Abstract
A fundamental understanding of the morphological development of cellulose fibers during fibrillation using micro grinder is very essential to develop effective strategies for process improvement and to reduce energy consumption. We demonstrated some simple measures for characterizing cellulose fibers fibrillated at different fibrillation times through the grinder. The morphology and degree of fibrillation of the samples at different stages of fibrillation were characterized. The fibrillation and mechanical properties reached a maximum in 2 h, and did not show any significant change with further grinding. The lateral dimensions of the smallest nanofibrils were between 15 and 40 nm. A slight reduction in the crystallinity and degree of polymerization did not lead to decrease in mechanical properties of cellulose films. The lower tensile properties at the initial stages of fibrillation are mainly due to the presence of limited refined and heterogeneously treated fibers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microfibrils or cellulose nanofibrils (CNFs) constitute the smallest fibrous component of cellulose fibers that have diameter in the range of 2–20 nm and lengths up to several micrometers depending on their origin (Stelte and Sanadi 2009). Owing to the abundance, smaller dimension, and high surface-to-volume ratio combined with excellent physical and mechanical properties, CNFs have been considered a prime candidate for many applications in material science field (Abraham et al. 2011; Kaushik and Singh 2011; He et al. 2012). The plant cell wall is a multilayered complex structure in which cellulose molecules are intimately associated with other polysaccharides, lignin, and extractives. These nano-scale elements which make up the cellulose fibers are held together by strong cohesive forces. Intensive mechanical treatment is required to disintegrate the cellulose fiber to nanofibrils (Uetani and Yano 2011). These mechanical treatments are very energy intensive (Siro and Plackett 2010). Several methods of mechanical fibrillation have been used for the production of CNFs such as homogenizers (Chinga-Carrasco et al. 2012; Chinga-Carrasco and Syverud 2012), microfluidizers (Henriksson et al. 2008), and grinders (Abe et al. 2007). Also, chemical means such as strong acidic hydrolysis to attack the noncrystalline fractions within the cellulose fiber produce low aspect ratio cellulose nanoelements known as cellulose nanocrystals (CNCs) or cellulose nanowhiskers (Dash et al. 2013).
Mechanical fibrillation using a micro grinder has several advantages compared to other means of nanofibrillation processes. The use of a micro grinder for mechanical fibrillation has a great potential for commercial scale-up as it can be used for microfibrillating large quantities of materials compared to other mechanical processes. Also, it can be used for microfibrillating sufficiently long fibers without any pretreatment and has no clogging tendency during mechanical fibrillation (Siro and Plackett 2010). Due to the high complexity and rigidity of plant cell wall, the fibers have to be repeatedly fed several times through the micro grinder in order to obtain uniform nanofibrils (Iwamoto and Nakagaito 2007). This results in high energy consumption for the process (Chinga-Carrasco and Syverud 2010; Syverud et al. 2011; Wang et al. 2012). Various studies using a micro grinder or other methods of mechanical fibrillation process such as homogenizer or micro fluidizers have shown that significant improvements in the mechanical properties occur after the internal fibrillation of the cellulose fibers into nanosized fibrils (Nakagaito and Yano 2004; Stelte and Sanadi 2009). Nanosized fibrils have more exposed surface area compared to bigger dimensions which leads to more fibril-to-fibril bonds within the sheets and thereby maximizing sheet density and better tensile properties (Stelte and Sanadi 2009). The degree of fibrillation or the exposed surface area obtained through nanosized fibrils using a micro grinder is relatively high compared to fibrillation using low-intensity refiners like a PFI mill. In general, refining or beating is a mechanical treatment carried out on cellulose fibers or pulp to make them suitable for desired paper-making properties. The major effects of refining are external fibrillation of a fiber yielding the formation of “hairs or fibrils” from the external fiber surface, increased swelling, and internal fibrillation leading to the disruption of hydrogen bonds (Oksanen et al. 1997). The sheets made from PFI mill-treated cellulose fibers showed tensile strength which is almost 75–80 percentage of the maximum tensile strength of sheets made from micro grinder-treated cellulose fibers. While most of the fibers are fibrillated to nanosized fibrils in micro grinder, the cellulose fibers retain their micron-sized dimensions in PFI mill. At a given density, the mechanical properties of sheets or films made from low-intensity refiners are much better than the micro grinder (Seth 1999; Kerekes 2005; Spence et al. 2011). This explains that there are certain factors other than the surface area of fibrillated fibers or the bonded area which affect the tensile properties. Also, the grinder treatment has to be optimized for high performance fibers. The continuous shearing forces generated by the grinding stones in micro grinding to sufficiently fibrillate pulp fibers can also degrade pulp fibers, thereby affecting their mechanical properties (Iwamoto and Nakagaito 2007). Therefore, a fundamental understanding of the morphological development of cellulose fibers during various stages of fibrillation process using micro grinding is essential to develop an effective means for process improvement and to reduce energy consumption of fibrillation. The objective of this work is to characterize pulp fibers at different stages of fibrillation using a commercial grinder and to evaluate the morphological aspects of fibers and its effect on the performance aspects of cellulose films made from various stages of fibrillation. Also, the performance of micro grinder at various stages will be compared to other low-intensity laboratory refiners like PFI mill.
