Abstract
Fe3O4/C nanocomposites have been prepared by one-pot PEG-assisted co-precipitation method. The structure and morphology of the as-prepared materials were analyzed by X-ray diffraction and transmission electron microscopy. The results showed that Fe3O4/C nanocomposites were well crystallized. Carbon nanoparticles dispersed among Fe3O4 particles forming a carbon layer, which prevent Fe3O4 particles from contacting each other. Electrochemical performance tests showed that Fe3O4/C nanocomposites keep at a high discharge capacity of 902.4 mAh g−1 at 1 C after 110 cycles. Furthermore, the samples showed much improved rate capability and better cycle stability compared with pure Fe3O4. The excellent electrochemical performance of Fe3O4/C nanocomposites can be attributed to unique nanostructure and existence of amorphous carbon in the composites. The existence of the amorphous carbon not only enhanced electric conductivity, but also buffered volume variation of Fe3O4/C nanocomposites during charge/discharge process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the daily depletion of petroleum resources, energy storage has become a crucial issue. Lithium-ion batteries (LIBs), as novel energy storage device, were extensively studied during past years. The properties of anode materials play a significant role in performance of LIBs (Bruce et al. 2008). Fe3O4 is an attractive candidate for anode material of LIBs due to its environment friendliness, high abundance and high theoretical capacities (924 mAh g−1) (Duan et al. 2012). Nevertheless, a large specific volume variation usually occurs in the host matrices of the metal oxide during charge/discharge process, and Fe nanocrystals come from lithiation process can catalyze decomposition the outer solid electrolyte interphase (SEI) (He et al. 2013), which will lead to rapid capacity decay and high-initial irreversible capacity loss. In previous research, two main kinds of strategies have been considered to improve the electrochemical performance of Fe3O4. Controlling Fe3O4 nanostructures is one way to partially accommodate the volume variation during Li+ insert/extract process and reduce diffusion path for Li+, which can improve theirs rate capability and capacity retention. Nanostructures, such as hollow particles (Chen et al. 2012b), porous nanoparticles (Chen et al. 2012a), nanorods (Chen et al. 2011b), nanotubes (Gao et al. 2011) etc., have been fabricated. Another strategy is to enhance electronic conductivity through synthesis of Fe3O4 nanocomposites. Many materials with high electronic conductivities, such as RuO2 (Liu et al. 2012), SnO2 (Wu et al. 2011), and carbon (C) (Zhang et al. 2008), have been selected to construct the conducting networks, among them, C is an appealing choice. C is a very stable anode material in LIBs due to small volume change during Li+ insert/extract process. It acts as a better cushioning layer to effectively buffer volume variation of the inner active materials during charge/discharge process. Furthermore, C layer has a vital role in enhancing electronic conductivity of electrode materials (Zhou et al. 2014).
However, there is less work focuses on combine controlling nanostructures with connecting C to improve the electrochemical properties of Fe3O4. And for preparation of Fe3O4/C nanocomposites, most methods involve many complicated steps, such as centrifugation or suction filtration for product collection, rinsing with water and ethanol, and oven drying (Zhong et al. 2011). It is therefore desirable to develop a high-yield and simple synthesis method to facilitate the applications of such material.
Here, Fe3O4/C nanocomposites were prepared by one-pot PEG-assisted co-precipitation method with simple steps by combine controlling nanostructures with connecting C. The precursors of Fe3O4/C nanocomposites were synthesized by heating the mixture of Fe(NO3)3·9H2O, PEG 400 (PEG: polyethylene glycol; 400: polymerization degree) and H2C2O4·2H2O in thermostatic water bath. In the reaction process, excess H2C2O4·2H2O acted as complexing agent with Fe(NO3)3·9H2O and formed soluble complex ([Fe(C2O4)3]3−), the PEG 400 chains surrounding the ([Fe(C2O4)3]3−) complex helped the complex dispersed in the solution and acted as C source afterward (Gong et al. 2014). After precipitation and calcination, Fe3O4/C nanocomposites were prepared. Herein, C nanoparticles have important role in improving electrochemical performance of Fe3O4/C nanocomposites, which enhance electric conductivity and buffer volume variation of the nanocomposites during charge/discharge process. Thus the as-synthesized Fe3O4/C nanocomposites exhibit excellent electrochemical performance, with ultra-high rate capabilities, which is much improved relative to that of pure Fe3O4. Compared with other Fe-based materials (Lang and Xu 2013; Zhao et al. 2012a), Fe3O4/C nanocomposites obtained in this work show better cycling performance.
