Abstract
Pd ion doping has influenced the band gap of SnO2 nanoparticles. Undoped and Pd ion-doped SnO2 nanoparticles were synthesized by chemical co-precipitation method. A tetragonal phase of SnO2 with a grain size range of 7–13 nm was obtained (studied by X-ray diffraction and transmission electron microscopy). A decreasing trend in the particle size with increasing doping concentration was observed. The presence of Pd in doped SnO2 was confirmed by chemical analysis carried out by energy-dispersive spectroscopy in the transmission electron microscope. Diffuse reflectance spectra showed a blue shift in absorption with increasing palladium concentration. Band gap of SnO2 nanoparticles was estimated from the diffuse reflectance spectra using Kubelka–Munk function and it was increasing with the increase of Pd ion concentration from 3.73 to 4.21 eV. The variation in band gap is attributed predominantly to the lattice strain and particle size. All the samples showed a broad photoluminescence emission centered at 375 nm when excited at 270 nm. A systematic study on the structural and optical properties of SnO2 nanoparticles is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tin oxide (SnO2) is a n-type wide band gap semiconducting material at room temperature with a band gap of 3.6 eV at 300 K (Robertson 1979). It has a wide range of linear and non-linear applications due to its structural (Wu et al. 1997), optical, and electrical properties in nanoscale dimensions, for example, gas sensors (Shen et al. 2009; Siciliano 2000; Lassesson et al. 2008), solar cells (Sun and Ouyang 2012; Tsai et al. 2011), LEDs (Morgan et al. 2000; Cirpan and Karasz 2006) due to good transparency and conductivity, Li ion batteries (Chandra Bose et al. 2002; Zhang et al. 2011; Zhu et al. 2011) as a anode material, capacitance/supercapacitors (Mu et al. 2011) for rapid transport of the electrolyte ions, electrochemical electrodes (Wang and Wang 2000; Ching et al. 2011), and as a support catalyst material (Marshall and Haverkamp 2012; Shao et al. 2009; Zhang et al. 2010).
With decreasing particle size in nanoscale regime, a drastic change is expected in morphology, structural and optical properties of tin oxide due to a large surface to volume ratio and surface-induced defects (Gleiter 1989). There are two major methods which have been used to tune the properties of SnO2 nanoparticles. The first is the heat treatment at different temperatures. In this method, the particle size may increase with calcinations at higher temperatures. The second one is doping of metal ions. There are many studies reported on tin oxide doped with transition metals (Co, Fe, and Ni) (Siciliano 2000; Ahmed et al. 2011; Sharma et al. 2011); pentavalent elements (Sb) (Marshall and Haverkamp 2012; Shao et al. 2009); and Ti and Zr (Postovalova et al. 2000) for various applications. Li-doped ITO was used to detect the Mn ions (Ching et al. 2011). There is a huge interest in doping catalytic metal ions, Pt and Pd in the SnO2 host material. These catalytic ions do not affect the structure of the host material but, in fact, improve the gas sensitivity and selectivity, lower the operating temperature, and improve the response time (Shen et al. 2009; Lassesson et al. 2008). Different dopants such as Zn, Ti, or V ions in SnO2 affect the structure and morphology; and are responsible for smaller crystallite size, creating high surface area and high packing density (Ahmed et al. 2011; Wang et al. 2010). SnO2 is also used as dilute magnetic semiconductor in spintronics when it is doped with magnetic ions. Recently such oxides having applications in spintronics were found to have room-temperature ferromagnetism (without any dopants) induced by surface oxygen atoms when the particle size is in the range 7–30 nm (Sundaresan et al. 2006). As mentioned before, many studies were reported on the Pd-doped SnO2 system (Pd:SnO2) but most of them have focused either directly on the gas-sensing properties or electrical properties (Matko et al. 1999). Reports on band gap tuning by Pd doping in SnO2 is hardly found and this is the motivation of this work. The objective of the present work is to study the effect of Pd ions on the band gap of SnO2 nanoparticles and to tune the same. Band gap tuning is successfully reported and supportive results of structural and optical properties were also systematically studied and reported here.