Experimental
Materials
Elementally chlorine-free (ECF) bleached kraft pulp from softwood (Loblolly pine) was obtained as a commercial sample. The chemical composition of the sample is listed in Table 1. Endoglucanase (Fibercare®) with an activity of 150 FPU/ml was provided by Novozymes North America (Franklinton, NC, USA). All other chemicals were ACS reagent grade obtained from Sigma-Aldrich (St.Louis, MO, USA).
Mechanical nanofibrillation
The pulp at 2 % solids was soaked in deionized water for 24 h and then disintegrated using a lab disintegrator (TMI, Ronkonkoma, NY, USA) for 10,000 revolutions. It was then fibrillated using a SuperMassColloider (MKZA6-2, Masuko Sangyo Co., Ltd., Japan) at 1,500 rpm. Pulp was fed continuously to the colloider consisting of two stone grinding disks positioned on top of each other. This was operated at contact grinding with the gap of the two disks adjusted to -100 μm. The zero gap corresponds to the starting point where the two disks just start to graze each other before loading pulp. The presence of pulp between the disks ensured that there is no direct contact between the disks even at the negative setting. The fibrillated samples were sampled periodically and the time-dependent energy consumption was recorded using a power meter attached to the colloider (Model KWH-3 Energy Meter, Load Control Inc., Sturbridge, MA, USA). The samples were treated with Kathon CP/ICP II (Rohm and Haas Company, Bellefonte, PA, USA) at a dose of 10 μl/ml of fibrillated suspension in order to avoid the mold growth.
Characterization of the nanofibrillated material
The morphology of fibrillated sample was analyzed by scanning electron microscope (SEM) (LEO EVO 40 SEM, Carl Zeiss NTS, Peabody, MA, USA) at 15 kV. Drops of the sample at ~0.1 % consistency were dried on polished aluminum mounts and were sputter-coated with gold to provide adequate conductivity. The morphology was also analyzed using a tapping mode atomic force microscopy (AFM) (AFM+, Anasys Instruments, Santa Barbara, CA, USA). Fibrillated sample suspension (0.1 % w/w) was sonicated and deposited by drop casting from solution onto a clean silicon wafer for imaging.
A modification of the TAPPI standard Method 256 was used to determine water retention value (WRV) for the fibrillated and unfibrillated sample. Samples at 4 % solids, with an o.d. (oven-dry) weight of 0.25 g, were centrifuged (Interactional centrifuge model EXD, International equipment CO, Boston, MA, USA) at 900 g for 30 min. The samples were weighed and oven dried at 105 °C until reaching constant weight. The % WRV was calculated as the amount of water retained by the fibers/fibrils after centrifugation relative to the weight of o.d. substrate (Dang et al. 2007).