Experimental section
Experimental
Fe3O4/C nanoparticles were prepared by one-pot PEG-assisted co-precipitation method with simple steps. All reagents are analytically pure and used without further purification. In a typical experiment, 2.52 g H2C2O4·2H2O and 2.00 g PEG 400 were dissolved in 100 mL deionized water, followed by addition of 2.02 g Fe(NO3)3·9H2O under magnetic stirring for 5 h to form a homogenous mixture solution. The mixture was transferred to a thermostat water bath and then maintained at 80 °C for 72 h and allowed to cool down to room temperature naturally. The faint yellow precipitate was prepared. After calcining the precipitate under argon atmosphere at 600 °C for 6 h, the Fe3O4/C nanoparticles were obtained. For comparison, pure Fe3O4 particles were prepared by the same procedure without addition of PEG 400.
Characterization of synthesized material
The crystal structures of the samples were investigated by X-ray diffraction (XRD, Bruker D8 Advance) with Cu Kα radiation. Raman measurement was employed to verify the chemical bonding characteristics of carbon with Raman microscope (Bruker Senterra R200-L) using a laser line of 532 nm as the excitation source. To understand the approximate content of carbon in the synthesized product, thermal gravimetric analysis (Netzsch Sta 449 F3) of the samples were employed from room temperature to 850 °C under air ventilation at a heating rate of 10 °C min−1. The morphological characteristics of the products were investigated by transmission electron microscopy (TEM, H-600, Hitachi, Japan). The working electrodes were prepared by mixing the active materials, acetylene black, and polyvinylidene fluoride at a weight ratio of 75:15:10 in N-methyl pyrrolidinone solvent. The mixtures were pasted on pure Cu foil, and then dried at 110 °C in a vacuum oven for 12 h for use in coin-type cells (2032), which were assembled in an argon-filled glove box. A Celgard 2300 microporous polyethylene membrane was used as the separator. The electrolyte was 1 M LiPF6 in a 1:1 (volume ratio) mixture of ethylene carbonate and dimethyl carbonate, and a lithium foil was as the counter electrode. The charge/discharge measurements were carried out on a battery test system (CT2001A, Land, China) in the voltage range between 0.0 and 3.0 V at room temperature. The specific capacities were calculated based on the total mass of active materials including Fe3O4 and C. Cyclic voltammetry (CV) was conducted in the voltage range of 0.0–3.0 V with a scan rate of 0.1 mV s−1 using a CHI660D electrochemical workstation. Electrochemical impedance spectroscopy (EIS) was measured on a Zahner Elektrik electrochemical workstation in the frequency range of 0.01–100 kHz, which applied a DC potential equal to the open circuit voltage of the cell and an AC oscillation of 5 mV. The impedance data were analyzed by Z-view 2.0 software.
Results and discussion
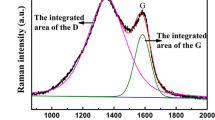
Figure 1 shows the XRD patterns of Fe3O4 particles and Fe3O4/C nanoparticles. All peaks of the two samples are coincident with the standard for face-centered Fe3O4 (JCPDS No. 65-3107). No peak for impurities is detectable in the patterns. The existence of C in Fe3O4/C nanoparticles was confirmed by Raman spectroscopy (Fig. 2). It is clearly seen that the as-synthesized Fe3O4/C nanoparticles show a typical two-band Raman spectrum with D and G bands. The D band at ~1,337 cm−1 is related to the vibrations of C atoms with dangling bonds in plane terminations of disordered graphite, confirming the amorphous C in the as-prepared material, while the G band at ~1,584 cm−1 consists with the E2g mode of graphite, namely, the stretching modes of C=C bonds of graphite (Dong et al. 2013). The result shows that there is amorphous C in the as-synthesized Fe3O4/C nanocomposites. Thermogravimetric analysis (TGA) was adopted to further confirm the C content in Fe3O4/C nanocomposites. Figure 3 is the TGA results of Fe3O4 particles and Fe3O4/C nanocomposites. As shown in Fig. 3, the mass loss of Fe3O4 particles and Fe3O4/C nanocomposites is little below 300 °C, indicating the two samples are stable under air atmosphere. The mass of Fe3O4 particles and Fe3O4/C nanocomposites shows a small increase approximately at 300 °C due to the oxidation of partial Fe3O4 to Fe2O3 (Liu et al. 2012). After 300 °C, the mass of Fe3O4 particles is stable, while the mass of Fe3O4/C nanocomposites decreases from 300 to 500 °C and remains stable after 500 °C, which corresponds to C decomposition. Since Fe3O4/C nanocomposites were dried at 110 °C in a vacuum oven for 12 h before TG analysis, the release of water was negligible (Hu et al. 2014). Thus, the carbon content was considered as the total weight loss, which was calculated to be 8.9 wt%.
