Abstract
The potential environmental impacts of engineered nanomaterials (ENMs), and their engineered nanoparticles (ENPs), have, in recent years, been a cause of concern. Life-cycle assessment (LCA) is a highly qualified tool to assess products and systems and has an increasing extent been applied to ENMs. However, still only 29 case studies on LCA of ENMs have been published in journals and this article investigates these studies. Generally, data on production of ENMs as well as the coverage of the life cycle are limited. In particular, within use and disposal stages data are scarce due to many unknowns regarding the potential release and fate of ENMs/ENPs to and in the environment. This study investigates the sensitivity of case studies with respect to ecotoxicity impacts through a quantification of the potential ecotoxicity impacts to algae, daphnia and fish as a result of direct release of Ag and TiO2 ENPs (mainly <200 nm in nominal diameter size) from various ENM products to the freshwater compartment. It was found that Ag and TiO2 release, from 1 g Ag or TiO2 ENM product, poses up to ca. 3.5 orders of magnitude higher ecotoxicity impact than the production of 1 g polymer (PP, PE and PET average) or 1 Wh of grid mix electricity from Scandinavia. ENMs from Ag had higher ecotoxic impact than those from TiO2 and there was a linear regression between Ag ENM content in the considered products and the potential ecotoxicity impacts to the freshwater species, according to release of total Ag during use (mainly washing).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanometer, the metric unit of length of a billion of a metre (10−9), has created a terminology for technologies that operate at the nanometer scale ranging from 0.1 to 1,000 nm, or 1 µm. Nanomaterials, also referred to as nanoobjects, by ISO (2008) and SCENIHR (2007), have been defined as materials with at least one dimension of 1–100 nm. According to ISO (2008), a nanoparticle needs to have all three dimensions within the 1–100 nm range. Nanomaterials and nanoparticles occur naturally in our surroundings as e.g. soil or salt particles and humans can be exposed to these on an everyday basis through air or food. Naturally occurring, or unintentionally produced, nanomaterials are known in general to cause little harm to humans (Buzea et al. 2007). Nanoparticles are also produced by activities as e.g. combustion. These anthropogenic particles have been an area of human health research for many years and are known to cause harm even though exact mechanisms are still being explored (Oberdörster et al. 2007). Engineered nanoparticles’ (ENPs) behaviour and potential impact to environment and humans are on the other hand to a larger extent unknown (Buzea et al. 2007; IITB 2012).
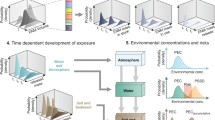
Through the life cycle of engineered nanomaterials (ENMs), i.e. from production of ENMs through their use and final disposal, their environmental and health impacts vary. A main cause of variation is the environmental exposure as a result of release of ENPs. This in turn depends on how ENMs are processed from raw materials and incorporated into a nanoproduct (commercial product containing ENMs) and how this product is affected by the surroundings in its lifetime (from use to disposal stage). The exposure is also a function of how the ENPs behave in the environment, prior to having an effect on biota and humans. To include the whole life cycle, for comparison and assessment of products and systems, life cycle thinking has been introduced (Som et al. 2010; Klöpffer et al. 2007), and one holistic albeit rigorous tool for that is Life-Cycle Assessment (LCA) (Fig. 1).
LCA framework and ENM release related impacts, based on USEtox™ (2012)
LCA of nanoproducts addresses all environmental impacts, but even though different studies have measured release from different products (e.g. Geranio et al. 2009; Künninger et al. 2010; Lorenz et al. 2012; Benn et al. 2010), there is currently no method available to quantitatively assess the environmental impacts deriving from ENM release. In chemicals risk assessment (RA), it is possible to identify the (eco) toxicological risk of a substance (provided the data are available) through calculating, measuring or modelling the exposure concentration and relates this to the effect concentrations observed in laboratory studies (Olsen et al. 2001; Grieger et al. 2012). LCA on the other hand is a relative impact assessment method (ISO 2006). The object of the assessment is a defined functional unit (quantified performance of a product system) making comparison between product systems possible but at the same time making it impossible to make risk estimates. Furthermore, a wide range of potential environmental impacts are taken into account. In theory, LCA can be applied to nanoproducts, but in practice the potential impacts of ENPs cannot be adequately assessed. A main challenge is that the physico-chemical characteristics of ENPs are not adequately understood in relation to environmental behaviour and effects, a challenge that is also faced by RA (Bauer et al. 2008; Grieger et al. 2012; Flemström et al. 2004).
Currently, a few reviews have been published on LCA of ENM products (Gavankar et al. 2012; Hischier and Walser 2012; Meyer et al. 2009; Upadhyayula et al. 2012). They addressed some of the observed barriers that need to be overcome in order to strengthen LCAs of ENMs (in particular, adequate and comprehensive life-cycle inventory data, especially for manufacturing and use, and the lack of data for nanospecific fate, transport and toxicity effects). Additionally, Grieger et al. (2012) reviewed the complementary use of RA and LCA, concluding that the approach is not mature for practical application but holds the potential to improve environmental assessment of ENMs. The reviews concluded that many studies performed until now are quite limited in their Life-Cycle Impact Assessment (LCIA). In comparison to the already performed reviews, this paper emphasises gaps of LCAs of ENM products with a focus on challenges for LCIA, mainly related to the potential toxicological impacts resulting from release of ENPs to freshwater.
Objectives of this paper
LCA of ENM products is still a rather new and developing application area for LCA and in this article we will review published scientific articles on LCAs of ENM products and systems (defined with ‘nano’ term as a prerequisite, not size). A particular focus is to identify and explain the challenges for LCIA, mainly those related to the ecotoxicological impact categories and to ENP behaviour in the environment. The potential release of ENPs and ENMs in the life cycle is also addressed, since this is considered central in the discussions of impacts from ENMs (e.g. Bauer et al. 2008). The main outcome of the review is an overview of the possibilities and limitations of LCA within this field, as well as of the challenges related to modelling of ENP behaviour in the freshwater environment. Finally, a sensitivity analysis is performed on Ag and TiO2 ENM products in order to estimate the potential ecotoxicological impacts of ENPs on freshwater species (algae, daphnia and fish)—an aspect that is missing in current approaches of LCIA of ENMs.
Life-cycle impact assessment and life-cycle assessment
LCA is a holistic, ISO 14040 series standardised, tool able to assess the potential environmental impacts of a product or a system throughout their entire life cycle (ISO 2006). Impact categories assessed include local (e.g. ecotoxicity), regional (e.g. acidification) and global (e.g. climate change, resource use) impacts. In LCIA, the impact potentials through the entire product life cycle must be considered in order to assess the overall environmental impact potentials (Wenzel et al. 1997). For each specific environmental impact category, contributing emissions are assessed using an environmental model to estimate the impact contribution compared to that of a reference amount of the substance. This relation is expressed as an equivalence factor, in LCIA terminology called a characterisation factor. The well-known example is CO2-equivalents for climate change impacts. Most LCIA methods are based on the following principles for calculation of environmental impacts (Wenzel et al. 1997; Guinée et al. 2002; Jolliet et al. 2003; European Commission Joint Research Centre (ECJRC) 2010):
The emission of a substance (i) has the magnitude of Qi and the characterisation factor for that substance to the environmental impact category (j) is CF(j)i. From this, the emissions potential contribution to the environmental impact is calculated (EP(j)i) and all emissions contributing to the impact category are summed (EP(j)) (Wenzel et al. 1997).
A main challenge in LCA of ENMs is how to derive the characterisation factors (CF) for ecotoxicological impacts of ENMs. The widely recognised USEtox™ model for deriving CFs on (eco-)toxicity is based on the general impact assessment framework (Eq. 2) (Rosenbaum et al. 2008). It considers a fate factor (FF) which is equal to the compartment specific residence time (in days) of a chemical. The exposure factor (XF) considered is in days−1 for human toxicity and unitless for ecotoxicity by indicating the dissolved and bioavailable fraction that biota is exposed to. Effects considered are expressed in the effect factor (EF) in cases/kg intake (EF) or in PAF m3/kg (PAF = potentially affected fraction). In ecotoxicology, XF can be neglected if the exposure is expected to be equal to the environmental concentration and the characterisation factor will then be FF × EF. The intake fraction (iF) for human toxicity expresses the fraction of released substance that will be taken in by humans. The derived characterisation factor (CF) is expressed in cases/kg emitted or in PAF m3/kg emitted (Rosenbaum et al. 2008):
The USEtox™ framework and calculation procedures could in theory be applied to derive CFs for ENMs. However, the USEtox™ framework is developed for organic chemicals and takes into account partitioning based primarily on octanol–water partitioning coefficient as well as degradation. So, for example, when applied to metals, the model fails to account for multiple and interconverting metal species and the sensitivity of metal-species distribution to ambient chemistry (Gandhi et al. 2010). Such issues will be relevant for ENMs as well and in order to calculate CFs for ENMs, the USEtox™ model needs to be adapted to model the fate of ENMs and take the aggregation and dissolution of ENPs into consideration. However, ENM behaviour in the environment is still not well understood, and data are not easily available (Lowry et al. 2012; Quik et al. 2011).
LCA has, still only to a limited extent, been applied to products containing ENMs and in the following the few published studies are reviewed. Almost none of the studies have directly considered fate of released ENMs due to the challenges described in “Difficulties in toxicological impact characterisation of ENPs” Section, where focus is on the LCIA challenges related to the physiochemical characteristics of ENMs and their behaviour in the environment.
Life-cycle assessment case studies of ENMs
In order to evaluate the current status of LCA application on ENMs and identify needs for improvement, a summary of published scientific articles containing LCA case studies performed on various products, components and systems containing ENMs are shown in Tables 1 and 2. The found case studies are divided into two tables addressing metal and their compounds, and carbon and composite materials case studies, respectively. The following interpretation addresses the strengths and weaknesses of these studies as well as the challenges still to be solved or even fully understood.
The identification of studies in Tables 1 and 2 was based on whether LCA was used. Some studies include life cycle thinking but do not directly claim to perform LCA. These have been included since they address one or several life-cycle stages, most often with a focus on energy consumption of ENM manufacturing.