Experimental procedure
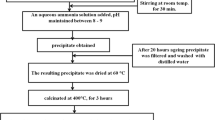
Undoped and Pd ion-doped SnO2 nanoparticles were prepared by chemical co-precipitation method. The advantages of the precipitation methods are the use of cheap salt precursor, simple operation, and ease of mass production. Moreover, the reaction conditions are mild and adjustable for different purposes (Dhannia et al. 2009). The chemical precursors were 99 % pure tin chloride dihydrate (SnCl2·2H2O) (Sigma Aldrich) and 99 % pure palladium chloride (Sigma Aldrich) and they were used as received without any further purification. Aqueous solution of 0.1 M tin chloride dihydrate and PdCl2 was placed in a beaker and stirred for 1 h at room temperature. Quantity of PdCl2 was chosen in such a way to give Pd ions concentrations to be 1, 3, and 5 mol% in SnO2. The homogenized solution was then neutralized by the addition of ammonia solution drop by drop. A white precipitate was formed immediately and that was washed well for a few times with distilled water and dried in air. All the samples were heat-treated at 400 °C for 1 h in air before carrying out all studies.
X-ray diffraction (XRD) patterns were recorded in a powder X-ray diffractometer (STOE diffractometer) by using a Cu-Kα (λ = 1.5406 Å) radiation in Bragg–Brentano geometry in a 2θ range from 0 to 70°. The voltage and current used in the X-ray generator were 40 kV and 30 mA, respectively. Transmission electron microscopy (TEM) studies were carried out in a high-resolution TEM microscope (LIBRA 200FE, Carl Zeiss) at an operating voltage of 200 kV, equipped with EDS (energy-dispersive spectroscopy) detector. It also contains an in-column Ω filter for spectroscopic studies and high-angle annular dark field detector (HAADF). The TEM specimens were prepared by dispersing the SnO2 nanoparticles in ethanol followed by ultrasonication and a drop of it was laid on a carbon-coated Cu TEM grid. Particle size distribution was obtained from the TEM image analysis. EDS was acquired with 17° tilt of the sample with respect to optic axis and the spectra were collected for 2 min of total collection time.
Fourier transform infrared (FTIR) spectra were recorded using Thermo Nicol model-6700 spectrometer with a resolution of 0.1 cm−1 on the standard pellet made from SnO2 with KBr. UV–Vis Spectra were recorded in absorption and diffuse reflectance (DRS) mode in a Varian 5000 UV–Vis spectrometer in the range from 200 to 800 nm with a resolution of 1 nm. Photoluminescence emission spectra were recorded in a fluorescence spectrophotometer (FluoroMax-P, Horiba Jobin Yvon) in the range of 200–500 nm. The emission spectra were recorded with the excitation wavelength λ ex = 270 nm. Powder samples were used for UV–Vis and PL spectra acquisition and they were packed in a standard sample holders provided by the company.
Results and discussion
Structure and microstructure studies
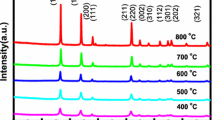
Figure 1 presents the XRD patterns of the undoped and Pd:SnO2 nanoparticles. The diffraction peaks were compared with the standard JCPDS data for SnO2 (JCPDS file number 41-1445) and they were indexed to the tetragonal rutile phase of SnO2 and marked in Fig. 1. All the samples were found to be crystalline. The samples are in single phase with no other secondary phases implying a well substitution of Pd ions in SnO2. Undoped SnO2 is referred as pure SnO2 and used as a control sample.
The crystallite size (t) was calculated from the FWHM of the 100 % intense diffraction peak of the XRD pattern by using the Debye–Scherrer formula given by
where λ is the wavelength of the X-rays used, β is the full width at half maximum (FWHM), θ is the Bragg angle, and t is the average crystallite size. The crystallite size of the undoped SnO2 is 11 nm; while on doping of Pd ion to the concentration of 1, 3, and 5 mol%, the particle sizes are 13, 10, and 8 nm, respectively. The crystallite size of 1 mol% Pd:SnO2 is observed to be increased to 13 nm as compared to the same of the pure SnO2 (11 nm). Perhaps, the following hypothesis is expected to occur during the chemical synthesis for the crystallite size change with Pd ions doping. It is known that the evolution of microstructure of materials depends mostly on the rate of ions transport during the chemical reaction. The rate is expected to be more for the heavier ions than the lighter one in the given medium. Therefore, the lighter Pd ions could have assisted faster assembly of slightly bigger grains of Pd:SnO2 compared to pure SnO2. But further increasing the Pd concentration as in case of 3 and 5 mol%, the reactions still become faster and should lead to the bigger crystallite but, due to scattering of ions that participated in nucleation and growth, could have caused the smaller crystallite size. There are reports in which particle size was controlled by the synthesis parameters such as concentration of the precursor materials (Ribeiro et al. 2004).