Figure 4 reveals the TEM images of Fe3O4 particles and Fe3O4/C nanocomposites. It can be seen from Fig. 4a that Fe3O4 particles are aggregated. While in Fig. 4b the Fe3O4 nanoparticles and C nanoparticles can be clearly differentiated. C nanoparticles disperse among Fe3O4 particles forming a C layer, which prevents the Fe3O4 particles from contacting each other. The special structure of C nanoparticles can relieve the volume variation of Fe3O4 during charge/discharge process availably (Huang et al. 2011). Furthermore, the existence of C nanoparticles limits the growth of Fe3O4 particles in the preparing procedure, which will prompt formation of Fe3O4/C nanocomposites. The nanostructure of Fe3O4/C is beneficial to shorten Li+ diffusion path (Liu et al. 2012). Moreover, the C nanoparticles will improve electrical conductivity (Zhang et al. 2008), suggesting that Fe3O4/C nanocomposites possess better electrochemical performance.
Figure 5 shows the first five CV curves of Fe3O4 particles and Fe3O4/C nanocomposites at room temperature in the voltage range of 0.0–3.0 V at a scanning rate of 0.1 mV s−1. In the first cycle, both samples have a wide anodic peak located around 1.7–2.1 V, which corresponds to the reversible oxidation of Fe0 to Fe2+, and part to Fe3+. Furthermore, two samples have a large cathodic polarization process (i.e., there is a voltage hysteresis during charge/discharge process) in the first cycle (about 0.58 V), which can be ascribed to electrolyte decomposition as well as SEI formation on the surface of the samples and Li+ intercalation reaction as follows:
(He et al. 2013; Zhou et al. 2014).
The large polarization could be due to the poor conversion reaction kinetics (lithium diffusion kinetics is limited during the intercalation process, for example) (Mitra et al. 2006). The phenomenon of polarization has been widely observed in a number of other Fe3O4/C composites (Zhang et al. 2008). Unfortunately, the application performance of LIBs is restricted by the large polarization. Therefore, it is very important to reduce the polarization of Fe3O4/C nanocomposites in the future work by improving the conversion reaction kinetics, such as reducing the particle size of Fe3O4/C nanocomposites (Lian et al. 2011). In the subsequent cycles, there is a substantial difference from the first cycle, which can be attributed to the decomposition of electrolyte to form a SEI layer. The peak current and integrated peak area of Fe3O4 are much lower than these of Fe3O4/C, indicating that Fe3O4/C has higher capacity and reactivity than Fe3O4. Besides the first cycle, the curves of Fe3O4/C overlapped better than that of Fe3O4, indicating that Fe3O4/C has more stable structure and more excellent reversibility (Duan et al. 2012).