Cradle-to-gate consideration
The case studies on metal, carbon and composite ENM products usually consider a cradle-to-gate LCA and focus on impacts caused by the energy-consuming manufacturing processes. The use and disposal stages, as well as the potential toxicity associated with the release of ENPs during the life cycle of the product, are most often neglected. Most studies in Tables 1 and 2 consider toxicity impacts, but only the case study from Walser et al. (2011) considers toxicity impacts from release of ENMs.
Some studies (Lloyd and Lave (2003), Lloyd et al. (2005), Babaizadeh and Hassan (2012), Manda et al. (2012), Roes et al. (2007), Steinfeldt et al. (2010a) and Walser et al. (2011)) consider the use and disposal stage, but the coverage of these stages is rather incomplete (excl. Walser et al. 2011). For example, Lloyd and Lave (2003) consider clay-polypropylene ENMs in car body panels and there they consider resource savings (fuel consumption) during the operation of a vehicle due to the lower weight of ENM, but no other supplementary materials are considered and thereby this study neglects the potential release of other agents in the use stage (Som et al. 2010). The disposal of ENMs is in general not properly dealt with due to lack of knowledge of whether end-of-life products would be landfilled, incinerated or recycled.
Functional unit
In order to perform a comparative LCA, the functional unit is central to ensure a comparable functionality of the two products. Studies as Joshi (2008), Kushnir and Sandén (2008), Grubb and Bakshi (2010) and others use a simplified functional unit relating just to the weight of the material, e.g. 1 kg of a certain material with ENMs. However, as also stated by Hischier and Walser (2012), a functional unit based on weight does not make sense in studies comparing ENMs with conventional materials, as functionality is not proportional with weight. If the use of ENMs results in improved functionality, this should be considered in the functional unit. ENM production tends to have high resource and energy use in the production stage (Lloyd and Lave 2003; Roes et al. 2007; Khanna et al. 2008), but an improvement in functionality can potentially justify the use of more resources in this stage in order to produce a better product with less environmental impact in the use stage.
For example, Roes et al. (2007) includes elasticity (Young modulus) and strength (tensile strength) in the functional unit when comparing PP/layered silicate nanocomposites with conventional PP, since the nanocomposite obtain the needed properties at a lower weight. In many LCAs of ENMs it may be difficult to identify the property that is the most important for defining the functional unit. To perform a fair LCA comparison, the functional properties must be central and it should be evaluated whether there is a need to scale the functional unit as Roes et al. (2007) does.
Inventory data
Production data of ENMs are scarce and the 29 studies in Tables 1 and 2 rely to a high extent on generic data e.g. those presented in Grubb and Bakshi (2010), Osterwalder et al. (2006), Tibbetts et al. (1994), Hwang et al. (2005) and Healy (2006), Healy et al. (2008). Primary process data of ENMs, coming from industry, are often not openly disclosed in this technology domain due to the relative novelty of the scientific field. This leads to a higher level of uncertainty as it needs to be covered by estimations and secondary and generic data, like the approach in Bauer et al. (2008), Joshi (2008), Merugula et al. (2010) and Isaacs et al. (2006). The lack of primary data limits the scope of the studies. Very often, the use and disposal stages of ENMs are unknown resulting in cradle-to-gate LCA study approaches, as seen in Tables 1 and 2.
Life-Cycle Inventory (LCI) date are crucial for making an LCA, and the relatively few case studies of ENMs underline the limited inventory data availability. In addition to the case studies presented in Tables 1 and 2, a number of scientific articles contribute to the slowly growing LCI. Some of the studies, contributing to the inventory, are Geranio et al. (2009), Köhler et al. (2008), Künniger et al. (2010), Som et al. (2011), Suppen et al. (2005), Durucan et al. (2006) and Gutowski et al. (2010). For example, Geranio et al. (2009) describes the release of Ag ENPs during textile washing, while Künninger et al. (2010) describes the nano-Ag release from facades due to weathering. There are several such studies that do not perform LCAs per se, but anyway present valuable LCI data that are needed when pursuing to do LCAs of ENMs and the products they are included in. These studies contribute in developing the needed inventory.
Impact hotspots
Currently, the conclusion, based on Tables 1 and 2, is that compared to their conventional counterpart products, the ENM products are more energy demanding and have an inferior cradle-to-gate environmental impact profile, e.g. in Khanna et al. (2007, 2008), where the production of nanofibres and polymer nanocomposites is more energy demanding than a steel product. On the other hand, Lloyd and Lave (2003) and Lloyd et al. (2005) show that the use stage for ENM products is more environmentally friendly than for their conventional counterpart products by assessing clay-polypropylene ENMs in car body panels and platinum-group metal particles in car catalysts, respectively. However, impacts such as potential ENP release are not considered. Gavankar et al. (2012) and Hischier and Wasler (2012) conclude that the assessment of nano-specific impacts must be developed but in order to do so the level of understanding of ENM behaviour, exposure and effects must be raised substantially.
Overall findings
On the basis of the 29 found studies, certain tendencies can be identified reflecting the current state of knowledge regarding LCA of ENMs:
-
Usual cradle-to-gate or manufacturing system boundary consideration.
-
Use and disposal life-cycle stages are poorly covered.
-
Common use of generic life-cycle inventory (LCI) data and assumptions.
-
Almost no consideration of release of ENMs (e.g. in the use or disposal stages) and the potential toxic impacts of these (fate, exposure and effect consideration). Walser et al. (2011) is an exception.
-
Cradle-to-gate LCA comparison of counterpart products (with ENMs and without) shows that ENM products are more energy demanding and, therefore, have a worse cradle-to-gate environmental profile, e.g. in polymer nanocomposites vs. steel and socks with and without nano (Moign et al. (2010); Meyer et al. 2010).
-
Cradle-to-grave LCA comparison of counterpart products (with ENMs and without) shows that the use phase is better for ENM products as usually an improved functionality is achieved, e.g. comparing clay-propylene nanocomposites with steel or aluminium in light-duty vehicles (Osterwalder et al. 2006).
Release of ENMs and assessment of their impacts contain a degree of complexity that makes them difficult to grasp scientifically and to include in a LCA approach. In the following, focus will be on the challenges related to assessment of the toxicological impacts, and afterwards a sensitivity analysis is performed on the potential toxicological impacts from release of ENMs/ENPs during use of products.
Difficulties in toxicological impact characterisation of ENPs
The potential toxicological impact of ENMs depends on the possible release of ENPs during an ENM products life, on their environmental fate and their potential effect when penetrating into living organisms. There is currently a lack in understanding of fate, exposure and effect of released ENPs in the environment—which is problematic especially in the freshwater compartment, as it is a common recipient (Quik et al. 2011; Lowry et al. 2012; Som et al. 2010). Some important parameters are particle appearance, transport, transformation and physico-chemical characteristics of ENPs. Current knowledge on these will be summarised below.
Particle appearance
Nanoparticles tend to agglomerate (coagulate), aggregate (fuse) or a combination thereof (e.g. like carbon black and TiO2), see Fig. 2. The bonding and interaction happen in order to reduce the high-surface energy. The interaction between two particles, in liquid and air, can in general be described by forces of van der Waals attractions and electrostatic repulsions (Rupasinghe R-A-TP 2011). The particle appearance influences the toxicity of ENMs in water (Oberdörster et al. 2007).
Primary particle (ENP) appearance. Reproduced from Oberdörster et al. (2007)
Transformation
The transformation upon release into freshwater environment can be performed biotically (interaction with plants, water flea, fish etc.) or abiotically (interaction with water, sand, light, etc.) and can alter shape, size, surface chemistry and ultimately the fate of ENMs. Physico-chemical properties of ENMs define their differentiated behaviour, and the processes considered important for ENMs after release to water are (Vonk et al. 2009; Lowry et al. 2012):
-
Dissolution
-
Change in surface structure of ENMs/ENPs
-
Aggregation/agglomeration
-
Sedimentation
ENMs commonly tend to sorb to high-surface-area colloids and then aggregate/agglomerate and sediment (Klaine et al. 2008).
Transport
Transport is partially controlled by the aggregation/agglomeration and subsequent deposition/sedimentation of ENMs. Aggregation of ENMs in the environment will depend on these parameters (Lowry and Casman 2009; Lowry et al. 2012):
-
Hydrophobicity
-
Chemical bonding between nanoparticles
-
Ionic strength
-
Ionic composition
The ionic strength, which is higher in marine than freshwaters, and pH and the presence of divalent cations such as Ca2+ and Mg2+ will influence the rate and extent of aggregation/agglomeration (Lowry and Casman 2009). Brant et al. (2005) suggest that if C60 fullerenes are released into natural waters with an ionic strength higher than 0.001 M, these may form large aggregates/agglomerates that will sorb to other particles or media and eventually become immobilised.
Important physico-chemical characteristics of ENPs
Based on the developing understanding of ENM fate in e.g. freshwater it seems that the following characteristics are important to consider (Batley and McLaughlin 2010) (Klaine et al. 2008):
-
Chemical composition
-
Mass
-
Particle number and concentration
-
Surface area concentration
-
Size distribution
-
Specific surface area
-
Surface charge/zeta potential
-
Surface contamination and the nature of any shell and capping
-
Solubility
-
Crystal structure
The natural conditions of the environment are also important along with the common fate consideration of aggregation/agglomeration and dissolution. However, these and other co-related mechanisms especially in water are neither fully understood nor well represented with characterisation data (Farré et al. 2011). An overview is shown in Fig. 3.
Toxicity
The toxic effect of ENP depends on a number of parameters, e.g. size, dissolution, surface structure and aggregation/agglomeration. Variation in ENP size does show difference in toxicity, e.g. Zhu et al. (2009) stated that a 48 h test showed a 143 mg/L LC50 on Daphnia magna from <20 nm TiO2 ENMs, while Heinlaan et al. (2008) observed a 48 h LC50 of 20,000 mg/L on Daphnia magna from 25 to 75 nm TiO2 in water. These indicate the variation in toxicity between different sizes of tested ENMs, but still different tests are difficult to compare due to variations in test conditions. Kashiwada (2006) also showed that particle size has an effect on accumulation of fluorescent nanoparticles in the Japanese medeka (Oryzias latipes), as smaller particles accumulate rapidly. On cell level, Hussain et al. (2009) showed that there is a size-dependent cellular interaction of silver nanoparticles in alveolar macrophages, and the cytotoxicity was 10 times higher for ENM particles of 15 nm than for those of 30 nm. The crystal structure of ENM materials also plays a role, e.g. TiO2 in anatase crystal structure form is more toxic to organisms than the rutile form (Hall et al. 2009; Wang et al. 2008).