Cellulose accessibility to cellulase for each fibrillated sample was evaluated from the measure of endoglucanase binding determined by UV–Vis spectrometry (Liu et al. 2011; Wang et al. 2012). The samples at a fixed basis o.d. weight of 0.10 g were treated with endoglucanase loading equivalent to 50 mg protein/l in 110 ml of acetate buffer (pH 4.8) at 4 °C. The sample suspension was circulated through a quartz cuvette of 10 mm optical path length using a flow loop and a peristaltic pump (Liu et al. 2011). The free enzyme concentration in the solution was continuously monitored by a UV–Vis spectrometer (Model 8453 Agilent, Palo Alto, CA) at wavelength 291 nm.
Production and testing of fibrillated cellulose films
Films of fibers resulting from various degrees of mechanical fibrillation were formed by ultrafiltration of fiber slurries using a 142-mm Millipore ultrafiltration apparatus with polyvinylidene difluoride (PVDF) membranes of 0.22-μm pore size (Millipore GVWP14250, Bedford, MA, USA). Fiber slurries of approximately 0.1 g/l were added to the ultrafiltration apparatus to make sheets with a target weight of 1.0 g. After dewatering, individuals were placed between membranes and placed between blotter papers. The films and membranes were pressed at 207 and 345 kPa for 3 min each and finally placed between smooth metal caul plates and allowed to dry at 50 °C under a pressure of ~14–20 kPa for 24 h. The mechanical properties of the films were measured according to TAPPI Standard Test Methods. The films were first conditioned according to TAPPI Method T 402, i.e., preconditioning at 22–40 C under 10–35 % relative humidity for at least 24 h, followed by conditioning under 50 ± 2.0 % RH at 23 ± 1.0 °C for at least 24 h. Three films for each experimental condition were prepared and sampled for mechanical testing. Three tensile specimens were obtained from each film. Tensile tests were performed using TAPPI T 494 in which a span of 6.4 cm, a width of 15 mm, and an elongation speed of 0.2 mm/min were used. The density was calculated by determining the weight, area, and thickness of the cellulose films. The weight of the films was determined using an analytic balance, and the thickness was determined using a micrometer gauge calculating the average of at least twelve independent measurements at different locations on the film. Canadian Standard Freeness (CSF) test was performed using TAPPI T 227 to measure the rate at which the fibrillated pulp drained. CSF is based on the principle that water from a dilute suspension of pulp with little surface area will drain rapidly through a specific screen plate. However, if the same pulp was to be subjected to a period of mechanical treatment, the increased surface area of the pulp would cause water to drain much more slowly.
Degree of polymerization and crystallinity of fibrillated samples
The degree of polymerization (DP) of the cellulosic solids was measured according to TAPPI T 230. 0.10 g of oven-dry cellulosic solid was first dissolved into 20 ml of 0.25 M cupriethylenediamine solution. The viscosity of the resultant solution was determined by a capillary viscometer. The DP of the cellulose was calculated using the following expression:
where X is the viscosity of the resultant solution.
The CNF crystallinity was measured by a FT-Raman spectroscopic method (Agarwal et al. 2010). Approximately 0.15 g of air-dried cellulosic sample pressed into a pellet was analyzed with a Bruker MultiRam spectrometer (Bruker Instruments Inc., Billerica, MA). Crystallinity index was calculated as
where I 380 and I 1096 are the Raman spectral intensities at 380 and 1096 wavenumbers (cm−1), respectively.