Figure 6 shows the initial charge/discharge curves of Fe3O4/C nanocomposites and Fe3O4 particles at a current density of 1 C. As shown in Fig. 6, in the beginning, the voltage of both samples drops to 0.75 V quickly, which can be attributed to the reaction of Fe3O4 + xLi++ xe− ↔ Li x Fe3O4 (Wang et al. 2008), and then obvious potential plateau of both samples at about 0.75 V versus Li+/Li, corresponding to the conversion reaction Li x Fe3O4 + (8 − x)Li+ → 3Fe0 + 4Li2O (Wang et al. 2010). The sloping curve down to the cut-off voltage of 0.0 V can be ascribed to the reaction between Fe and the electrolyte that forms a gel-like solid electrolyte interphase (SEI) film (Laruelle et al. 2002). The Fe3O4/C nanocomposites show a high discharge capacity of 1,480.4 mAh g−1 and a charge capacity of 903.5 mAh g−1 with a Coulombic efficiency of 61 % at the 1st cycle. While the Fe3O4 particles shows a discharge capacity of 1,255 mAh g−1 and a charge capacity of 857.7 mAh g−1, the Coulombic efficiency of Fe3O4 particles is 68 % at the 1st cycle. The poor Coulombic efficiency for the first cycle of both samples is ubiquitous for electrochemical reactions with lithium through a conversion reaction. (Cabana et al. 2010). Compared to Fe3O4 particles, Fe3O4/C nanocomposites have lower first Coulomb efficiency, the Fe3O4/C nanocomposites with lower first Coulomb efficiency possibly originates from the existence of amorphous C, which has a high surface area (Zhao et al. 2012b). The reason needs further investigation. However, lower first Coulombic efficiency of the Fe3O4/C nanocomposites needs further improvement. The cycling performance of Fe3O4 particles and Fe3O4/C nanocomposites was evaluated between 0.0 and 3.0 V at 1 C for 110 cycles. As shown in Fig. 7, the first discharge capacity of Fe3O4 particles is as high as 1,255 mAh g−1. However, the discharge capacity quickly fades and only 317.4 mAh g−1 remains at the 110th cycle, indicating Fe3O4 particles have a poor cycling performance (Chen et al. 2011a). In contrast, the first discharge capacity of Fe3O4/C nanocomposites is as high as 1,480.4 mAh g−1, and stabilizes at 902.4 mAh g−1 after 110 cycles. Obviously, the cycling performance and specific capacity of Fe3O4/C nanocomposites are much better than those of Fe3O4 particles and many other similar materials reported (Lang and Xu 2013; Li et al. 2012b; Zhao et al. 2012a). Fe3O4/Fe/C, which prepared by Zhao et al., exhibited about 600 mAh g−1 capacity at 50 mA g−1 after 40 cycles; Lang et al. prepared porous Fe3O4/C microbelts, which exhibited a 710 mAh g−1 capacity at 0.2 C after 50 cycles; Fe3O4@C core–shell rings prepared by Li et al. showed 801 mAh g−1 at 0.2 C after 100 cycles. Compared to the materials, the cycling performance of Fe3O4/C nanocomposites in this paper is even better, indicating that the one-pot PEG-assisted co-precipitation method could be an effective and simple way to synthesize Fe3O4/C nanocomposites with excellent electrochemical performance. Herein, both of the initial discharge capacities of Fe3O4 particles (1,255 mAh g−1) and Fe3O4/C nanocomposites (1,480.4 mAh g−1) exceed the theoretical capacity of Fe3O4 (924 mAh g−1). The extra capacities of the two samples beyond the theoretical values are probably due to the decomposition of non-aqueous electrolyte during the discharge process (Huang et al. 2011). It has been reported that, during the charge/discharge process, the Fe3O4-based anode surface would be covered by SEI film, which formed due to the reductive decomposition of the organic electrolyte. And the capacity of Fe3O4 mainly comes from the reversible conversion reaction between Li+ and Fe3O4. During lithiation, a Li2O matrix was formed, and Fe nanocrystals dispersed in this matrix [Li2O and Fe formed from above Eqs. (1) and (2)]. The Fe nanocrystals can catalyze decomposition of the SEI. For Fe3O4/C nanocomposites obtained in this work, the C layer which dispersed among Fe3O4 particles can prevent Fe nanocrystals from catalyzing decomposition of the outer SEI. Thus, a relative stable SEI film would form on the materials surface without the rupturing and reformation, which reduced depletion of the electrolyte. Furthermore, the C layer can accommodate the volume expansion/contraction of Fe3O4 particles upon lithiation/delithiation without breaking down the structure of Fe3O4/C nanocomposites. So Fe3O4/C nanocomposites possess excellent cycling performance and high capacity (He et al. 2013). However, both Fe3O4 particles and Fe3O4/C nanocomposites have a large polarization in the first cycle may be caused by conversion of Fe3O4 to Fe and the consumption of the electrolyte to form SEI, this characteristic is agreed well with the CV results.