During dissolution, ENMs become smaller and may completely dissolve, depending on material and environmental conditions, which may influence the toxicity significantly. The toxicity, in the case of Ag ENMs released to freshwater, will depend on the intrinsic toxicity potential of ENMs and the ions formed through oxidative dissolution (Scheringer et al. 2010). In continuation, Scheringer et al. (2010) mentions that the high toxicity potential of free Ag ions in natural waters may be disrupted by the presence of complexing ligands, as they will reduce the silver ion concentration and the related bioavailability of those. The toxicological effects are also related to change in surface structure of ENPs which may be caused by removal/alteration of the coating. Change in surface structure, e.g. by natural and anthropogenic chemicals in the environment, may result in enhanced mobility, bioavailability, aggregation (mainly hydrophobic surfaces), sedimentation, dissolution and dispersion (mainly hydrophilic surfaces), and consequently the actual exposure and toxicity may increase (Vonk et al. 2009; Lowry and Casman 2009). Also, solution pH and the presence of adsorbing molecules and ions have an influence. The closer the pH is to the isoelectric point, the particle charge becomes lower and a change in the repulsive forces is able to promote aggregation/agglomeration (Franklin et al. 2007; Illés and Tombácz 2006). The correlation of these processes, in contrast to single-chemical behaviour, means that single-chemical impact models are usually not suitable to use for ENMs (Lowry and Casman 2009).
After aggregation, the gravitational forces cause sedimentation and ENMs are removed from the water layer and the material is less available to certain water organisms, but more to the benthic organisms in the water environment (Klaine et al. 2008; Lowry et al. 2012). Turbulent motion in benthos and bio-turbulation of sediments can cause re-suspension and make the material more available to organisms in the water layer (Klaine et al. (2008).
Sensitivity analysis—potential ecotoxicological impacts from Ag and TiO2 ENM products
The few LCA studies that mention the release of ENPs, mainly in the use phase, have not taken the step further towards assessment of the effects, e.g. Meyer et al. (2010). Only Walser et al. (2011) takes this step even though a nanomaterials specific impact characterisation factor was not calculated. Meyer et al. (2010) concludes that ENPs are released from Ag ENM-fabrics during washing, and Geranio et al. (2009) quantifies the potential release of silver during washing. However, the potential impact of the release is not assessed and that is the aim of the sensitivity analysis in this study. In a LCA perspective, the effect factor (EF) should be applied along with the fate factor and exposure factor in order to derive the characterisation factor (see Eq. 2). Due to the challenges in modelling the fate and exposure, described in “Difficulties in toxicological impact characterisation of ENPs” Section, a scenario of direct emission to freshwater with subsequent exposure has been made in this sensitivity analysis. Direct emission to freshwater is a worst-case scenario, while the fate factor (FF) set to 1 day is a best-case scenario due to the short substance residence time in freshwater. The EF for Ag and TiO2 ENM was calculated from the potentially affected fraction (PAF) of species (Larsen and Hauschild 2007a, b). In comparative assessments like LCA, the effect factors should be based on HC50 (hazardous concentration for 50 % of the species) and PAF (Rosenbaum et al. 2008; Larsen and Hauschild 2007a, b):
HC50 values
HC50 is defined as the hazardous concentration in kg/m3 at which 50 % of species in an aquatic environment are exposed to a concentration above their EC50. As normally in standardised testing, three trophic levels are included and it is recommended that at least three toxicity values are available at each trophic level (Rosenbaum et al. 2008; Larsen and Hauschild 2007a, b). In this study, the HC50chronic values are based on chosen EC50acute values according to standards from Rosenbaum et al. (2008) and Larsen and Hauschild (2007a, 2007b). In the supporting information, in Tables 3 and 4, the calculation of HC50EC50 for Ag and TiO2 ENMs (mainly <200 nm diameter size, see Tables 3 and 4 in supporting information) from freshwater species data for three trophic levels is shown, according to Larsen and Hauschild (2007a, 2007b) and Rosenbaum et al. (2008).
Freshwater species sensitivity distribution
Following the derived HC50EC50 geometric mean results for Ag and TiO2 ENM, the aquatic species sensitivity distributions can be derived, where C is the concentration of Ag or TiO2 ENM, α is the average chronic logHC50 for Ag or TiO2 ENM and calculated on the basis of Tables 3 or 4 (supporting information), β is the scale parameter and σ is the standard deviation of HC50EC50 values in Tables 3 or 4 (supporting information) (Larsen and Hauschild 2007a, b):
The PAF curve is thus derived and shows the species sensitivity, on the basis of Tables 3 and 4 from supporting information, in freshwater in relation to ENM Ag and TiO2 concentrations (Figs. 4, 5).
Freshwater characterisation factor
The PAF curves show that in order to affect 50 % of species in freshwater, the concentration must be 5.83E − 5 kg Ag ENM/m3 and 1.91E − 2 kg TiO2 ENM/m3 (HC50chronic). Hereby, Ag ENMs are considered more toxic towards the freshwater species than TiO2 ENMs, in accordance to e.g. Klaine et al. (2008). From the HC50chronic, the effect factor (EF) can be derived with the aim of generating characterisation factors (CF) expressing the PAF resulting from release of 1 kg of Ag or TiO2 ENM. As mentioned in "Life-cycle impact assessment and life-cycle assessment" Section, the exposure factor (XF) is neglected for ecotoxicity and the fate factor (FF) is due to severe challenges (mainly many unknowns) simplified to 1 day assuming rapid transformation, aggregation and sedimentation of ENMs:
Characterisation factors applied on Ag and TiO2 ENM case studies
The derived characterisation factors, in Freshwater characterisation factor Section, for Ag and TiO2 ENM were applied to the release in the case studies (case studies details can be seen in supporting information). These studies represent the actual release, achieved through laboratory testing. The release is based on the total silver or titanium(-dioxide) content, and thus may not be in ENM or ENP size definition (ISO 2008; SCENIHR 2007). The release is assumed to be in the ENM size, even if not all may be within that range, and the release is assumed to directly being led to freshwater environment. Thus, this scenario represents the worst case as it is based on total Ag and TiO2 content released, and assumes that washing water will be led directly to a freshwater recipient without passing a waste-water treatment facility. Tables 5 and 6, in the supporting information, summarise the results, comprising potential release and characterised ecotoxicity impact results from ENM products.
Figures 6 and 8 show the characterised impact results, also seen in the last column in Tables 5 and 6 in the supporting information. The ecotoxicity impact from 1 g of Ag ENM products varies within the range of 1E − 7 to 1E4 PAF*m3*day, dependent on the amount released from the products. For Ag ENM, according to the linear regression, the ecotoxicity impact is 1E − 6 PAF*m3*day per µg Ag ENM content in the products (Fig. 7).
Ecotoxicity impact per gram of Ag ENM product and also compared to non-ENM commodities as ecotoxicity impact per gram of polymer (PP, PE, PET European (RER) average, Ecoinvent v2.2 and Buwal database) and per Wh electricity generated (Scandinavian countries grid mix average, Ecoinvent v2.2 database). Based on Table 3 and 5 from supporting information (see also Figs. 4 and 6). Cross (+) indicates the geometric mean
The TiO2 ENM ecotoxicity impact, per gram product, is within the range of 2E − 6 to 4E − 5 PAF*m3*day, while the linear regression based on these products indicates an increase of 2E − 9 PAF*m3*day per µg TiO2 ENM content in product.
The conclusion based on the Ag and TiO2 ENM case studies and the ENM characterisation factors is that the ecotoxicity impact is higher for Ag than for TiO2 cases. This can be seen from the derived characterisation factors in “Freshwater characterisation factor” Section and the impacts displayed in Figs. 6 and 8, as these show a higher potential ecotoxicity impact to freshwater organisms (the ones included in Tables 3 and 4 that can be seen in the supporting information). Also, as seen in Fig. 6, there is a linear regression for Ag cases, while for TiO2 in Fig. 8 this cannot be concluded (based on only 11 release cases found). This means, for Ag cases, that the content of Ag ENMs in products is determining for amount of Ag released (mainly due to washing), and consequently to the ecotoxicity impacts to the freshwater species.
In Fig. 7, it is shown for Ag ENM products that the most common products (textiles, socks and shirts) represent the highest impacts, except for the medical mask and cloth that represents only two datasets. These impacts, only originating from the Ag release, are ca. 1–3.5 orders of magnitude higher according to median (excl. baby plush toys & baby blanket) than the impacts from production of 1 g PP, PE and PET polymer average or 1 Wh of grid mix electricity from Scandinavia. The polymer and electricity production data were attained from Ecoinvent v2.2 and Buwal database, and the ecotoxicity impacts were derived through Impact 2002+ LCA method (Jolliet et al. 2003). The ecotoxicity impact potential from the polymer and energy production originates from the entire production chain, in contrast to the ENM products where the accounted impacts are based solely on the release of Ag in the use-stage (see Fig. 7). These are provided in order to place the potential impacts of released Ag ENM in perspective. Even though assumptions are made in order to quantify the impact potential from e.g. washing of t-shirts containing different Ag ENM textiles, it should still be clear that the release will occur (Geranio et al. 2009) and the related freshwater impacts will be created if the waste-water treatment is not suited for the complete removal of Ag ENM. Also, impacts of Ag ENM in freshwater could be related to dissolution into silver ions as according to Ratte (1999) the silver ion (Ag+) is defined as the most toxic form of silver in water. Silver speciation commonly occurs in freshwater with sulphide and chloride and this reduces the silver bioavailability, but even though this is the most realistic fate, the toxicity studies are quite simple and may to a lesser extent include speciation in the laboratory setup for toxicity measurements to freshwater organisms (Scheringer et al. 2010; Fabrega et al. 2011; Jones and Grainger 2009). Due to difficulties in empirically testing different scenarios, biological models have been introduced and one of those is the Free Ion Activity Model (FIAM) and the Biotic Ligand Model (BLM) used to predict acute metal toxicity to aquatic vertebrates and invertebrates (Fabrega et al. 2011). The main issue with such a model is that it is equilibrium based and not always suitably models what happens in real life (Slaveykova and Wilkinson 2005; Fabrega et al. 2011). Further, it is unknown how suitable some of the assumptions are for ENMs, e.g. dominance of free ion activities in determining bioavailability, aqueous phase chemistry is at equilibrium and that uptake and flux across the membrane is rate limiting (Fabrega et al. 2011).