The crystallite size calculation was also done by Williamson–Hall (W–H) (Pal and Chauhan 2007) method. The W–H plot is a plot of βcosθ versus 4sinθ. Crystallite size can be obtained from the y-intercept whereas the strain value is obtained from the slope of the straight line fit to the data. When the particle size becomes smaller, more lattice strain can be expected in the nanoparticles. This lattice strain will lead to a broadening in the XRD peaks. The width of the diffraction peak depends on particle size, shape, inhomogeneous strain, and instrumentation errors. Strain has a major impact on variation of grain size. Therefore, W–H plot, by taking take care of the peak broadening by strain, gives a more accurate particle size estimation. In addition, one can get the strain present in the crystal lattice. The Williamson–Hall formula is given below as,
where t and ε are the effective particle size and effective strain, respectively, and other symbols hold the same meaning as in the Scherrer formula. The W–H plot for undoped and Pd:SnO2 nanoparticles is presented in Fig. 2.
The grain size obtained from the W–H plot for the undoped SnO2 is 10 nm, and the same for the 1, 3, and 5 mol% Pd:SnO2 is 11, 9, and 7 nm, respectively. The trend followed by the grain sizes obtained from W–H plot is similar as the crystallite sizes calculated by the Debye–Scherrer formula. The negative slope of the fitted lines in the W–H plot indicates the presence of compressive strain in the lattice of undoped and Pd:SnO2 nanoparticles. In 1 mol% Pd-doped SnO2, the compressive strain is less as compared to the pure one. On increasing Pd concentration, the compressive strain increases from −0.01193 to −0.01746. This behavior of strain with Pd concentration is presented in Fig. 3. The grain size and the strain calculated from the W–H plot are listed in Table 1.
Microstructure of pure and Pd:SnO2 nanoparticles was studied by TEM microscopy. Typical bright field TEM micrographs of Pd:SnO2 nanoparticles are presented in Fig. 4. For each sample, low-magnification and high-resolution TEM micrographs are presented along with a selected area diffraction pattern as inset. The shape of the Pd:SnO2 nanoparticles are nearly spherical in all cases. Particle size distribution in Pd:SnO2 was estimated from the TEM image analysis by using the ImageJ software. The analysis was carried out on both the bright and dark field images. The histograms of the particle size distribution for the undoped, 1, 3, and 5 mol% Pd:SnO2 nanoparticles are presented in Fig. 5a–d. The histograms were fitted to the log-normal distribution function and the peak values were taken as average particle size that is ranging from 8.8 to 13.1 nm. As we obtained earlier from the XRD analysis, the particle size of the 1 mol% Pd:SnO2 is slightly higher than the pure SnO2 and then decreases with further increase of doping concentration. A comparison was made between the particle sizes (see Table 1) calculated from the Debye–Scherrer’s formula, W–H plot, and TEM, and in all cases, they agreed well with each other.
Typical high-resolution TEM images for the Pd:SnO2 nanoparticles are presented in Fig. 4b, d, f, and h. Inter-planar distance of 0.33 and 0.23 nm have been calculated from the lattice fringes obtained in the HRTEM images and they correspond to (110) and (111) planes, respectively, of the tetragonal phase of SnO2. The inset of the HRTEM image shows the selected area electron diffraction (SAED) patterns that were indexed to the tetragonal SnO2. Elemental analysis was performed by EDS spectroscopy and the EDS spectra of Pd:SnO2 are presented in Fig. 6. The presence of Sn, O, and Pd was studied. The EDS peak at 2.838 eV is present only in the Pd ion-doped samples and absent in the undoped SnO2. Thus EDS results confirm the presence of Pd in the Pd-doped SnO2 nanoparticles.