Besides the high capacity and stable cycling performance, a good rate performance is also an important criterion for high performance of LIBs. Figure 8 is cycling performance of Fe3O4 particles and Fe3O4/C nanocomposites at various current densities (100–1,500 mA g−1) at room temperature. As show in Fig. 8, Fe3O4/C nanocomposites are capable of delivering discharge capacity of 1,053.4, 981.1, 894.4, 866.9, 808.9, and 698.3 mAh g−1 at the current density of 100, 200, 500, 700, 1,000, and 1,500 mA g−1, respectively. After charge/discharge cycling at high current density, the current density is reduced stepwise to 100 mA g−1, with a specific capacity as high as 1,086.7 mAh g−1 recovered, indicating that Fe3O4/C nanocomposites have good kinetic properties (Lian et al. 2010). Remarkably, the discharge capacities of Fe3O4/C nanocomposites at current densities of 100 and 200 mA g−1 are higher than the theoretical capacity of Fe3O4. The extra capacity may be due to the synergistic effect between Fe3O4 nanoparticles and C layer in the nanocomposites under small current densities (Paek et al. 2009), and the real reason needs further investigation. However, as the current density increases from 100 to 200, 500, 700, 1,000, and 1,500 mA g−1, the reversible capacity of Fe3O4 decreases from 722.5 to 496.2, 411.2, 317.3, 250.7, and 173.2 mAh g−1, respectively. Fe3O4/C nanocomposites display superior rate capability and reversibility compared with previously reported results. Fe3O4/Fe/C (Zhao et al. 2012a) was prepared by sol–gel method with ultrasonication and vacuum drying, it exhibited about 500 mAh g−1 capacity at the current density of 500 mA g−1; porous Fe3O4/C microbelts (Lang and Xu 2013) were prepared by electrostatic spinning, which including complicated steps such as selection of viscosity and ultrasonic. And the prepared Fe3O4/C microbelts exhibited a capacity of 300 mAh g−1 at the current rate of 1 C. By contrast, herein Fe3O4/C nanocomposites were prepared by a simple one-pot PEG-assisted co-precipitation method, and the as-prepared Fe3O4/C nanocomposites have ultra-high rate capabilities. The much improved electrochemical performance of Fe3O4/C nanocomposites could be explained as follows: Firstly, Raman spectroscopy data show that there are amorphous C nanoparticles in Fe3O4/C nanocomposites. The existence of amorphous C in the composite materials not only buffers the volume expansion/contraction of Fe3O4 during charge/discharge process, but also restrains the growth of Fe3O4 particles to easily form Fe3O4/C nanocomposites (Huang et al. 2011). Nanocomposites with small size will shorten Li+ diffusion path and make the permeation of electrolyte easier to surface of electrode, which will accelerate electrochemistry reaction (Duan et al. 2012). Secondly, the amorphous C can form a conducting network to enhance the electrical conductivity of Fe3O4/C nanocomposites, which will improve the electrochemical performance of the nanocomposites (Zhang et al. 2008). Thirdly, The TEM shows that C surrounding the Fe3O4 particles; the C layer which surrounded Fe3O4 nanoparticles build a special nanostructure, which can prevent Fe nanocrystals from catalyzing decomposition the outer SEI. Thus, a relatively stable SEI film would form on the material surface without rupturing and reformation. The stable SEI guaranteed excellent cycling performance and high capacity of Fe3O4/C nanocomposites (He et al. 2013).
In order to understand the impedance changes of Fe3O4/C nanocomposites, electrochemical impedance spectroscopy (EIS) measurements of the composites conducted on the fresh battery and the battery after CV tested. The impedance data were analyzed with Z-view 2.0 software by fitting to an equivalent electrical circuit, which show in Fig. 9. R s is the bulk solution resistance; R ct is charge-transfer resistance; W o is the Warburg impedance describing the diffusion of Li+ in Fe3O4/C; CPE reflects the interfacial capacitance (Li et al. 2012a). Each spectrum is consists of a semicircle in the high-frequency region and a straight line in the low-frequency region. The depressed semicircle in high-frequency region is attributed to the charge-transfer resistance (R ct) of the electrochemical reaction and the straight line is related to the diffusion-controlled Warburg impedance. From the fitted results, the charge-transfer resistance of the fresh battery is (~148.1 Ω) higher than that of the battery after CV tested (~82.6 Ω), due to the wetting of Fe3O4/C by electrolyte. After CV tested, the battery aging as well as continuous electrochemical interaction, the electrode of fully wetting by electrolyte provided continuous transportation paths, which enhanced Li+ diffusion and decreased the impedance as well as improved the electrochemical performance of Fe3O4/C (Tummala et al. 2012).