Regarding TiO2 ENM products, as seen from the boxplot in Fig. 9, the t-shirts washed 10-times have higher ecotoxicity impact due to higher release, than e.g. after one wash. The impacts, only originating from the TiO2 release, are ca. 1–2 orders of magnitude higher (median) than the impacts from production of 1 g PP, PE and PET polymer average or 1 Wh of grid mix electricity from Scandinavia. The release of Ag or TiO2 in the use stage has in almost all cases higher impacts than the total ecotoxicity impacts from production of 1 g polymer (PP, PE and PET average) and 1 Wh of Scandinavian electricity (grid mix). This sensitivity underlines the need to consider the release of ENPs and/or ENMs during use, solely due to potential ecotoxicity impacts.
Ecotoxicity impact per gram of TiO2 ENM product and also compared to non-ENM commodities as ecotoxicity impact per gram of polymer (PP, PE, PET European (RER) average, Ecoinvent v2.2 and Buwal database) and per Wh electricity generated (Scandinavian countries grid mix average, Ecoinvent v2.2 database). Based on Tables 4 and 6 from supporting information (see also Figs. 5 and 8). Cross (+) indicates the geometric mean
According to Jones and Grainger (2009), the main hurdle is still to model and predict the actual fate of ENPs when they are released to the environment since more products with ENMs are introduced to the consumer market. For example, if more washable products contain Ag ENM, the actual hazard may be higher in terms of PAF, but this of course also depends on the actual fate of ENMs as described in “Difficulties in toxicological impact characterisation of ENPs” Section.
Limitations of LCA of ENMs—what needs to be considered
In order to perform an LCA of ENMs, certain aspects need to be considered and some are more important than others. As described in “Life Cycle Assessment case studies of ENMs” and “Difficulties in toxicological impact characterisation of ENPs” Section, the potential ENP/ENM release is commonly not included in LCAs. This is partly due lack of knowledge about the release, partly due to a missing understanding of how to quantify the ecotoxicity as a function of particle release from ENM products during its life cycle.
Figure 10 illustrates LCA limitations regarding the impact assessment of ENMs. The limitations are linked to the limited knowledge on fate and effects of released ENPs. Currently, the LCAs performed on ENMs disregard the potential (eco-)toxicity impacts of ENP release to environmental compartments. In order to include these, there is a need to develop characterisation factors in collaboration with the RA field. Currently, if aiming at performing an LCA one should be aware of the following:
-
Goal and scope: If possible, consider the whole life cycle and set goals according to LCA possibilities (see Fig. 10). The functional unit needs to take into account the functionality and property differences of the product when using ENMs.
-
LCI: Data are difficult to acquire, so either collaborate with the industry or base the data on already published studies and generic processes from databases as e.g. Swiss Ecoinvent.
-
LCIA: A completely holistic impacts assessment cannot be performed, mainly due to the unanswered questions regarding accounting of ENM/ENP release and the related impacts (Som et al. 2010; Bauer et al. 2008):
-
How much ENM/ENP is released to environmental compartments (e.g. water) and technosphere (e.g. waste-water treatment)?
-
Which exposure to ENMs/ENPs occur in the environment and what are the effects on biota and humans?
-
At different times, what appearance (size, shape and composition) do the ENMs/ENPs take in the environment (primary particles (ENPs), agglomerated ENPs, aggregated ENPs, agglomerated aggregates)?
-
What are the environmental consequences from different end-of-life treatment of ENM products?
These impact assessment-related problems need to be uncovered and solutions to these included in future LCA. The general approach, as mentioned previously, will be to include RA developments in LCA, so fate and end-point effects can be quantified.
Conclusion
Currently, the application of LCA of ENMs is not well developed and there are different reasons for this. Firstly, due to the relative novelty of ENMs, the inventory data available are limited making it difficult to establish the actual inventory data of ENM raw material extraction and production. Secondly, the potential release of ENP during the life cycle is not yet well investigated (partly due to measuring difficulties). Finally, after release of ENMs to the environment, there is still an incomprehensive understanding of processes controlling the fate and effect of released ENPs. In this context, only one study managed to include impacts related to ENM release in their LCA of a nanoproduct, although a nanomaterial specific impact characterisation factor was not calculated (Walser et al. 2011). To make LCA more robust when assessing ENMs the factors controlling transport, transformation, fate (e.g. aggregation and deposition) and effect of released ENPs must be uncovered and corresponding LCA environmental impact characterisation factors should be developed. Due to constraints of unknowns and time the prospect is to develop simplified models that may serve in preliminary fate and effects assessments of released ENMs. There is also a need to develop a better LCI database for ENMs, as Bauer et al. (2008) also underlines.
The conclusion of the sensitivity analysis underlines the need to consider this potential impact source during a products lifetime, as the quantified ecotoxicity impacts in this study for Ag and TiO2 ENM products are higher compared to other frequently applied processes such as polymer production (European PP, PE and PET average) and electricity (Scandinavian grid mix). Further, Ag ENMs, compared to TiO2 ENMs, contribute to a higher ecotoxicity to freshwater algae, daphnia and fish. The ecotoxicity impacts originating from the Ag and TiO2 release from Ag and TiO2 ENM product, pose up to ca. 3.5 orders of magnitude higher (median) ecotoxicity impact for Ag and ca. 1–2 orders of magnitude higher (median) ecotoxicity impact for TiO2 than production of 1 g polymer (European PP, PE and PET average) or 1 Wh of grid mix electricity from Scandinavia. Additionally, for Ag ENM products, a linear regression was observed between Ag ENM content in the products and the release of total Ag during use (mainly washing), consequently leading to potential ecotoxicity impacts to algae, daphnia and fish freshwater species.
Some may question the need to apply LCA on ENM products, but LCA has been proven as one of the only holistic tools to understand the overall impact of products and systems—meaning that it covers broadly in terms of including the whole life cycle, all potential impacts and their range of categories. Thus, LCA has good potential to be developed into a robust tool for assessing a wide range of impacts deriving from ENM product life cycles. Based on the review, we observe that LCAs have been applied on ENM products, but so far mainly to assess the accountable production-related emissions. Thus, we find that the future aims should be to further map, develop and validate the application of LCA of ENMs and, importantly, collect inventory data and develop impact characterisation factors to further the assessment of potential environmental and toxicological impacts from released ENMs/ENPs. To supplement this massive task that lies ahead, the sensitivity analysis performed in this study on Ag and TiO2 identifies some of the aspects needed in development of impact characterisation factors, but the approach to calculate, i.e. the freshwater fate factor for each ENM especially needs more attention. In this sense, LCA should be used with caution as release, fate and effect of ENM/ENP is currently not properly accounted for and the actual environmental implications not enough investigated.
References
Althaus H-J, Chudacoff M, Hellweg S, Hischier R, Jungbluth N, Osses M, Primas A (2003) Life Cycle Inventories of Chemicals. Final report ecoinvent 2000 No. 8. EMPA Dübendorf, Swiss Centre for Life Cycle Inventories, Dübendorf, Zürich, Switzerland. http://www.poli.br/~cardim/PEC/Ecoinvent%20LCA/ecoinventReports/08_Chemicals.pdf. A slightly newer version from 2007, Accessed 5 October 2013
Amatayakul W (1999) Life cycle assessment of a catalytic converter for passenger cars. Master Thesis. Dept. of Chemical Environmental Science Chalmers University of Technology. Goteborg. Sweden
Amatayakul W, Ramnäs O (2001) Life cycle assessment of a catalytic converter for passenger cars. J Clean Prod 9(5):395–403(9)
Andersen PJ, and Ballinger TH (1999) Improvements in Pd:Rh and Pt:Rh three way catalysts. Society of Automotive Engineers Technical Paper 1999-01-0308. doi:10.4271/1999-01-0308
Angellier H, Choisnard L, Molina-Boisseau S, Ozil P, Dufresne A (2004) Optimization of the preparation of aquwous suspensions of waxy maize starch nanocrystals using a response surface methodology. Biomacromolecules 5:1545–1551
APME (2000) Data collected by Boustead consulting: ecoprofiles of chemicals and polymers. APME Brussels.
