mimic photosynthesis. Pure Appl Chem 73:459–467
Gratzel M (2003) Dye-sensitized solar cells. J Photochem Photobiol C Photochem Rev 4:145–153
Gratzel M (2005) Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 44:6841–6851
Gratzel M, Hagfeldt A (2000) Molecular photovoltaics. Acc Chem Res 33:269–277
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Hummers WS, Offeman RE (1958) Preparation of graphene oxide. Chem Soc 80:1339
Hwang JO, Lee DH, Kim JY, Han TH, Kim BH, Park M, No K, Kim SO (2011) Vertical ZnO nanowires/graphene hybrids for transparent and flexible field emission. J Mater Chem 21:3432–3437
Kamat PV (2010) Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J Phys Chem Lett 1:520–527
Kongkanand A, Dominguez RM, Kamat PV (2007) Single wall carbon nanotube scaffolds for photoelectrochemical solar cells. Capture and transport of photogenerated electrons. Nano Lett 7:676–680
Lee JS, You KH, Park CB (2012) Highly photoactive, low bandgap TiO2 nanoparticles wrapped by graphene. Adv Mater 24:1084–1088
Li B, Cao H (2011) ZnO@graphene composite with enhanced performance for the removal of dye from water. J Mater Chem 21:3346–3349
Lightcap IV, Kamat PV (2013) Graphitic design: prospects of graphene-based nanocomposites for solar energy conversion, storage, and sensing. Acc Chem Res 46:2235–2243
Liu X, Pan L, Lv T, Lu T, Zhu G, Sun Z, Sun C (2011) Microwave-assisted synthesis of ZnO–graphene composite for photocatalytic reduction of Cr(VI). Catal Sci Technol 1:1189–1193
Luo QP, Yu XY, Lei BX, Chen HY, Kuang DB, Su CY (2012) Reduced graphene oxide-hierarchical ZnO hollow sphere composites with enhanced photocurrent and photocatalytic activity. J Phys Chem C 116:8111–8117
Lv R, Wang X, Lv W, Xu Y, Ge Y, He H, Li G, Wu X, Li X, Li Q (2014) Facile synthesis of ZnO nanorods grown on graphene sheets and its enhanced photocatalytic efficiency. J Chem Technol Biotechnol. doi:10.1002/jctb.4347
Marlinda AR, Huang NM, Muhamad MR, Anamt MN, Chang BYS, Yusoff N, Harrison I, Lim HN, Chia CH, Kumar SV (2012) Highly efficient preparation of ZnO nanorods decorated reduced graphene oxide nanocomposites. Mater Lett 80:9–12
Moser J, Gratzel M (1984) Photosensitized electron injection in colloidal semiconductors. J Am Chem Soc 106:6557–6564
Nayak P, Anbarasan B, Ramaprabhu S (2013) Fabrication of organophosphorus biosensor using ZnO nanoparticle-decorated carbon nanotube-graphene hybrid composite prepared by a novel green technique. J Phys Chem C 117:13202–13209
Nazeeruddin Md K, Baranoff E, Gratzel M (2011) Dye-sensitized solar cells: a brief overview. Sol Energy 85:1172–1178
Omar A, Abdullah H, Yarmo MA, Shaari S, Taha (2013) Morphological and electron transport studies in ZnO dye-sensitized solar cells incorporating multi- and single-walled carbon nanotubes. J Phys D Appl Phys 46:165503/1–165503/8
Omar FS, Ming HN, Hafiz SM, Ngee LH (2014) Microwave synthesis of zinc oxide/reduced graphene oxide hybrid for adsorption-photocatalysis application. Int J Photoenergy 2014:1–8
Rani S, Suri P, Shishodia PK, Mehra RM (2008) Synthesis of nanocrystalline ZnO powder via sol–gel route for dye-sensitized solar cells. Sol Energy Mater Sol C 92:1639–1645
Sahoo AK, Srivastava SK, Raul PK, Gupta AK, Shrivastava R (2014) Graphene nanocomposites of CdS and ZnS in effective water purification. J Nanopart Res 16:2473/1–2473/17
Shahzad N, Pugliese D, Lamberti A, Sacco A, Virga A, Gazia R, Bianco S, Shahzad MI, Tresso E, Pirri CF (2013) Monitoring the dye impregnation time of nanostructured photoanodes for dye sensitized solar cells. J Phys Conf Ser 439:012012/1–012012/12
Tang YB, Lee CS, Xu J, Liu ZT, Chen ZH, He Z, Cao YL, Yuan G, Song H, Chen L, Luo L, Cheng HM, Zhang WJ, Bello I, Lee ST (2010) Incorporation of graphenes in nanostructured TiO2 films via molecular grafting for dye-sensitized solar cell application. ACS Nano 4:3482–3488
Wahab R, Ansari SG, Kim YS, Seo HK, Shin HS (2007) Room temperature synthesis of needle-shaped ZnO nanorods via sonochemical method. App Surf Sci 253:7622–7626
Xiang Q, Yu J, Jaroniec M (2012) Graphene-based semiconductor photocatalysts. Chem Soc Rev 41:782–796
Xu C, Wang X, Zhu J (2008) Graphene–metal particle nanocomposites. J Phys Chem C 112:19841–19845
Xu T, Zhang L, Cheng H, Zhu Y (2011) Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. App Catal B 101:382–387
Yang N, Zhai J, Wang D, Chen Y, Jiang L (2010) Two-dimensional graphene bridges enhanced photoinduced charge transport in dye-sensitized solar cells. ACS Nano 4:887–894
Zeferino RS, Flores MB, Pal U (2011) Photoluminescence and Raman scattering in Ag-doped ZnO nanoparticles. J Appl Phys 109:014308/1–014308/6
Zhang L, Cheng H, Zong R, Zhu Y (2009a) Photocorrosion suppression of ZnO nanoparticles via hybridization with graphite-like carbon and enhanced photocatalytic activity. J Phys Chem C 113:2368–2374
Zhang Q, Dandeneau CS, Zhou X, Cao G (2009b) ZnO nanostructures for dye-sensitized solar cells. Adv Mater 21:4087–4108
Zhang H, Lv X, Li Y, Wang Y, Li J (2010a) P25-graphene composite as a high performance photocatalyst. ACS Nano 4:380–386
Zhang Y, Tang ZR, Fu X, Xu YJ (2010b) TiO2–graphene nanocomposites for gas phase photocatalytic degradation of volatile aromatic pollutant: is TiO2–graphene truly different from other TiO2–carbon composite materials? ACS Nano 4:7303–7314
Zhao N, Cheng X, Zhou Y, Yang M, Yang J, Zhong T, Zheng S (2014) Synthesis of flexible free-standing silver nanoparticles–graphene films and their surface-enhanced Raman scattering activity. J Nanopart Res 16:2335/1–2335/11
Acknowledgments
This work was financially supported by DST-PURSE Programme of Madurai Kamaraj University. The authors thank DST-FIST for providing Powder XRD facility, UGC-UPE for providing micro-Raman and solar simulator facilities. The authors PJ & JM acknowledge Mr. Suresh, School of Energy Science, Madurai Kamaraj University for his assistance in I–V measurement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayabal, P., Gayathri, S., Sasirekha, V. et al. Preparation and characterization of ZnO/graphene nanocomposite for improved photovoltaic performance. J Nanopart Res 16, 2640 (2014). https://doi.org/10.1007/s11051-014-2640-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2640-7