Results and discussion
Morphological characterization
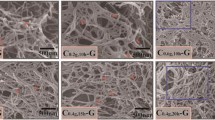
The original pulp fibers have an average width of 25–40 μm (Fig. 1a), while most of the fibers turned to submicro- and nanosized fibrils after 6 h of fibrillation by the grinder (Fig. 1b). Figure 2 shows the SEM images of fibrillated pulp fibers of samples taken at 15 and 30 min of fibrillation through the grinder. There were large amount of fibers still retained in 15 and 30 min fibrillated samples with the same dimensions (Fig. 2a, d) of the original pulp fibers (25–40 μm) with limited mechanical treatment. Some of fibers (Fig. 2b, e) were mildly fibrillated externally to produce fibrils from the external surface, indicating that fibrillation was restricted to the surface of fibers; while some fibers were flattened due to high shearing force. Some of the fibers (Fig. 2c, f) were highly fibrillated internally to submicro- and nano-fibrils. This heterogeneity in treatment among the fibers can be due to different flow patterns within the grinder and different force distributions within a single floc or aggregation of fibers (Hietanen and Ebeling 1990; Seth 1999). Even within a single fiber, there is heterogeneity in the mechanical stressing along the length of fiber. This is quite evident from the highly fibrillated fibers (Fig. 2c, f). Some parts of cell wall were still held together to form submicron-sized fibrils, while at the other end the internal fibrillation of these submicron-sized fibrils continued to produce nanofibrils. With continuing fibrillation time to 1 h, and increase in the number of passes through the grinder ensured that more fibers were mechanically fibrillated and further split to smaller fibrils and also the number density of fibers retaining original pulp dimensions were substantially reduced (Fig. 3a, b). With 2 h of fibrillation, there were negligible variations (Fig. 3b) in the fiber morphology indicating that a homogeneous fibrillation was almost completely accomplished. When the number of h of fibrillation was increased from two to six, there was no significant change in the morphology of the samples, as indicated by the SEM imaging. (Compare Figs. 1b, 3b).
Due to limited resolution of the SEM imaging, the cellulose structures to the scale of <100 nm were obscured in the images. AFM imaging can zoom into the ultra-fine morphology of the fibers at nanoscale. Figure 4 shows the AFM image of fibrillated sample for 15 min and 2 h, respectively. The lateral dimensions of the smallest nanofibril aggregates were between 15 and 40 nm for both of the samples as imaged by AFM (Fig. 4a, b). This shows that with the current grinder set up, with the available tensile, compressive, and shearing forces, a very limited number of pass or impact can cause sufficient stress on the fibers to fibrillate to the smallest nanofibril aggregates. Figure 5 shows the number of passes through the grinder at different fibrillation times.
Mechanical properties
Figure 6 shows the change in tensile strength and strain with fibrillation time. From the morphological characterization, it was quite clear that as the fibrillation time proceeds, more and more pulp fibers were disintegrated to smaller fibers. Table 2 shows the degree of fibrillation as indicated by WRV and cellulase adsorption. WRV is a measure of fiber swelling capacity; as this test quantifies the water that remains within the pore spaces in the cell walls of cellulose fibers after a centrifugal force expels the free water present between the fibers and inside the cell lumen (Weise et al. 1996). Cell walls in cellulose fiber consist of long nanofibrils closely aggregated together by strong inter fibril hydrogen bonds (Gardner and Blackwell 1974). The mechanical treatment results in peeling of fibrils from external surface of fibers, and internal fibrillation leading to the partial delamination followed by fibrillation of fibers to submicro- and nano-fibrils. All these lead to the breakage of inter fibril bonds creating more pore spaces in fibers. Therefore, WRV increases with the external and internal fibrillation. The WRV increased markedly between 0 and 2 h of grinding time for the pulp fibers and thereafter reached a plateau. This indicates that all the pore spaces within the cell wall are open and no more water accessible surface can be created through continued fibrillation after 2 h. This also indicates that the size reduction of cellulose fibers to nanofibril aggregates was not significant after 2 h of fibrillation (Cheng et al. 2007). The highly fibrillated samples showed higher amount of cellulase adsorbed at equilibrium compared to the lesser fibrillated samples (Table 2). The surface area was a limiting factor for the samples till 2 h of fibrillation for cellulase adsorption (Hoeger et al. 2013). Figure 7a shows the increase in film density with fibrillation time. Density increased with fibrillation and reached a maximum with 2 h of fibrillation and thereafter did not show any significant change. The increase in fibrillation leads to more exposed surface area of fibril which leads to more fibril-to-fibril bonds within the films when prepared by pressing and thereby the maximum density. This leads to an appreciable increase in bond strength per unit area. These bonds act in cooperation during straining of a film and most of them remain intact until the failure stress is reached. Thus, the tensile strength increased with the increasing bond strength per unit area and relative bonded area (Page 1969; Stelte and Sanadi 2009). Also, Fig. 6b shows that strain increased with the increasing fibrillation and reached a maximum for samples grinded for 2 h and thereafter did not show any significant change. Figure 7b shows the increase in modulus with the fibrillation time which reached a maximum with 2 h of fibrillation, and did not show any significant change with further fibrillation time. It can be observed from the SEM, WRV, and cellulase adsorption results that the average fiber diameter decreased with fibrillation time to a minimum with 2 h of fibrillation and thereafter did not show any significant variation. This decrease in fiber diameter leads to more fibers bridging the network of the film and results in increased hydrogen bonding with fibrillation time. The modulus increases with the increase in the number of hydrogen bonds within the films and the effective fraction of hydrogen bonds which resists mechanical strain is directly proportional to the apparent density of the cellulose films (Nissan and Sternstein 1964; Henriksson et al. 2008; Stelte and Sanadi 2009).