Aridi TN, Al-Daous MA (2009) HDS of 4,6-dimethyldibenzothiophene over MoS2 catalysts supported on macroporous carbon coated with aluminosilicate nanoparticles. Appl Catal A 359(1–2):180–187
Babaizadeh H, Hassan M (2012) Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr Build Mater 40(March):314–321
Ballari MM, Hunger M, Hüsken G, Brouwers HJH (2010) NOx photocatalytic degradation employing concrete pavement containing titanium dioxide. Appl Catal B 95(3–4):245–254
Bartley G, Bykowski B, Welstand S, Lax D (1999) Effects of catalyst formulation on vehicle emissions with respect to gasoline fuel sulfur level. Society of Automotive Engineers Technical Paper 1999-01-3675. doi:10.4271/1999-01-3675
Batley EG, McLaughlin JM (2010) Fate of manufactured nanomaterials in the Australian environment. CSIRO Niche Manufacturing Flagship Report. Department of the Environment. Water. Heritage and the Arts. http://www.environment.gov.au/system/files/pages/371475a0-2195-496d-91b2-0a33f9342a6d/files/manufactured-nanomaterials.pdf. Accessed 5 October 2013
Bauer C, Burchgeister J, Hischier R, Poanietz WR, Schebek L, Warsen J (2008) Towards a framework for life cycle thinking in the assessment of nanotechnology. J Clean Prod 16(8–9):910–926
Benn T, Cavnagh B, Hristovski K, Posner JD, Westerhoff P (2010) The release of nanosilver from consumer products used in the home. J Environ Qual 39(6):1875–1882
Berghaus JO, Legoux JG, Moreau C, Terasi F, Chrâska T (2007) Mechanical and thermal transport properties of suspension alumina-zirconia composite coatings. J Therm Spray Technol 17(1):91–104
BLS (2011) Discount rate of nano TiO2 and glass window material. U.S. Bureau of Labor Statistics. http://www.bls.gov/cpi/home.htm. Accessed 11 November 2013
Brant J, Lecoanet H, Wiesner M (2005) Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanopart Res 7(4–5):545–553
Braun J, Hauber T, Többen H, Windmann J, Zacke P, Chatterjee D, Correa C, Deutschmann O, Maier L, Tischer S, Warnatz J (2002) Three-dimensional simulation of the transient behavior of a three-way catalytic converter. Society of Automotive Engineers Technical Paper 2002-01-0065. doi:10.4271/2002-01-0065
Brisley RJ, Collins NR, French C, Morris D, Twigg MV (1999) Development of advanced platinum-rhodium catalyst for future emissions requirements. Society of Automotive Engineers Technical Paper 1999-01-3627. doi:10.4271/1999-01-3627
Burch SD, Keyser MA, Colucci CP, Potter TF, Benson DK, Biel JP (1996) Applications and benefits of catalytic converter thermal management. Society of Automotive Engineers Technical Paper 961134. doi:10.4271/961134
Buzea C, Blandino Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: sources and toxicity. Department of Physics. Gastrointestinal Diseases Research Unit & Department of Physiology. Queens University at Kingston General Hospital. Kingston. Ontario. Canada. http://arxiv.org/pdf/0801.3280v1. Accessed 20 June 2013
Capello C, Hellweg S, Badertscher B, Betschart H, Hungerbühler K (2007) Environmental assessment of waste-solvent treatment options. J Ind Ecol 11(4):26–38
Carnegie Mellon (2008) Economic input-output life cycle assessment (EIO-LCA). Carnegie Mellon University Green Design Institute (http://www.eiolca.net)
Cetinkunt S (2007) Mechatronics. John Wiley and Sons. Inc., Hoboken
Cetinkunt S (2008) Mechanical engineering. Personal Communication. University of Illinois, Chicago (done by Sengül & Theis (2010))
Chafik TOD, Gass JL, Bianchi D (1998) Heat of adsorption of carbon monoxide on a Pt/Rh/CeO2/Al2O3 three-way catalyst using in-Situ infrared spectroscopy at high temperatures. J Catal 179(2):503–514
Chatterjee D, Deutschmann O, Warnatz J (2001) Detailed surface reaction mechanism in a three-way catalyst. Faraday Discuss 119:371–384
Cheng F, Kelly SM (2010) Production of TiO2 by chemical precipitation, Personal communication by Manda et al. (2012). University of Hull. United Kingdom
Chilson M (2008) Telephone and E-mail Communication. ME Baker Inc. (done by Sengül & Theis (2010))
Consumersearch (2009) Laundry detergent: full report. http://www.consumersearch.com/laundry-detergent/best-laundry-detergent. Accessed 5 October 2013
Corbiere-Nicollier T, Laban BG, Lundquist L, Leterrier Y, Manson J-AE, Jolliet O (2001) Life cycle assessment of biofibres replacing glass fibres as reinforcement in plastics. Res Cons Recycl 33(4):267–287
Cornelius SJ (2001) Modelling and Control of Automotive Catalysts. Ph.D. Dissertation. Sidney Sussex College. University of Cambridge. United Kingdom. http://library.certh.gr/libfiles/PDF/EKETA-1325-MODELING-AND-CONTROL-AUTOMOTIVE-CATALYSTS-CAM-AC-UK-Y2001-PP182-PhD-Thesis.pdf. Accessed 13 June 2013
Dahlben LJ, Isaacs JA (2009) Environmental assessment of manufacturing with carbon nanotubes. International symposium on sustainable systems and technology 2009 (ISSST). IEEE International Symposium on, vol., no., pp.1,5, 18–20 May 2009. doi: 10.1109/ISSST.2009.5156767
Dahlben LJ, Eckelman Jensen M, Hakimian A, Somu S, Isaacs JA (2013) Environmental life cycle assessment of a carbon nanotube-enabled semiconductor device. Environ Sci Technol 47(15):8471–8478
Dean KA, Coll BF, Talin AA, von Allmen PA, Wei Y, Rawlett AM et al (2005) Field emission display and methods of forming a field emission display. U.S. Patent 7,070,472 B2. Filed Oct. 25 2004, issued Jul. 4, 2006
Defra (2004) Environmental reporting guidelines for company reporting on greenhouse gas emissions. Department for Environment Food and Rural Affairs (DEFRA). United Kingdom. http://www.thecarbontrust.co.uk/energyCMS/CarbonTrust/pages_preview/page_64.asp. Accessed in 2005 (by Osterwalder et al. 2006)
Demesne (2009) Appliance life expectancy: how long should an appliance last? http://www.demesne.info/Home-Maintenance/Appliance-Life-Expectancy.htm. Accessed 5 October 2013
Deorsola FA, Russo N, Blengini GA, Fino D (2012) Synthesis, characterization and environmental assessment of nanosized MoS2 particles for lubricants applications. Chem Eng J 195–196:1–6
DOE (1994) Voluntary reporting of greenhouse gases under section 1605(b) of the energy policy act of 1992: general guidelines. U.S. Department of Energy, Washington
DOE (2011) U.S. Department of energy. Industrial assessment centers database. http://iac.rutgers.edu/database/assessments/. Accessed 13 November 2013
Doka G (2003) Life cycle inventories of waste treatment services. Ecoinvent report No 13. EMPA Dübendorf, Swiss Centre for Life Cycle Inventories, Dübendorf, Zürich, Switzerland. http://www.doka.ch/13_I_WasteTreatmentGeneral.pdf. Accessed 5 October 2013
Dornburg V, Lewandowski I, Patel M (2004) Comparing the land requirements, energy savings, and greenhouse gas emissions reduction of biobased polymers and bioenergy. J Ind Ecol 7(3–4):93–116
Durfee DJ, Tomlinson JJ (2001) Boston washer study. Energy Division. Oak Ridge National Lab. Oak Ridge. http://www.ornl.gov/~webworks/cppr/y2002/rpt/112217.pdf. Accessed 13 June 2013
Durucan S, Korre A, Munoz-Melendez G (2006) Mining life cycle modelling: a cradle-to-gate approach to environmental management in the minerals industry. J Clean Prod 14(12–13):1057–1070
Duyvesteyn WP, Spitler TM, Sabacky BJ, Prochazka J (2000a) Processing aqueous titanium chloride solutions to ultrafine titanium dioxide. U.S. Patent 6440383. filed Feb. 14, 2000, issued Aug. 27, 2002
Duyvesteyn WP, Sabacky BJ, Verhulst DEV, West-Sells PG, Spitler TM, Vince A, Burkholder JR, Huls BJPM (2000b) Processing titaniferous ore to titanium dioxide pigment. U.S. Patent 6375923. filed Feb. 7, 2000, issued April 23, 2002
Duyvesteyn WP, Spitler TM, Sabacky BJ, Vince A, Prochazka J (2000c) Processing aqueous titanium solutions to titanium dioxide pigment. U.S. Patent 6548039. filed Feb. 14, 2000, issued April 15, 2003
Ekchian JA, Balles EN, Christeller DL, Cowart JS, Fuller WD (1999) Use of non-thermal plasma generated by a corona discharge device (CDD) to improve the efficiency of a 3-way catalyst. Fuel economy & after-treatment development session. Proc Glob Powertrain Congr Stuttg, Ger 9:1–12
Energy Efficiency Office (1993) Energy consumption guide 31; the moulding of thermo-plastic containers by the extrusion-blow moulding process. Department of the Environment. Energy Efficiency Office
Etchart-Salas R (2007) Suspension plasma spraying. Analytical and experimental approach of the phenomena imply in the reproducibility and the quality of the deposits. PhD thesis No. 50–2007. University of Limoges
European Commission (EC) (2001) Integrated pollution prevention and control: reference document on best available techniques in the pulp and paper industry. European Commission. http://www.umweltbundesamt.de/sites/default/files/medien/419/dokumente/bvt_zellstoff-papierindustrie_zf_0.pdf. Accessed 16 January 2013
European Commission Joint Research Centre (ECJRC) (2010) ILCD handbook: general guide for life cycle assessment—detailed guidance. European Commission. Joint Research Centre. Institute for Environmental and Sustainability, European Union
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37(2):517–531
Fan Y, Cheng H, Wei Y, Su G, Shen Z (2000) Tailoring the diameters of vapor-grown carbon nanofibers. Carbon 38(6):921–927
Farré M, Sanchís J, Barceló D (2011) The fate and the behavior of nanomaterials in the environment, Analysis and assessment of the occurrence. TrAC, Trends Anal Chem 30(3):517–527