FTIR studies
In the nanoscale materials, the surface is highly sensitive and greatly modified easily. In order to understand the effects of surface modification and removal of impurities from the synthesis, FTIR studies were crucial and carried out. The chemical species present in SnO2 nanoparticles were investigated by infrared spectra. The FTIR spectra of all the Pd:SnO2 nanoparticles are presented in Fig. 7. There are several bands present in the wavenumber range 4,000–400 cm−1. The band in the range 3,110–3,431 cm−1 and at 1,635 cm−1 were assigned to the vibration of hydroxyl group due to the absorbed/adsorbed water and showing a stretching vibrational mode of O–H group (Gondal et al. 2010). The bands at 2,356; 2,852; and 2,923 cm−1 belong to the stretching vibrations of C–H bonds that could be from the adsorption and interaction of atmospheric carbon dioxide with water (Gnanam and Rajendran 2010; Tan et al. 2011). The band appeared in the region 400–700 cm−1 is assigned to the Sn–O anti-symmetric vibrations. In that region, the peaks at 560 and 670 cm−1 are assigned to Sn–O and Sn–O–Sn vibrations, respectively (Gondal et al. 2010). In all samples, the vibrations associated to C–H and O–H bonds are seen. This implies that the surface is highly active and adsorbed these molecules. Therefore, if any surface modification is to be done on these samples, de-hydrolysis should be done by heating. Changes observed in the shape, width, and positions of FTIR peaks were attributed to the variation in the local defects, grain size and shape of the nanoparticles (Srinivas et al. 2011).
UV–Vis spectroscopy studies
The effect of Pd ions doping on the band gap of SnO2 nanoparticles was studied by UV–Vis absorption spectroscopy. The UV–Vis diffuse reflection spectra of the pure and Pd ion-doped SnO2 nanoparticles are presented in Fig. 8 and its inset shows the magnified view of the region marked with dotted square for better clarity. With Pd ions concentration, the onset of the reflection spectra is blue shifted.
Experimentally, optical absorption coefficient α of a semiconductor close to the band edge can be expressed by the Tauc plot (Pankove 1971) as given in Eq. 3.
where α is the absorption coefficient, A is a constant, E g is the absorption band, hν is the photon energy, and n depends on the nature of the transition. That is, n can be 1/2, 2, 3/2, and 3 corresponding to the allowed direct, allowed indirect, forbidden direct, and forbidden indirect transitions, respectively. In this case, n = 1/2 that is for direct allowed transition. Figure 9 presents the UV–Vis diffuse reflectance spectra of pure and Pd ion-doped SnO2 nanoparticles. The band gap energies were calculated using Kubelka–Munk (K–M) model (Tandon and Gupta 1970) which at any wavelength is given by,
where F(R) = α is the K–M function and R is the percentage of reflectance. A graph is plotted between [F(R)*hν]2 versus hν and the x-intercept value yields the band gap energy. Though the Eq. (3) is enough to calculate the band gap energy from the UV–Vis spectra, we have used the K–M plot for this purpose. Since K–M function is better to be used to derive this parameter from the diffused reflectance spectra of the powder sample (Tandon and Gupta 1970). The K–M plot for the Pd:SnO2 nanoparticles is presented in Fig. 7. The band gap energies were calculated by extrapolating the absorption to zero absorption coefficients which is calculated from the Eq. (3). The band gap energies thus obtained are 3.73, 4.03, 4.12, and 4.21 eV for the 0, 1, 3, and 5 mol% Pd:SnO2 nanoparticles, respectively. Change in band gap energy with doping concentration is shown in the inset of Fig. 9. The band gap energy shows a blue shift with increasing Pd concentration. This increase in band gap with Pd concentration can be attributed either to the decreasing grain size or to strain present in the samples.
