Abstract
A colorimetric system using noble metal nanoparticles (NPs), for the detection of small concentrations of mercury ion (Hg2+) in water is described. Fluorescein-functionalized Au and Ag NPs were self-assembled into networks of one-dimensional chains in the presence of mercury(II) by chelation process; this causes an easily optical measurable change in the absorption spectrum of the particles in aqueous solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years there has been growing interest among chemists in the preparation of new materials that are ordered on length scales that extend beyond the molecule (Mann et al. 2000). Particularly, one-dimensional (1D) assemblies of nanoparticles (NPs), i.e. chains, can significantly help in the understanding of some processes in living organisms (Walker et al. 2002; Alivisatos 2004) and fundamental quantum mechanics of nanometre-scale systems (Maier et al. 2001, 2003). A directional organization process is critical for the preparation of 1D NP assemblies. The use of linear templates (organic polyelectrolyte (Minko et al. 2002; Kiriy et al. 2002; Yu and Cölfen 2004; Cölfen and Qi 2001; Yu et al. 2002, 2003; Zhang et al. 2001) and biomolecules (Warner and Hutchinson 2003; Mann et al. 2000; Lui and Lu 2003; Si et al. 2007a, b; Walsh et al. 2003; Lee et al. 2002), inorganic wires (Ajayan and Lijima 1993; Yin et al. 2004; Han and Zettl 2003; Wang et al. 2004) and nanotubes (Ugarte et al. 1998), pores (Martin 1996) and step-edges (Fort et al. 2003)) is a vital and feasible approach for the preparation of 1D assemblies of NPs (Tang and Kotov 2005).
On the other hand, among the various MNPs, gold (Au) and silver (Ag) NPs can be easily used as optical probes, since they present an intense surface Plasmon resonance band (SPR) in the visible region (ca. 520 nm for Au and ca. 400 nm for Ag). However, the localization of the SPR band depends of some parameters, as size, shape and refractive index of the environment. Depending of the conditions the NPs could present different colours. The NPs colour could also change upon the coupling of SPR of adjacent Nps (Chechik and Crooks 1999; Lowe et al. 2002; Si et al. 2007a, b). Furthermore, AuNPs and AgNPs are known to dramatically change the optical properties of nearby fluorophores (Hong and Kang 2006; Aslan et al. 2007; Cheng and Xu 2007). Studies involving AuNPs and AgNPs coated with fluorophores on surface, such as fluorescein, often show an emission fluorescence quenching (Dulkeith et al. 2005; Schneider and Decher 2006) or enhancement (Aslan et al. 2004, 2006; Lakowicz et al. 2002; Zhang and Lakowicz 2006; Xu et al. 2011), which highly depended on fluorophore–metal distance (Cheng and Xu 2007; De and Kundu, 2011; Saha et al. 2009).
Having in mind this aspect, and following our research project in new fluorescent and/or colorimetric chemosensors (Lodeiro, et al. 2010; Lodeiro and Pina 2009), herein we describe the design of colorimetric probes based on fluorescein-directed assembly of gold and silver NPs, and their use for sensitive and selective detection and quantification of mercury metal ion by the formation of 1D chains without templates. For this study, a novel compound L with formula C26H24O5S2 was synthesized and fully characterized by reaction of fluorescein (free acid) as precursor with 3-chloro-1-propanethiol, yielding 95 % of an orange powder (L) (Scheme 1).
Experimental details
Physical measurements
Elemental analyses were carried out with Fisons Instruments EA1108 microanalyzer at the University of Vigo (CACTI), Spain. Infrared spectra were recorded in KBr windows using a JASCO FT/IR-410 spectrophotometer. 1H and 13C NMR were carried out in a BrukerAvance III400 at an operating frequency of 400 MHz for 1H NMR and 100.6 MHz for 13C NMR using the solvent peak as an internal reference at 25 ºC.
The MALDI-MS analyses have been performed with a MALDI-TOF-TOF-MS model Ultraflex II (Bruker, Germany) equipped with nitrogen from the BIOSCOPE group. Each spectrum represents accumulations of 5 × 50 laser shots. The reflection mode was used. The ion source and flight tube pressure were less than 1.80 × 10−7 and 5.60 × 10−8 Torr, respectively. The MALDI mass spectra of the soluble samples (1 or 2 μg/μL) were recorded using the conventional sample preparation method for MALDI-MS. One microlitre was put on the sample holder on which the ligand had been previously spotted. The sample holder was inserted in the ion source.
The nanoparticles size distributions were measured using a dynamic light scattering system, Malvern Nano ZS instrument with a 533-nm laser diode from the Faculty of Science at Ourense Campus, University of Vigo. To perform the transmission electron microscopy (TEM) images the samples were prepared dropping 1 μL of the colloidal suspension onto a copper grid coated with a continuous carbon film and allowing the solvent to evaporate. TEM images were obtained through a JEOL JEM 1010F TEM operating at 100 kV. To perform the Fourier transformations we used the Digital Micrograph (Gatan) software (Sánchez-Iglesias et al. 2006).
UV–Vis absorption spectra (220–900 nm) were performed using a JASCO-650 UV–Vis spectrophotometer and fluorescence spectra on a HORIBA JOVIN-IBON Spectramax 4. The linearity of the fluorescence emission vs. concentration was checked in the concentration range used (10−5–10−6 M). A correction for the absorbed light was performed when necessary. The spectrophotometric characterizations and titrations were performed as follows: the stock solutions of the compounds (ca. 10−3 M) were prepared by dissolving an appropriate amount of the compounds in a 10-mL volumetric flask and diluting them to the mark with absolute ethanol. The solutions were prepared by appropriate dilution of the stock solutions, which were still 10−5–10−6 M. All of the measurements were performed at 298 K.