Micro grinder is found to be very effective in producing nanosized fibril aggregates. This is in accordance with studies reported in the literature (Abe et al. 2007; Hoeger et al. 2013). The CSF of the pure pulp was 740 ml and after 15 min of fibrillation it dropped to 40 ml and thereafter with further fibrillation, the pulp did not show any CSF at all for the micro grinder. The sudden drop in the CSF after 15 min of fibrillation is mainly due to high external and internal fibrillation of pulp fibers to produce nanofibrils. The energy consumption for the fibrillation using mechanical grinder was found to be linearly related to the fibrillation time (Fig. 8). The PFI mill shows strength values around 100–110 nm/g around a freeness of 200–300 ml, approximately (Seth 1999; Kerekes 2005). The currently used micro grinder has to fibrillate the samples for at least one h to obtain tensile strength around 120 MPa (which is equivalent to 105 nm/g) at an energy expenditure of 2,300 kwh/tonne. The specific energy and intensity of micro grinder can be considered very high in comparison to the PFI mill. At a given specific energy, a low-intensity mechanical device creates more homogenous impacts on fibers or more fibers receive impacts than a high-intensity device (Kerekes 2005). At higher intensity, fibers receive heterogeneous impacts or fewer fibers receive impacts. Low-intensity refiners like PFI mill give much more homogenous treatment to pulp fibers and also result in more number of fibers receiving mechanical treatment. Mechanical treatment swells up the fibers though internal delamination, making the fibers more conformable resulting in more fiber–fiber bonds, and also shrinkage stresses help to get rid of various deformations within fiber during film drying (Seth 2006). This explains why the pulps refined at low-intensity refiners like PFI mill produce a better bonded sheet at a higher tensile strength compared to the initial stages of micro grinder. The low tensile properties for the films made from initial stages of fibrillation i.e., 15 and 30 min of fibrillation compared to other low-intensity refiners like PFI mill (Seth 1999; Kerekes 2005) are mainly due to high number density of fibers with limited mechanical treatment and due to the heterogeneously treated fibers in the films (Fig. 2). The significantly reduced fiber length during the initial 15-30 min micro grinding reduced fiber network strength to result in reduced strength. These different types of treated fibers can cause uneven segments within the network. The unbeaten or limited treated fibers can have lot of deformations such as curls, crimps, and kinks in the unbonded segments. This can lead to uneven stress distribution within the network leading to stress concentration and thereby a premature failure. Also, the effective modulus of the segment bonded in the network with these type of fibers is appreciably lowered (Seth 2006). However, with continuing fibrillation, an increase in the number of passes through the micro grinder ensured that more fibers get mechanical stressing and a high internal fibrillation and split to smaller uniform nanofibrils and thereby acquire much homogeneity between the treated fibers resulting in a substantial increase in the tensile strength.