FEA (2001) Large Volume Solid Inorganic Chemicals. Titanium Dioxide. Final Report. Institute for environmental technique and management. Federal Environmental Agency (Germany-Austria). European Commission .http://www.google.dk/url?qwww.prtr-es.es/data/images/BREF%2520Industria%2520Qu%25C3%25ADmica%2520Inorg%25C3%25A1nica%2520de%2520gran%2520volumen%2520de%2520producci%25C3%25B3n%2520(s%25C3%25B3lidos%2520y%2520otros%2520productos)-02FDB2732F82B5AE.pdf&saeiAI0U7SfM6SN4ATjtYHgBw&vedusg. Accessed 22 May 2013
Fiengo G, Glielmo L, Santini S, Caraceniz A (2004) A Fault Diagnosis Algorithm for Three-Way Catalytic Converters. http://www.ing.unisannio.it/fiengo/Download/Pers/Avec2000.pdf. Accessed 14 February 2013
Finegan IC, Tibbetts GG, Glasgow DG, Ting JM, Lake ML (2003) Surface treatments for improving the mechanical properties of carbon nanofiber/thermoplastic composites. J Mater Sci 38(16):3485–3490
FIRE (2011) Factor information retrieval (FIRE). online software. http://cfpub.epa.gov/webfire/. Accessed 11 November 2011
Flemström, Carlson R, Erixon M (2004) Relationships between life cycle assessment and risk assessment—potentials and obstacles. Industrial Environmental Informatics (IMI). Chalmers University of Technology. Naturvårdsverket. http://www.naturvardsverket.se/Documents/publikationer/620-5379-5.pdf. Accessed 5 February 2013
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41(24):8484–8490
Frischknecht R, Jungbluth N (2007) Implementation of life cycle impact assessment methods. Data v2.0—Ecoinvent Report No. 3. Swiss Centre for Life Cycle Inventories. Dübendorf. Zürich, pp. 755. http://www.ecoinvent.org/fileadmin/documents/en/03_LCIA-Implementation.pdf. Accessed 15 January 2013
Fthenakis V (2008) E-mail communication. Columbia University, New York (done by Sengül & Theis (2010))
Fthenakis VM, Kim HC, Alsema E (2008) Emissions from photovoltaic life cycles. Environ Sci Technol 42(6):2168–2174
Gandhi N, Diamond ML, van de Meent D, Huijbregts MAJ, Peijnenburg W, Guinee J (2010) New method for calculating comparative toxicity potential of cationic metals in freshwater: application to copper, nickel, and zinc. Environ Sci Technol 44(13):5195–5201
Gang Z (2007) Preparation, structure, and properties of advanced polymer composites with long fibers and nanoparticles, Ph.D. thesis, The Ohio State University
Ganter MJ, Seager TP, Schauerman CM, Landi BJ, Raffaelle RP (2010) A life-cycle energy analysis of single wall carbon nanotubes produced through laser vaporization. IEEE, Proceedings of International Symposium Sustainable Systems and Technology 18–20 May 2009. doi: 10.1109/ISSST.2009.5156708
Gavankar S, Suh S, Keller AF (2012) Life cycle assessment at nanoscale: review and recommendations. Int J Life Cycle Assess 17(3):295–303
Geranio L, Heuberger M, Nowack M (2009) The behaviour of silver nanotextiles during washing. J Environ Sci Technol 43(21):8113–8118
Giessmann A (2002) Substrat- und Textilbeschichtung, 1st edn. Springer, Berlin, p 180
Gordeyev SA, Macedo FJ, Ferreira JA, van Hattum FWJ, Bernardo CA (2000) Transport properties of polymer-vapour grown carbon fibre composites. Phys B: Cond Matt 279(1–3):33–36
Grieger KD, Laurent A, Miseljic M, Christensen F, Baun A, Olsen SI (2012) Analysis of current research addressing complementary use of life-cycle assessment and risk assessment for engineered nanomaterials: have lessons been learned from previous experience with chemicals? J Nanopart Res 14:958
Groner MD, Fabreguette FH, Elam JW (2004) George SM Low-temperature Al2O3 atomic layer deposition. Chem Mater 16(4):639–645
Gröning P, Ruffieux P, Schlapbach L, Gröning O (2003) Carbon nanotubes for cold electron sources. Adv Eng Mater 5(8):541–550
Grubb FG, Bakshi RB (2010) Life cycle of titanium dioxide nanoparticle production. J Ind Ecol 15(1):81–95
Guinée JB, Gorrée M, Heijungs R, Huppes G, Kleijn R, De Koning A et al (2002) Handbook on life cycle assessment. Kluwer Academic Publishers, Operational guide to the ISO standards. Dordrecht, p 704
Gur I, Fromer NA, Geier ML, Alivisatos AP (2005) Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 310(5747):462–465
Gutowski TG, Liow JYH, Sekulic DP (2010) Minimum exergy requirements for the manufacturing of carbon nanotubes. Sustainable Systems and Technology (ISSST). 2010 IEEE International Symposium 1(6):17–19
Ha SC, Choi E, Kim SH, Roh JS (2005) Influence of oxidant source on the property of atomic layer deposited Al2O3 on hydrogen-terminated Si substrate. Thin Solid Films 476(2):252–257
Hall S, Bradkey T, Moore JT, Kuykindall T, Minella T (2009) Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 3(2):91–97
Hassan MM (2010) Quantification of the environmental benefits of ultrafine/nano titanium dioxide photocatalyst coatings for concrete pavement using hybrid life cycle assessment. ASCE J Infrastruct Syst 16(2):160–166
Healy M (2006) Environmental and economic comparison of single-wall carbon nanotube production alternatives. Master’s thesis. Northeastern University. Boston. United States of America
Healy ML, Tanwani A, Isaacs JA (2006) Economic and environmental tradeoffs in SWNT production: NSTI-Nanotech. Nano Science and Technology Institute, Boston
Healy ML, Dahlben LJ, Isaacs JA (2008) Environmental assessment of single-walled carbon nanotube processes. J Ind Ecol 12(3):376–393
Hegemann D, Amberg M, Ritter A, Heuberger M (2009) Recent developments in Ag metallised textiles using plasma sputtering. Mater Technol 24(1):41–45
Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71(7):1308–1316
Helmer M (2002) News and views: cleaning up catalysts. Nature 418(6894):138
Hensel C, Konieczny R, Brück R (2000) Recycling technology for metallic substrates: a closed cycle. Society of Automotive Engineers Technical Paper 2000-01-0596. doi:10.4271/2000-01-0596
Hilliard HE (1998) Silver. U.S. Geological Survey Commodity Report. http://pubs.usgs.gov/of/2004/1251/2004-1251.pdf. A newer version from 2007, Accessed 5 October 2013
Hischier R, Walser T (2012) Life cycle assessment of engineered nanomaterials: state of the art and strategies to overcome existing gaps. Sci Total Environ 425(15):271–282
Home depot (2009) Average price and capacity of 18 Energy Star certified washing machines. http://www.homedepot.com. Accessed 5 October 2009
Howe JY, Tibbetts GG, Kwag C, Lake ML (2006) Heat treating carbon nanofibers for optimal composite performance. J Mater Res 21(10):2646–2652
Hung LS, Tang CW (1999) Interface engineering in preparation of organic surface emitting diodes. Appl Phys Lett 74(21):3209–3211
Hussain SM, Braydich-Stolle LK, Schrand AM, Murdock RC, Yu KO, Mattie DM, Schlager JJ, Terrones M (2009) Toxicity evaluation for safe use of nanomaterials: recent achievements and technical challenges. Adv Mater 21(16):1549–1559
Hwang C-L, Ting J, Chiang J-S, Chuang C (2005) Process of direct growth of carbon nanotubes on a substrate at low temperature. U.S. Patent 6,855,376, Chutung (Taiwan): Industrial Technology Research Institute. http://www.freepatentsonline.com/6855376.html. Accessed 20 November 2012
IITB (2012) Bio-nanotechnology. Biomaterials and bio-Interfaces Laboratory. School of Biosciences and Bioengineering, Indian Institute of Technology Bombay (IITB). http://www.btc.iitb.ac.in/~/biomatlab/nanotech.html. Accessed 20 November 2012
Illés E, Tombácz E (2006) The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J Colloid Interface Sci 295(1):115–123
Isaacs AJ, Tanwani A, Healy LM (2006) Environmental assessment of SWNT production. Proceedings of the 2006 IEEE International Symposium on Electronics and the Environment. ISEE, 38–41
ISO (2006) 14040: environmental management—life cycle assessment—principles and framework. International Organization for Standization
ISO (2008) Technical specifications ISO/TS 27687:2008 (E): nanotechnologies—terminology and definitions for nano-objects—nanoparticle, nanofibre and nanoplate. International Organization for Standization, Berlin
Jimenez JL, Nelson DD, Zahniser MS, McManus JB, Kolb CE, Koplow MD, Schmidt S (1997) In The 7th On-Road Vehicle Emissions Workshop: Washington, D.C., 1997
JIS (2004) Japanese Industrial Standard (JIS)—Fine ceramics (advanced ceramics, advanced technical ceramics)—test method for air purification performance of photocatalytic materials—part 1: removal of nitric oxide. JIS R 1701-1. 2004;1701-1:1–9
Jolliet O, Margni M, Charles R, Humbert S, Payet J, Rebitzer G, Rosenbaum R (2003) IMPACT 2002+: a new life cycle impact assessment methodology. Int J Life Cycle Assess 8(6):324–330
Jones CF, Grainger DW (2009) In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev 61(6):438–456
Joshi S (2008) Can nanotechnology improve the sustainability of biobased products? J Ind Ecol 12(3):474–489
Kammler HK, Mädler L, Pratsinis SE (2001) Flame synthesis of nanoparticles. Chem Eng Technol 24(6):583–596
Kandabarow AM (2006) Gas Injection Techniques for Al2O3 Atomic Layer Deposition. Micro/Nano Fabrication Laboratory Publication. Princeton Institute for the Science and Technology of Materials. Princeton University. http://w3.pppl.gov/ppst/docs/kandabarow.pdf. Accessed 13 October
Kashiwada S (2006) Distribution of nanoparticles in the see-through medeka (Oryzias latipes). Environ Health Perspect 114(11):1697–1702
Kato K, Hibino T, Komoto K, Ihara S, Yamamoto S, Fujihara H (2001) A life-cycle analysis on thin-film CdS/CdTe PV modules. Sol Energy Mater Sol Cells 67(1–4):279–287
Khanna V, Bakshi BR, Lee LJ (2007) Life cycle energy analysis and environmental life cycle assessment of carbon nanofibers production. Proceedings of the 2007 IEEE International Symposium on Electronics & the Environment. 7-10 May 2007. pp.128–133
Khanna V, Bakshi BR, Lee LJ (2008) Assessing life cycle environmental implications of polymer nanocomposites. IEEE Computer Society Washington, DC, USA. Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment. 1–6