Once the increase in E g is attributed only to the grain size, it is contradicted in the case of 1 mol% Pd:SnO2; Because, the 1 mol% Pd:SnO2 sample, by having a higher particle size (than the pure SnO2), also has the higher band gap energy of 4.03 eV. Therefore, increasing band gap is not only due to the quantum confinement effect, but also due to other factors such as doping concentration (impurity), lattice strain, surface effect, etc. (Smith et al. 2009; Chen et al. 2003), which might be dominated by the later effects. Another reason of increasing band gap energy is the charge transfer transitions between the Pd atom and the SnO2 conduction or valence band. Similar results have been reported earlier by others researchers (Fang et al. 2008). Generally when the particle radius of the semiconductor is equal or less than excitonic Bohr radius, quantum confinement effect is dominantly observed. When the size of the particle is higher than the excitonic radius, the region is called a weakly confined region and the contribution from the particle size to the confinement effect will be less. The excitonic Bohr radius of the SnO2 is 2.7 nm (Ribeiro et al. 2004). In our case, the particle size is greater than the excitonic Bohr radius of SnO2, which means that they are in the weakly confining region. Hence in our samples, the contribution to be band gap from the particle size is only slight but some other factors are influencing, may be, dominantly. Lattice strain is the next major factor to influence the energy gap of the materials. With further decreasing particle size, more and more compressive lattice strain is expected to be introduced in the lattice. Compressive strain is attributed to the imperfection in the coordination number and also to the bond order loss in nanoparticles (Zhu et al. 2010). This lattice strain is basically due to imperfection or lattice dislocation causing the increment of band gap energy. The lattice strain calculated earlier for pure and Pd:SnO2 nanoparticles (Table 1) is evident for its presence and contributed to the variation in band gap. The lattice strain was compressive in nature and increasing with the Pd ion concentration. Similar kind of change in band gap was observed in carbon nanotubes and that change was induced by the uniaxial strain (Maki et al. 2007).
Photoluminescence studies
Figure 10 shows the PL emission spectra of Pd:SnO2 nanoparticles acquired at room temperature when excited at 270 nm. All the samples show a strong and broad emission in the ultraviolet region. In literature there were only a few studies made on the Pd ion-doped SnO2 nanoparticles. It is to be noted that the intensity of the 3 mol% Pd:SnO2 is lower than the rest of the samples. Intensity is not necessarily to be compared as such because the samples were in powder form and packed manually in the holder and, therefore, only qualitative analysis is to be considered in this case. The emission bands have been observed at 3.69 eV at 250-nm excitation (Pan et al. 2006) for nitrogen-incorporated SnO2 thin film and 3.16 eV for SnO2 (Tan et al. 2011) at 275-nm excitation. In the literature, various reports can be found with the emission peaks of SnO2 that occurred in a range from 318 to 640 nm (Gu et al. 2003; Ahmed et al. 2011; Gondal et al. 2010). Even then the mechanism of PL emission in SnO2 is not properly understood. In general, one can expect a sharp peak along with a broad spectrum in oxide semiconductors. Generally, two mechanisms of emission in metal oxides are expected. One is a direct recombination of a conduction electron in a Si 4p band and a hole in the O 2p valence band and usually this gives rise to a sharp emission peak. This is a band edge transition across the band gap of 3.6 eV (for bulk SnO2). If this is a case, then the emission corresponding to these transitions should lie in the range 345–350 nm. But, in the present case, the emission peak was observed at 375 nm (3.31 eV). Therefore, the emission through direct recombination across the band gap is not feasible and ruled out. Another mechanism is due to transitions between localized electronic states, which could be due to lattice defects created by impurities, interstitial defects, and dangling bonds (Gu et al. 2003; Hu et al. 2002; Brovelli et al. 2006). This usually gives a broad emission peak assumed to be originated from multiple luminescent centers. It is well known that oxygen vacancies in SnO2 generate localized states (act as radiative centers in PL) in the form of shallow donor levels approximately 0.03–0.3 eV below the bottom of the SnO2 conduction band (Brovelli et al. 2006). The considerable number of trap states can also be formed by the Sn vacancies and interstitials. Therefore, the emission related to the transitions with the participation of these shallow levels should be around 375 nm in SnO2. Based on the discussion above, it is implied that the observed luminescence bands can be correlated to the transitions (shown in Fig. 10) with the participation of shallow acceptor and shallow donor levels.
Conclusions
Nanocrystalline rutile-structured SnO2 with a size range of 8–13 nm was prepared by a cost-effective preparation method. Grain size obtained by three methods namely Debye–Scherrer formula, W–H plot, and TEM image analysis agree well with each other. FTIR study showed the presence of the adsorbed species implying the importance of heat treatment for high-end applications of these materials. The important feature of this work is the band gap tuning that was achieved by doping Pd ions. The dominant cause for the band gap change is the lattice strain introduced by the doping. Strong PL emission was observed in the UV region. A good optical emitting SnO2 nanoparticles with band gap tunability was demonstrated in this work.