Crystallinity and degree of polymerization (DP)
Figure 9 shows the effects of fibrillation time on the crystallinity and DP for the pulp fibers. The overall reduction in crystallinity and DP was in the order of 14–20 and 13–21 %, respectively. Both the crystallinity and DP decreased continuously with the fibrillation time. As fibrillation progresses, the fiber wall opens and there is an increase in the swelling of the fibers making them more flexible and the crystalline zone more accessible to mechanical deconstruction. The continuous shearing force generated by the grinding disks can destroy the cellulose crystals and shorten the chain length resulting in smaller crystals, thereby reducing the crystallinity of samples. Several studies have demonstrated the strong correlation between DP and length of cellulose fibers (Henriksson et al. 2008; Shinoda et al. 2012). In our results, there was a small dip in mechanical properties after 4 h. This could be due to reduction in the length of nanofibrils. Increased fibrillation tends to reduce the size of nanofibrils (Wang et al. 2012). These low aspect fibers yield more end groups per unit volume, thereby more defects per unit volume (Kulachenko et al. 2012). Also, these fibers are rigid and easy to pull out from the aggregation of fibers making sheets more brittle than those sheets made form high aspect ratio fibers (Nakagaito and Yano 2004). Degradation of pulp fibers during the grinding treatment was reported in another study (Iwamoto and Nakagaito 2007). The fibrillated nanosized fibers obtained had a negative effect on the mechanical properties of sheets and composites. The results proved that with the use of current disk grinder, we achieved the maximum fibrillation and increase in mechanical properties in 2 h under the conditions studied.
Conclusions
Micro grinder was found to be very effective in producing nanosized microfibril aggregates. With the current micro grinder setup, it took multiple passes through the grinder for 2 h to obtain individualized uniform cellulose nanofibers of 15–40 nm in diameter. The modulus, strength, and strain to failure for the cellulose films made from pulp fibers at different stages of fibrillation reached a maximum in 2 h of fibrillation, and did not show any significant change with any further fibrillation. The disintegration of the cell wall of the pulp fibers into nanofibrils is evident from the SEM and AFM images. Also, WRV and cellulase adsorption method showed that the degree of fibrillation did not show any significant change after 2 h. A slight reduction in the crystallinity or DP did not affect the performance of CNFs. A very limited number of passes or impact through the micro grinder can cause sufficient stress on the fibers to fibrillate to the smallest nanofibril aggregates. However, it was quite clear that the high-intensity grinder created a lot of heterogeneity among the treated fibers and within a single fiber along its length with limited number of passes through the micro grinder. The high density of fibers with limited treatment and the heterogeneity in treatment resulted in very low tensile properties for the cellulose films. Thus, it required many passes through the refiner for 2 h in obtaining highly uniform nanosized fibrils. Even though, 15 and 30 min of fibrillation in treatment resulted in high increase in density, cellulose films made from these fibers did not show better mechanical properties when compared to films made from low-intensity refiners like PFI mill. This shows that homogeneity in treatment between the fibers and within the fibers is very essential for better mechanical properties.
References
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 8(10):3276–3278
Abraham E, Deepa B, Pothan LA, Jacob M, Thomas S, Cvelbar U, Anandjiwala R (2011) Extraction of nanocellulose fibrils from lignocellulosic fibers: a novel approach. Carbohydr Polym 86(4):1468–1475
Agarwal UP, Reiner RS, Ralph SA (2010) Cellulose I crystallinity determination using FT-Raman spectroscopy: univariate and multivariate methods. Cellulose 17(4):721–733
Cheng Q, Wang SQ, Rials TG, Lee SH (2007) Physical and mechanical properties of polyvinyl alcohol and polypropylene composite materials reinforced with fibril aggregates isolated from regenerated cellulose fibers. Cellulose 14(6):593–602