Kim C-W, Choi K-S, Lee S-J, Kim J-M, Nam J-W (2000) Composition for electron emitter of field emission display and method for producing electron emitter using the same. USA: Samsung Display Devices Co., Ltd. p. 6. U.S. Patent number 6146230 A
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27(9):1825–1851
Klöpffer W, Curran MA, Frankl P, Heijungs R, Köhler A, Olsen SI (2007) Nanotechnology and life cycle assessment—synthesis of results attained from a workshop in Washington. D.C. 2-3 October 2006—A Systems Approach to Nanotechnology and the Environment. Woodrow Wilson International Center for Scholars. project on Emerging Nanotechnologies, EU
Koehler A, Wildbolz C (2009) Comparing the environmental footprints of home-care and personal-hygiene products: the relevance of different life-cycle phases. Environ Sci Technol 43(22):8643–8651
Köhler RA, Som C, Helland A, Gottshalk F (2008) Studying the potential release of carbon nanotubes throughout the application life cycle. J Clean Prod 16(8–9):927–937
Körner E, Hegemann D (2008) Personal Communication. St. Gallen, 2008. (done by Walser et al. (2011))
Koroneos C, Stylos N, Moussiopoulos N (2006) LCA of multicrystalline silicon photovoltaic systems e Part 1: present situation and future perspectives. Int J Life Cycle Assess 11(2):129–136
Krishnan N, Boyd S, Somani A, Raoux S, Clark D, Dornfeld D (2008) A hybrid life cycle inventory of nano-scale semiconductor manufacturing. Environ Sci Technol 42(8):3069–3075
Kumar R, Münstedt H (2005) Silver ion release from antimicrobial polyamide/silver composites. Biomaterials 26(14):2081–2088
Künninger T, Fischer A, Gerecke A, Heeb M, Kunz P, Ulrich A, Vonbank R (2010) Release of conventional and nano-sized biocides from coated wooden façades during weathering: consequences for functionality and aquatic environment Proceedings of the International Convention of Society of Wood Science and Technology and United Nations Economic Commission for Europe—Timber Committee October 11-14, 2010, Geneva, Switzerland
Kushnir D, Sandén BA (2008) Energy requirements of carbon nanoparticle production. J Ind Ecol 12(3):360–375
Lafyatis DS, Bennett CJ, Hales MA, Morris D, Cox JP, Rajaram RR (1999) Comparison of Pd-only vs. Pd-Rh catalysts: effects of sulfur. Temperature and Space Velocity. Society of Automotive Engineers: Warrendale PA. 1999; SAE 1999-01-0309
Larsen HF, Hauschild M (2007a) Evaluation of ecotoxicity effect indicators for use in LCIA, Int J LCA 12(1):24–33 (Erratum for p. 32 in Int J LCA 12(2):92)
Larsen HF, Hauschild M (2007b) GM-troph: a low data demand ecotoxicity effect indicator for use in LCIA. Int J LCA 12(2):79–91
Laurijssen J, Marsidi M, Westenbroek A, Worrell E, Faaij A (2010) Paper and biomass for energy? The impact of paper recycling on energy and CO2 emissions. Resour Conserv Recycl 54(12):1208–1218
LeCorre D, Hohenthal C, Dufresne A, Bras J (2012) Comparative sustainability assessment of starch nanocrystals. J Polym Environ 21(1):71–80
Lloyd MS, Lave BL (2003) Life cycle economic and environmental implications of using nanocomposites in automobiles. Environ Sci Technol 37(15):3458–3466
Lloyd MS, Lave BL, Matthews HS (2005) Life cycle benefits of using nanotechnology to stabilize platinum-group metal particles in automotive catalysts. Environ Sci Technol 39(5):1384–1392
Lorenz C, Windler L, von Goetz N, Lehmann RP, Schuppler M, Hungerbuhler K, Heuberger M, Nowack B (2012) Characterization of silver release from commercially available functional (nano)textiles. Chemosphere 89(7):817–824
Lowry GV, Casman EA (2009) Nanomaterial transport, transformation, and fate in the environment. NATO Science for Peace and Security Series. Nanomaterials: Risks and Benefits 125–137
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol 46(13):6893–6899
Lucena P, Vadillo JM, Laserna JJ (2001) Mapping of platinum group metals in automotive exhaust three-way catalysts using laser-induced breakdown spectrometry. Anal Chem 71(19):4385–4391
Manda BM, Blok K, Patel MK (2012) Innovations in papermaking: an LCA of printing and writing paper from conventional and high yield pulp. Sci Total Environ 15(439):307–320
Merugula AL, Khanna V, Bakshi RB (2010) Comparative life cycle assessment: reinforcing wind turbine blades with carbon nanofibers. Proceedings of the 2010 IEEE International Symposium on Sustainable Systems and Technology. ISSST 2010. 5507724
Meyer ED, Curran MA, Gonzales MA (2009) An examination of existing data for the industrial manufacture and use of nanocomponents and their role in the life cycle impact of nanoproducts. Environ Sci Technol J 43(5):1256–1263
Meyer ED, Curran MA, Gonzalez MA (2010) An examination of silver nanoparticles in socks using screening-level life cycle assessment. J Nanopart Res 13(1):147–156
Moeller G, Coe-Sullivan S (2006) Quantum-dot light-emitting devices for displays. Inf Disp 22(2):2–6
Moign A, Vardelle A, Themelis NJ, Legoux JG (2010) Life cycle assessment of using powder and liquid precursors in plasma spraying: the case of yttria-stabilized zirconia. Surf Coat Technol 205(2):668–673
Noijuntira I, Kittisupakorn P (2009) Life Cycle Assessment for the Activated Carbon Production by Coconut Shells and Palm-Oil Shells. The 2nd RMUTP International Conference 2010. Thailand. http://www.google.dk/url?qrepository.rmutp.ac.th/bitstream/handle/123456789/708/33.%2520I-sika%2520%2520Noijuntira.pdf%3Fsequence%3D1&saei&ved=&usg. Accessed 12 September 2013
Nanosolar Inc. (2008) Designed to last. http://www.nanosolar.com/Designedtolast.htm. Accessed May 2008
NLV (2010) Regional authority for consumer protection and food control lower saxony (Niedersaechsisches Landesamt für Verbraucherschutz).Triclosan and Silver in Textiles—Reply on Information Request, 2010. (done by Walser et al. (2011))
Oberdörster G, Stone V, Donaldson K (2007) Toxicology of nanoparticles: a historical perspective. Nanotoxicology 1(1):2–25
Oh SH, Bissett EJ, Battiston PA (1993) Mathematical modeling of electrically heated monolith converters: model formulation, numerical methods, and experimental verification. Ind Eng Chem Res 32(8):1560–1567
Olsen SI, Christensen FM, Hauschild M, Pedersen F, Larsen HF, Tørsløv J (2001) Life cycle impact assessment of chemicals— a methodological comparison. Environ Impact Assess Rev 21(4):385–404
Orbital Engine Corporation (1999) Orbital Direct Injection. A Technology Update from the Orbital Engine Corporation. http://www.orbeng.com.au/orbital/customersProducts/pdf/4smoneng.pdf. Accessed 14 February 2004
Osterwalder N, Capello C, Hungerbühler K, Stark JW (2006) Energy consumption during nanoparticle production: how economic is dry synthesis? J Nanopart Res 8(1):1–9
Phylipsen D, Kerssemeeckers M, Blok K, Patel M, de Beer J (2002) Clean technologies in the materials sector – current and future environmental performance of material technologies. Report Commissioned by European Commission. http://www.google.dk/url?q=,http://ftp.jrc.es/EURdoc/eur20515en.pdf&sa=U&ei=SFQxU76sN6mk4gTU-4GQCQ&ved=0CB8QFjAA&usg=AFQjCNHGLuePf5WpqWOLVhnqaLygupNe0w. Accessed 16 October 2013
Qiu W, Mai K, Zeng H (1998) Effect of macromolecular coupling agent on the property of PP/GF composites. J Appl Polym Sci 71(10):1537–1542
Quik TKJ, Vonk AJ, Hansen FS, Baun A, Van De Meent D (2011) How to assess exposure of aquatic organisms to manufactured nanoparticles? Environ Int 37(2011):1068–1077
Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem 18:89–108
Reimann DO (2006) CEWEP energy report: results of specific data for energy. Efficiency rates and coefficients. plant efficient factors and NCV of 97 EuropeanW-t-E plants and determination of the main energy results. Confederation of European Waste-to-Energy plants (CEWEP). Bamberg, Germany. http://www.cewep.com/storage/med/media/statements/106_11_07_06_CEWP-Report_Final_Version.pdf. Accessed 5 June 2013
Riondel A (1998) Process for the preparation of isobornyl(meth)acrylate. Atochem Elf SA. European Patent Application EP0759423, p3
Roes AL, Marsili E, Nieuwlaar E, Patel MK (2007) Environmental and cost assessment of a polypropylene nanocomposite. J Polym Environ 15(3):212–226
Roscheisen MR, Pichler K (2006) (Oct. 3) High throughput surface treatment on coiled flexible substrates. U.S. Patent number 7,115,304 B2
Rosenbaum KR, Bachmann MT, Gold SL, Huijbregts AJM, Jolliet O, Juraske R, Koehler A, Larsen FH, MacLeod M, Margni M, McKone ET, Payet J, Schuhmacher M, Van De Meent D, Hauschild ZM (2008) USEtox—the UNEP-SETAC toxicity model: recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int J Life Cycle Assess 13(7):532–546
Rüdenauer I, Eberle U, Griesshammer R (2006) Ökobilanz und Lebenszykluskostenrechnung Wäschewaschen. Oeko-Institut e.V.: Freiburg, p 147. http://www.oeko.de/oekodoc/289/2006-008-de.pdf. Accessed 5 June 2013
Rupasinghe R-A-TP (2011) Dissolution and aggregation of zinc oxide nanoparticles at circumneutral pH; a study of size effects in the presence and absence of citric acid. Master thesis. University of Iowa, 2011
Santini S (2003) On Board Diagnosis for Three-Way Catalytic Converters; Group for Research on Automotive Control Engineering: http://www.ing.unisannio.it/glielmo/Bertinoro/Santini.pdf. Accessed 14 February 2003
Saouter E, van Hoof G, Feijtel TCJ, Owens JW (2002) The effect of compact formulations on the environmental profile of northern European granular laundry detergents—Part II: life cycle assessment. Int J Life Cycle Assess 7(1):27–38
SCENIHR (2007) The existing and proposed definitions relating to products of nanotechnologies. Scientific Committee on Emerging and Newly Identified Health Risks. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_012.pdf. Accessed 20 September 2012