References
Ahmed AS, Muhamed SM, Singla ML, Tabassum S, Naqvi AH, Azam A (2011) Band gap narrowing and fluorescence properties of nickel doped SnO2 nanoparticles. J Lumin 131:1–6
Brovelli S, Chiodini N, Meinardi F, Lauria A, Paleari A (2006) Ultraviolet free-exciton light emission in Er-passivated SnO2 nanocrystals in silica. Appl Phys Lett 89:153126
Chandra Bose A, Kalpana D, Thangadurai P, Ramasamy S (2002) Synthesis and characterization of nanocrystalline SnO2 and fabrication of lithium cell using nano-SnO2. J Power Sources 107:138–141
Chen X, Lou Y, Samia AC, Burda C (2003) Coherency strain effects on the optical response of core/shell heteronanostructures. Nano Lett 3:799–803
Ching YM, Tee TW, Zainal Z (2011) Electrochemical studies of Mn(II) mediated by Li+ doped indium tin oxide (ITO) electrode. Int J Electrochem Sci 6:5305–5313
Cirpan A, Karasz FE (2006) Indium tin oxide nanoparticles as anode for light-emitting diodes. J Appl Polym Sci 99:3125–3129
Dhannia T, Jayalekshmi S, Santhosh Kumar MC, Prasada Rao T, Chandra Bose A (2009) Effect of aluminium doping and annealing on structural and optical properties of cerium oxide nanocrystals. J Phys Chem Solids 70:1443–1447
Fang LM, Zu XT, Li ZJ, Zhu S, Liu CM, Zhou WL, Wang LM (2008) Synthesis and characteristics of Fe3+-doped SnO2 nanoparticles via sol–gel-calcination or sol–gel-hydrothermal route. J Alloys Compd 454:261–267
Gleiter H (1989) Nanocrystalline materials. Prog Mater Sci 33:233–315
Gnanam S, Rajendran V (2010) Preparation of Cd-doped SnO2 nanoparticles by sol–gel route and their optical properties. J Sol–Gel Sci Technol 56:128–133
Gondal MA, Drmosh QA, Saleh TA (2010) Preparation and characterization of SnO2 nanoparticles using high power pulsed laser. Appl Surf Sci 256:7067–7070
Gu F, Wang SF, Song CF, Lv MK, Qi YX, Zhou GJ, Xu D, Yuan DR (2003) Synthesis and luminescence properties of SnO2 nanoparticles. Chem Phys Lett 372:451–454
Hu JQ, Ma XL, Shang NG, Xie ZY, Wong NB, Lee CS, Lee ST (2002) Large-scale rapid oxidation synthesis of SnO2 nanoribbons. J Phys Chem B 106:3823–3826
Lassesson A, Schulze M, van Lith J, Brown SA (2008) Tin oxide nanocluster hydrogen and ammonia sensors. Nanotechnology 19:015502–015508
Maki H, Sato T, Ishibashi K (2007) Direct observation of the deformation and the band gap change from an individual single-walled carbon nanotube under uniaxial strain. Nano Lett 7:890–895
Marshall AT, Haverkamp RG (2012) Nanoparticles of IrO2 or Sb–SnO2 increase the performance of iridium oxide DSA electrodes. J Mater Sci 47:1135–1141
Matko I, Gaidi M, Hazemann JL, Chenevier B, Labeau M (1999) Electrical properties under polluting gas (CO) of Pt- and Pd-doped polycrystalline SnO2 thin films: analysis of the metal aggregate size effect. Sens Actuators B 59:210–215
Morgan DV, Al-Ofi IM, Aliyu YH (2000) Indium tin oxide spreading layers for AlGaInP visible LEDs. Semicond Sci Technol 15:67–72
Mu J, Chen B, Guo Z, Zhang M, Zhang Z, Shao C, Liu Y (2011) Tin oxide (SnO2) nanoparticles/electrospun carbon nanofibers (CNFs) heterostructures: controlled fabrication and high capacitive behaviour. J Colloid Interface Sci 356:706712
Pan SS, Ye C, Teng XM, Li L, Li GH (2006) Localized exciton luminescence in nitrogen-incorporated SnO2 thin films. Appl Phys Lett 89(251911):1–3
Pankove J (1971) Optical processes in semiconductors. Dover Publications, New York
Postovalova GG, Roginskaya YE, Zav SA, Yalov, BSh Galyamov, Kilmasenko NL (2000) Formation of high conductivity regions in nanostructured SnO2–AOx (A = Ti4+, Zr4+, Sb3+, Sb5+) films exposed to UV Radiation or H2. Inorg Mater 36:364–374