Conclusion
Fe3O4/C nanocomposites were prepared by one-pot PEG-assisted co-precipitation method with simple steps. The XRD and TEM results show that Fe3O4/C nanocomposites are well crystallized and a matrix is formed by conductive C surrounding Fe3O4 particles. The as-synthesized Fe3O4/C nanocomposites exhibit excellent electrochemical performance with a stable specific discharge capacity of 902.4 mAh g−1 after 110 cycles at 1 C and ultra-high rate capabilities. Compared with other nanosized Fe-based materials, the electrochemical performance of Fe3O4/C nanocomposites is improved obviously. Taking the excellent electrochemical performance and facile synthesis method into consideration, Fe3O4/C nanocomposites prepared by one-pot PEG-assisted co-precipitation method could be a competitive candidate anodic material for high-performance LIBs in the future.
References
Bruce PG, Scrosati B, Tarascon JM (2008) Nanomaterials for rechargeable lithium batteries Angew Chem. Int Ed 47:2930–2946. doi:10.1002/anie.200702505
Cabana J, Monconduit L, Larcher D, Palacín MR (2010) Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 22:E170–E192. doi:10.1002/adma.201090116
Chen JS, Zhang YM, Lou XW (2011a) One-pot synthesis of uniform Fe3O4 nanospheres with carbon matrix support for improved lithium storage capabilities. ACS Appl Mater Interfaces 3:3276–3279
Chen YJ, Xiao G, Wang TS, Ouyang QY, Qi LH, Ma Y, Gao P, Zhu CL, Cao MS, Jin HB (2011b) Porous Fe3O4/carbon core/shell nanorods: synthesis and electromagnetic properties. J Phys Chem C 115:13603–13608. doi:10.1021/jp202473y
Chen Y, Song B, Tang X, Lu L, Xue J (2012a) One-step synthesis of hollow porous Fe3O4 beads-reduced graphene oxide composites with superior battery performance. J Mater Chem 22:17656–17662. doi:10.1039/c2jm32057f
Chen Y, Xia H, Lu L, Xue J (2012b) Synthesis of porous hollow Fe3O4 beads and their applications in lithium ion batteries. J Mater Chem 22:5006. doi:10.1039/c2jm15440d
Dong Y, Hu M, Ma R, Cheng H, Yang S, Li YY, Zapien JA (2013) Evaporation-induced synthesis of carbon-supported Fe3O4 nanocomposites as anode material for lithium-ion batteries. CrystEngComm 15:1324. doi:10.1039/c2ce26459e
Duan L, Huang Y, Jia D, Wang X, Guo Z (2012) Fe3O4 fuzzy spheroids as anode materials for lithium-ion batteries. Mater Lett 71:151–153. doi:10.1016/j.matlet.2011.12.051
Gao G et al (2011) One-step synthesis of Fe3O4@C nanotubes for the immobilization of adriamycin. J Mater Chem 21:12224. doi:10.1039/c1jm12535d
Gong C, Xue Z, Wang X, Zhou XP, Xie XL, Mai YW (2014) Poly(ethylene glycol) grafted multi-walled carbon nanotubes/LiFePO4 composite cathodes for lithium ion batteries. J Power Sources 246:260–268. doi:10.1016/j.jpowsour.2013.07.091
He C, Wu S, Zhao N, Shi C, Liu E, Li J (2013) Carbon-encapsulated Fe3O4 nanoparticles as a high-rate lithium ion battery anode material. ACS Nano 4:4459–4469
Hu M, Jiang Y, Yan M (2014) Scalable synthesis of Fe3O4/C composites with enhanced electrochemical performance as anode materials for lithium-ion batteries. J Alloys Compd 582:563–568. doi:10.1016/j.jallcom.2013.08.098
Huang Y, Dong Z, Jia D, Guo Z, Cho WI (2011) Preparation and characterization of core–shell structure Fe3O4/C nanoparticles with unique stability and high electrochemical performance for lithium-ion battery anode material. Electrochim Acta 56:9233–9239. doi:10.1016/j.electacta.2011.07.141
Lang L, Xu Z (2013) In situ synthesis of porous Fe3O4/C microbelts and their enhanced electrochemical performance for lithium-ion batteries. ACS Appl Mater Interfaces 5:1698–1703. doi:10.1021/am302753p
Laruelle S, Gugon S, Poizot P, Dollé M, Dupont L, Tarascon JM (2002) On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential. J Electrochem Soc 149:A627–A634. doi:10.1149/1.1467947