Scher EC (2006) Nanostructure and nanocomposite based compositions and photovoltaic devices. U.S. Patent 7,087,832
Scheringer M, Macleod M, Behra R, Sigg L, Hungerbühler K (2010) Environmental risk associated with nanoparticulate silver used as biocide. H and PC Compendium on Detergency - Vol. 6(2) April/June 2011. 27–29
Sengül H, Theis LT (2009) Life cycle inventory of semiconductor cadmium selenide quantum dots for environmental applications. In: Savage et al (eds) Nanotechnology applications for clean Water. William Andrew Inc., Norwich, pp 561–582
Sengül H, Theis LT (2010) An environmental impact assessment of quantum dot photovoltaics (QDPV) from raw material acquisition through use. J Clean Prod 19(1):21–31
Seyler C, Hofstetter T, Hungerbuhler K (2005) Life cycle inventory for thermal treatment of waste solvent from chemical industry: a multiinput allocation model. J Clean Prod 13(13–14):1211–1224
Shan Y, Coyle TW, Mostaghimi J (2007) Numerical simulation of droplet breakup and collision in the solution precursor plasma spraying. J Therm Spray Technol 16(5–6):698–704
Shan Y, Coyle TW, Mostaghimi J (2010) Modeling the influence of injection modes on the evolution of solution sprays in a plasma jet. J Therm Spray Techol 19(1–2):251
Sheats JR (2004) Manufacturing and commercialization issues in organic electronics. J Mater Res 19(7):1974–1989
Sheats JR, Capps P, Adriani P (2007) Individually encapsulated solar cells and solar cell strings having a substantially inorganic protective layer. Nanosolar Inc. U.S. Patent Number: 2007/0295390 A1
Slaveykova VI, Wilkinson KJ (2005) Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environ Chem 2(1):9–24
Som C, Berges M, Chaudry Q, Dusinska M, Fernandes FT, Olsen SI, Nowack B (2010) The importance of life cycle concepts for the development of safe nanoproducts. Toxicology 269(2–3):160–169
Spielmann M, Kägi T, Stadler P, Tietje O (2004) Life cycle inventories of transport services. Ecoinvent report No 14. EMPA Dübendorf, Swiss Centre for Life Cycle Inventories, Dübendorf, Zürich, Switzerland. http://www.poli.br/~cardim/PEC/Ecoinvent%20LCA/ecoinventReports/14_Transport.pdf. Accessed 13 October 2013
Stamatelos AM, Koltsakis CC, Kandylas IP (1998) Computer aided engineering in SI engine exhaust aftertreatment systems design. In proceeding of FISITA World Automotive Congress 1998. Paris France
Stamminger R. (2007) Information brochure “Energieverbrauch der Waschmaschine”. In: Landesweiter Aktionstag Nachhaltiges Waschen
Steinfeldt M, von Gleich A, Henkle JLL, Endo M, Morimoto S, Momosaki E (2010a) Environmental relief effects of nanotechnologies by the example of CNT composite materials and films. International Conference; 9th, Ecobalance; Towards and beyond 2020
Steinfeldt M, von Gleich A, Petschow U, Pade C, Sprenger R.-U (2010b) Entlastungseffekte für die Umwelt durch nanotechnische Verfahren und Produkte (Environmental Relief Effects through Nanotechnological Processes and Products). UBA-Texte 33/2010, Dessau. http://www.umweltbundesamt.de/sites/default/files/medien/461/publikationen/3777.pdf. Accessed 16 May 2012
Suppen N, Carranza M, Huerta M, Hernández AM (2005) Environmental management and life cycle approaches in the Mexican mining industry. J Clean Prod 14(12–13):1101–1115
Tanwani A (2005) Carbon nanotube production: An economic and environmental assessment of alternative technologies. Master’s thesis. Northeastern University, Boston
Tibbetts GG, Gorkiewicz DW (1993) A new reactor for growing carbon fibers from liquid-and vapor-phase hydrocarbons. Carbon 31(5):809–814
Tibbetts GG, Bernardo CA, Gorkiewicz DW, Alig RA (1994) Role of sulfur in the production of carbon fibers in the vapor phase. Carbon 32(4):569–576
Tremeac B, Meunier F (2008) Life cycle analysis of 4. 5 MW and 250 turbines. Renew Sustain Enerey 13(8):2104–2110
Upadhyayula VKK, Meyer DE, Curran MA, Gonzalez MA (2012) Life cycle assessment as a tool to enhance the environmental performance of carbon nanotube products: a review. J Clean Prod 26(May):37–47
U.S. Environmental Protection Agency (1990) Titanium tetrachloride production, from report to congress on special wastes from mineral processing. vol. II, Office of Solid Waste, July 1990, p. 13-1–31
U.S. Environmental Protection Agency (1997) Benefits and Cost of Potential Tier 2 Emission Reduction Technologies: Final Report. Energy and Environmental Analysis Inc. Office of Mobile Sources, 1997
U.S. Census Bureau (2009) Socks production—summary 2008: Report MQ315B(08)-5. Washington DC. U.S. Department of Commerce
USEtoxTM (2012) Background of the USEtoxTM model, USEtoxTM homepage. http://www.usetox.org/background.aspx. Accessed 7 February 2012
van Hattum FWJ, Leer C, Viana JC, Carneiro OS, Lake ML, Bernardo CA (2006) Conductive long fibre reinforced thermoplastics by using carbon nanofibres. Plast Rubber Comp 35(6–7):247–252
Verhulst D, Sabacky B, Spitler T, Duyvesteyn W (2002) The Altair TiO2 pigment process and its extension to the field of nanomaterials. CIM Bull 95:89–94
Verhulst D, Sabacky B, Spitler T, Prochazka J (2003) New developments in the Altair TiO2 hydrochloride pigment process. In Proceedings of the 5th International Conference in Honor of Professor Ian Ritchie
Verhulst D, Sabacky B, Lang J, Ellsworth D (2006a) Iron control in the Altair hydrochloride pigment process. In Proceedings of the 3rd International Symposium on Iron Control in Hydrometallurgy.
Verhulst D, Sabacky B, Marganski R, Wang B, Ellsworth D (2006b) Optimization of the critical steps of the Altair hydrochloride pigment process. In Proceedings of the Sohn Symposium on Advanced Processing of Metals and Materials. Volume 6. Warrendale, PA: TMS
Vestas (2004) Life Cycle Assessment of onshore and offshore sited wind farms. LCA on V80-2.0 turbines. Vestas. https://www.google.com/url?qvestas.com/~/media/vestas/about/sustainability/pdfs/lca_v80_2004_uk.pdf&saeivedusg. Accessed 18 October 2012
Vestas (2006) Life Cycle Assessment of offshore and onshore sited wind power plants. LCA on V90-3.0 turbines. Vestas. http://www.google.com/url?qvestas.com/~/media/vestas/about/sustainability/pdfs/lca_v90_june_2006.pdf&saei=JFYxU6D4BqGm4ATrtYHQDA&ved=0CB0QFjAA&usg=AFQjCNH3MMlj7s8Hich8YGA-A7zeLmE7fw. Accessed 18 October 2012
Vonk JA, Struijs J, van de Meent D, Peijnenburg WJGM (2009) Nanomaterials in the aquatic environment: toxicity. exposure and risk assessment. RIVM Report 607794001/2009, RIVM Bilthoven, Nederlands. http://nl.sitestat.com/rivm/rivm-nl/s?link.en.documents_and_publications.scientific.reports.2010.april.nanomaterials_in_the_aquatic_environment_toxicity_exposure_and_risk_assessment.download_pdf&ns_type=pdf&ns_url=http%3A%2F%2Fwww.rivm.nl%2Fdsresource%3Fobjectid=rivmp:54941&type=org&disposition=inline&ns_nc=1. Accessed 10 October 2012
Walser T, Demou E, Lang JD, Hellweg S (2011) Prospective environmental life cycle assessment of nanosilver t-shirts. Environ Sci Technol 45(10):4570–4578
Wang J, Chen C et al (2008) Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett 183(1–3):72–80
Weil M, Dura H, Shimon B, Baumann M, Zimmermann B, Ziemann S, Lei C, Markoulidis F, Lekakou T, Decker M (2012) Ecological assessment of nano-enabled supercapacitors for automotive application. Conf Ser Mater Sci Eng 40:012013
Weinzettel J, Reenaas M, Solli C, Hertwich EG (2009) Life cycle assessment of a floating offshore wind turbine. Renew Enerey 34(3):742–747
Wenzel H, Hauschild MZ, Alting L (1997) Environmental assessment of products, vol 1: Methodology, tools and case studies in product development. Chapman and Hall, UK
Wernet G, Papadokonstantakis S, Hellweg S, Hungerbühler K (2009) Bridging data gaps in environmental assessments: modeling impacts of fine and basic chemical production. Green Chem 11:1826–1831
Wickboldt P (2008) US DC (Display Consortium). Telephone Communication. (done by Sengül & Theis (2010))
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40(14):4336–4345
Worrell E, Galitsky C, Masanet E, Graus W (2008) Energy efficiency improvement and cost saving opportunities for the glass industry. An ENERGY STAR guide for energy and plant managers. Environmental Energy Technologies Division. Berkely (California): Ernest Orlando Lawrence Berkeley National Laboratory. http://www.energystar.gov/buildings/sites/default/uploads/tools/Glass-Guide.pdf. Accessed 21 August 2014
Wu S, Natsuki T, Kurashiki K, Ni Q, Iwamoto M, Fujii Y (2007) Conductivity stability of carbon nanofiber/unsaturated polyester nanocomposites. Adv Comp Mater 16(3):195–206
Yamagata C, Ussui V, Andrade JD, Paschoal JOA (2005) Synthesis of nanosilica powders by recovering an effluent from pure zirconia powder production process via wet chemical processing. http://pintassilgo2.ipen.br/biblioteca/2005/ptech/11091.pdf (can be accessed through), http://ebookbrowsee.net/21-19-pdf-d452004477. Accessed 15 October 2013
Yu HG, Lee SC, Yu J, Ao CH (2006) Photocatalytic activity of dispersed TiO2 particles deposited on glass fibers. J Mol Catal A: Chem 246(1–2):206
Zhu X, Zhu L, Chen Y, Tian S (2009) Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J Nanopart Res 11:67–75
Acknowledgments
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no 263946.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miseljic, M., Olsen, S.I. Life-cycle assessment of engineered nanomaterials: a literature review of assessment status. J Nanopart Res 16, 2427 (2014). https://doi.org/10.1007/s11051-014-2427-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2427-x