Chinga-Carrasco G, Syverud K (2010) Computer-assisted quantification of the multiscale structure of films made of nanofibrillated cellulose. J Nanoparticle Res 12:841–851
Chinga-Carrasco G, Syverud K (2012) On the structure and oxygen transmission rate of biodegradable cellulose nanobarriers. Nanoscale Res Lett 7:192
Chinga-Carrasco G, Kuznetsova N, Garaeva M, Leirset I, Galiullina G, Kostochko A, Syverud K (2012) Bleached and unbleached MFC nanobarriers: properties and hydrophobisation with hexamethyldisilazane. J Nanopart Res 14:1280
Dang Z, Zhang JG, Ragauskas AJ (2007) Characterizing TEMPO-mediated oxidation of ECF bleached softwood kraft pulps. Carbohydr Polym 70(3):310–317
Dash R, Foston M, Ragauskas AJ (2013) Improving the mechanical and thermal properties of gelatin hydrogels cross linked by cellulose nanowhiskers. Carbohydr Polym 91(2):638–645
Gardner KH, Blackwell J (1974) The structure of native cellulose. Biopolymers 13:1975–2001
He L, Li X, Li W, Yuan J, Zhou H (2012) A method for determining reactive hydroxyl groups in natural fibers: application to ramie fiber and its modification. Carbohydr Res 348:95–98
Henriksson M, Berglund LA, Isaksson P, Lindstroem T, Nishino T (2008) Cellulose nanopaper structures of high toughness. Biomacromolecules 9(6):1579–1585
Hietanen S, Ebeling K (1990) A new hypothesis for the mechanics of refining. Pap Puu 72(2):172–179
Hoeger IC, Nair SS, Ragauskas AJ, Deng Y, Rojas OJ, Zhu JY (2013) Mechanical deconstruction of lignocellulose cell walls and their enzymatic saccharification. Cellulose 20(2):807–818
Iwamoto S, Nakagaito AN, Yano H (2007) Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. App Phys A Mater Sci Process 89(2):461–466
Kaushik A, Singh M (2011) Isolation and characterization of cellulose nanofibrils from wheat straw using steam explosion coupled with high shear homogenization. Carbohydr Res 346:76–85
Kerekes RJ (2005) Characterizing refining action in PFI mills. TAPPI 4(3):9–14
Kulachenko A, Denoyelle T, Galland S, Lindstroem SB (2012) Elastic properties of cellulose nanopaper. Cellulose 19(3):793–807
Liu H, Zhu JY, Chai XS (2011) In situ, rapid, and temporally resolved measurements of cellulase adsorption onto lignocellulosic substrates by UV–Vis spectrophotometry. Langmuir 27(1):272–278
Nakagaito AN, Yano H (2004) The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl Phys A Mater Sci Process 78(4):547–552
Nissan AH, Sternstein SS (1964) Cellulose-fiber bonding. TAPPI 47(1):1–6
Oksanen T, Pere J, Buchert J, Viikari L (1997) The effect of trichoderma reesei cellulases and hemicellulases on the paper technical properties of never-dried bleached kraft pulp. Cellulose 4:329–339
Page DH (1969) Theory for the tensile strength of paper. TAPPI 52(4):674–681
Seth RS (1999) Beating and refining response of some reinforcement pulps. TAPPI 82(3):147–155
Seth RS (2006) The importance of fiber straightness for pulp strength. Pulp Pap Can 107(1):34–41
Shinoda R, Saito T, Okita Y, Isogai A (2012) Relationship between length and degree of polymerization of tempo-oxidized cellulose nanofibrils. Biomacromolecules 13(3):842–849
Siro I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17(3):459–494
Spence KL, Venditti RA, Rojas OJ, Habibi Y, Pawlak JJ (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18(4):1097–1111
Stelte W, Sanadi AR (2009) Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind Eng Chem Res 48(24):11211–11219
Syverud K, Xhanari K, Chinga-Carrasco G, Yu Y, Stenius P (2011) Films made of cellulose nanofibrils: surface modification by adsorption of a cationic surfactant and characterization by computer-assisted electron microscopy. J Nanopart Res 13:773–782
Uetani K, Yano H (2011) Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 12(2):348–353
Wang QQ, Zhu JY, Gleisner R, Kuster TA, Baxa U, McNeil SE (2012) Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 19(5):1631–1643
Weise U, Maloney T, Paulapuro H (1996) Quantification of water in different states of interaction with wood pulp fibres. Cellulose 3(4):189–202
Acknowledgments
This work was partially supported by the USDA Forest Service R&D special funding on Cellulose Nano-Materials (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, S.S., Zhu, J.Y., Deng, Y. et al. Characterization of cellulose nanofibrillation by micro grinding. J Nanopart Res 16, 2349 (2014). https://doi.org/10.1007/s11051-014-2349-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2349-7