Li L, Wang T, Zhang L, Su Z, Wang C, Wang R (2012a) Selected-control synthesis of monodisperse Fe3O4@C core-shell spheres, chains, and rings as high-performance anode materials for lithium-ion batteries. Chem Eur J 18:11417–11422. doi:10.1002/chem.201200791
Li R, Ren X, Zhang F, Du C, Liu J (2012b) Synthesis of Fe3O4@SnO2 core-shell nanorod film and its application as a thin-film supercapacitor electrode. Chem Commun 48:5010–5012. doi:10.1039/c2cc31786a
Lian P, Zhu X, Liang S, Li Z, Yang W, Wang H (2010) Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim Acta 55:3909–3914. doi:10.1016/j.electacta.2010.02.025
Lian P, Zhu X, Liang S, Li Z, Yang W, Wang H (2011) High reversible capacity of SnO2/graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim Acta 56:4532–4539. doi:10.1016/j.electacta.2011.01.126
Liu J, Zhou Y, Liu F, Liu C, Wang J, Pan Y, Xue D (2012) One-pot synthesis of mesoporous interconnected carbon-encapsulated Fe3O4 nanospheres as superior anodes for Li-ion batteries. RSC Adv 2:2262–2265. doi:10.1039/c2ra01241c
Mitra S, Poizot P, Finke A, Tarascon JM (2006) Growth and electrochemical characterization versus lithium of Fe3O4 electrodes made by electrodeposition. Adv Funct Mater 16:2281–2287. doi:10.1002/adfm.200500753
Paek SM, Yoo EJ, Honma I (2009) Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure. Nano Lett 9:72–75
Tummala R, Guduru RK, Mohanty PS (2012) Binder free, porous and nanostructured Co3O4 anode for Li-ion batteries from solution precursor plasma deposition. J Power Sources 199:270–277. doi:10.1016/j.jpowsour.2011.10.048
Wang L, Yu Y, Chen PC, Zhang DW, Chen CH (2008) Electrospinning synthesis of C/Fe3O4 composite nanofibers and their application for high performance lithium-ion batteries. J Power Sources 183:717–723. doi:10.1016/j.jpowsour.2008.05.079
Wang SQ, Zhang JY, Chen CH (2010) Fe3O4 submicron spheroids as anode materials for lithium-ion batteries with stable and high electrochemical performance. J Power Sources 195:5379–5381. doi:10.1016/j.jpowsour.2010.03.035
Wu P, Du N, Zhang H, Yu J, Yang D (2011) Carbon nanocapsules as nanoreactors for controllable synthesis of encapsulated iron and iron oxides: magnetic properties and reversible lithium storage. J Phys Chem C 115:3612–3620. doi:10.1021/jp1113653
Zhang WM, Wu XL, Hu JS, Guo YG, Wan LJ (2008) Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv Funct Mater 18:3941–3946. doi:10.1002/adfm.200801386
Zhao X, Xia D, Zheng K (2012a) Fe3O4/Fe/carbon composite and its application as anode material for lithium-ion batteries. ACS Appl Mater Interfaces 4:1350–1356. doi:10.1021/am201617j
Zhao X, Xia D, Zheng K (2012b) An Fe3O4–FeO–Fe@C composite and its application as anode for lithium-ion battery. J Alloys Compd 513:460–465. doi:10.1016/j.jallcom.2011.10.081
Zhong Y, Wang X, Jiang K, Zheng JY, Guo Y, Ma Y, Yao J (2011) A facile synthesis and lithium storage properties of Co3O4/C hybrid core–shell and hollow spheres. J Mater Chem 21:17998–18002. doi:10.1039/c1jm12765a
Zhou Q, Zhao Z, Wang Z, Dong Y, Wang X, Gogotsi Y, Qiu J (2014) Low temperature plasma synthesis of mesoporous Fe3O4 nanorods grafted on reduced graphene oxide for high performance lithium storage. Nanoscale 6:2286–2291. doi:10.1039/c3nr05423c
Acknowledgments
This work was supported by the Natural Science Foundation of Xinjiang Province (2013211A004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, Y., Huang, Y., Wang, X. et al. One-pot synthesis of Fe3O4/C nanocomposites by PEG-assisted co-precipitation as anode materials for high-rate lithium-ion batteries. J Nanopart Res 16, 2614 (2014). https://doi.org/10.1007/s11051-014-2614-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2614-9