Ribeiro C, Lee EJH, Giraldi TR, Longo E, Varela JA, Leite ER (2004) Study of synthesis variables in the nanocrystal growth behavior of tin oxide processed by controlled hydrolysis. J Phys Chem B 108:15612–15617
Robertson J (1979) Electronic structure of SnO2, GeO2, PbO2, TeO2 and MgF2. J Phys C 12:4767–4776
Shao Y, Liu J, Wang Y, Lin Y (2009) Novel catalyst support materials for PEM fuel cells: current status and future prospects. J Mater Chem 19:46–59
Sharma A, Varshney M, Kumar S, Verma KD, Kumar R (2011) Magnetic properties of Fe and Ni doped SnO2 nanoparticles. Nanomater Nanotechnol 1:29–33
Shen Y, Yamazaki T, Liu Z, Meng D, Kikuta T (2009) Hydrogen sensors made of undoped and Pt-doped SnO2 nanowires. J Alloys Compd 488:L21–L25
Siciliano P (2000) Preparation, characterisation and applications of thin films for gas sensors prepared by cheap chemical method. Sens Actuators B 70:153–164
Smith AM, Mohs AM, Nie S (2009) Tuning the optical and electronic properties of colloidal nanocrystals by lattice strain. Nat Nanotechnol 4:56–63
Srinivas K, Manjunath Rao S, Venugopal Reddy P (2011) Structural, electronic and magnetic properties of Sn0.95Ni0.05O2 nanorods. Nanoscale 3:642–653
Sun K, Ouyang J (2012) Polymer solar cells using chlorinated indium tin oxide electrodes with high work function as the anode. Solar Energy Mater Solar Cells 96:238–243
Sundaresan A, Bhargavi R, Rangarajan N, Siddesh U, Rao CNR (2006) Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys Rev B 74:161306R
Tan L, Wang L, Wang Y (2011) Hydrothermal synthesis of SnO2 nanostructures with different morphologies and their optical properties. J Nanomater. doi:10.1155/2011/529874
Tandon SP, Gupta JP (1970) Measurement of forbidden energy gap of semiconductors by diffuse reflectance technique. Phys Status Solidi 38:363–367
Tsai CH, Hsu SY, Huang TW, Tsai YT, Chen YF, Jhang YH, Hsieh L, Wu CC, Chen YS, Chen CW, Li CC (2011) Influences of textures in fluorine-doped tin oxide on characteristics of dye-sensitized solar cells. Org Electron 12:2003–2011
Wang WY, Wang CM (2000) Nanocrystalline tin-oxide modified electrodes and their electrochemical characterization. J Chin Chem Soc 47:405–414
Wang CT, Lai DL, Chen MT (2010) Surface and catalytic properties of doped tin oxide nanoparticles. Appl Surf Sci 257:127–131
Wu X, Zou B, Xu J, Yu B, Tang G, Zhang G, Chen W (1997) Structural characterization and optical properties of nanometer-sized SnO2 capped by stearic acid. Nanostructured Mater 8:179–189
Zhang P, Huang SY, Popov BN (2010) Mesoporous tin oxide as an oxidation-resistant catalyst support for proton exchange membrane fuel cells. J Electrochem Soc 157:B1163–B1172
Zhang M, Lei D, Du Z, Yin X, Chen L, Li Q, Wang Y, Wang T (2011) Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J Mater Chem 21:1673–1676
Zhu Z, Ouyang G, Yang G (2010) Bandgap shift in SnO2 nanostructures induced by lattice strain and coordination imperfection. J Appl Phys 108:083511–083514
Zhu X, Zhu Y, Murali S, Stoller MD, Ruoff RS (2011) Reduced graphene oxide/tin oxide composite as an enhanced anode material for lithium ion batteries prepared by homogenous coprecipitation. J Power Sources 196:6473–6477
Acknowledgments
The financial supports from the UGC-DAE-CSR (CSR-KN/CRS-14/2011-12/586) and the start-up grant (PU/PC/Start-up Grant/2011-12/311) of Pondicherry University are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nandan, B., Venugopal, B., Amirthapandian, S. et al. Effect of Pd ion doping in the band gap of SnO2 nanoparticles: structural and optical studies. J Nanopart Res 15, 1999 (2013). https://doi.org/10.1007/s11051-013-1999-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1999-1