Luminescence quantum yield was measured using a solution of fluorescein in sodium hydroxide solution (0.1 M) as a standard ([Φ] = 0.79) (Turkevich et al. 1951; Enüstün and Turkevich 1963; Turkevich 1985a, b) for L. All values were corrected for the refraction index of the solvents.
Synthesis of chemosensor L
A solution of 3-chloro-1-propanethiol (0.333 g, 3.01 mmol) in acetone (50 mL) was added dropwise to a solution of fluorescein (free acid) (0.5 g, 1.505 mmol) in acetone (100 mL). The resulting solution was gently maintained with magnetic stirring for ca. 48 h at room temperature. After that, the solution was gently refluxed with magnetic stirring for ca. 2 h. The solvent was evaporated under reduced pressure, yielding an orange oil. The oil was washed with hexane, leading to an orange powder that was characterized as chemosensor L. L was soluble in acetone, methanol, ethanol and DMSO and insoluble in chloroform, dichloromethane, ethyl acetate and water.
L: Colour: orange powder. (95 %): Anal. Calc. for C26H26O6S2: C, 62.6; H, 5.2; S, 12.8. Found: C, 62.1; H, 4.7; S, 13.0 %. IR (KBr windows) cm−1: ν(C=C) 1,595, ν(S–H) 2,560. NMR spectra: δ H (DMSO-d 6, 400 MHz) ppm: 1.96 (t, J = 7.0 Hz, 2H, H2), 2.06 (t, J = 6.8 Hz, 2H, H1), 2.19 (s, 2H, SH24), 2.70 (t, J = 7.0 Hz, 2H, H2′), 2.82 (t, J = 6.8 Hz 2H, H1′), 3.73 (m, J = 6.5, Hz 4H, H3), 6.54 (s, 2H, H9 and H15), 6.68 (s, 2H, H5 and H13), 7.25 (s, 2H, H6 and H12), 7.70–7.72 (m, 2H, H20 and H18); 7.76–7.80 (m, 1H, H19); 7.99 (d, 1H, H21); δ C (DMSO-d 6, 400 MHz) ppm: 26.61 (C1), 31.32 (C2), 34.40 (C3), 83.05 (C10), 102.14 (C9, C15), 109.49 (C7, C11), 112.51 (C5,C13), 124.49 (C18), 126.08 (C22), 128.92 (C21, C20), 129.95 (C6, C12), 135.47 (C19), 151.76, 152.37 (C4, C14), 159.39 (C8, C16, C17), 168.59 (C23). MALDI-TOF/MS (m/z): 481.60 [L+H]+. The value of the luminescence quantum yield of L was 0.6989. This value was measured using a solution of fluorescein in sodium hydroxide solution (0.1 M) as a standard ([Φ] = 0.79).
Synthesis of gold and silver nanoparticles in aqueous solution
Preparation of AuNPs@L
Au@citrate NPs were prepared in aqueous solution following the Turkevich method (Turkevich et al. 1951; Enüstün and Turkevich, 1963; Turkevich 1985a, b). An aqueous solution (125 mL) of hydrogen tetrachloroaurate (0.125 mmol, 49.5 mg) was added rapidly to a solution of 1 % (P/V) sodium citrate (12.5 mL) that was heated under reflux. Heating under reflux was continued for an additional 5 min, during which time the colour changed to deep red. An ethanolic solution (10 mL) of chemosensor L (0.096 mmol, 4.8 mg) was added to 50 mL of AuNPs@citrate and stirred for 24 h (Fig. 1). After that, the resulting mixture was collected by centrifugation three times at 9,000 rpm for 30 min and the supernatant was carefully removed up. The pellet containing the AuNPs@L were re-suspended in 20 mL of milli-Q water. The formation of the AuNPs@L can be observed because of the appearance of the gold plasmonic resonance band (PRB) at 500–600 nm, that is a characteristic of well-dispersed NPs with sizes in the range of 20 ± 5 nm. The absorbance of bound fluorescein at ca. 500 nm leads to a broadening of Au plasmon band (Fig. S1).
Preparation of AgNPs@L
An aqueous solution (20 mL) of silver nitrate (0.008 mmol, 1.37 mg) was mixed with a solution of 0.3 % (P/V) polyvinylpyrrolidone (PVP) (90 mg) in milli-Q water (5 mL). The mixture was vigorously stirred and a fresh aqueous solution (10 mL) of sodium borohydride (0.050 mmol, 1.891 mg) was added quickly while stirring, during which time the colour changed to yellow.
After that, the resulting mixture was collected by centrifugation three times at 9,000 rpm for 30 min and the supernatant was carefully removed up to eliminate the NaBH4 and PVP in excess. The pellet containing the AgNPs@PVP was re-suspended in 30 mL of milli-Q water.
The formation of the NPs can be observed because of the appearance of the silver SPR at ca. 400 nm, that is a characteristic of well-dispersed NPs with sizes in the range of 10 ± 5 nm (Figure S2).
The formation of AgNPs@L from AgNPs@PVP in aqueous solution was followed by UV–Vis absorption and emission spectroscopy (Fig. 2).
Results and discussion
Small stable gold and silver NPs stabilized with chemosensor L were synthesized to explore their potential application for the detection of silent metal ions (Hg2+, Hg+, Cd2+, Zn2+, Pb2+ and Ag+) as well as Cu2+ in aqueous solution.
First, the formation of AuNPs@L was made by exchanging of the citrate molecules located in the NPs surface of AuNPs@citrate (Turkevich 1985a) for chemosensor L (Fig. 1). After 24 h, the Zeta potential changed from −33.0 mV/cm (AuNPs@citrate) to −35.0 mV/cm (AuNPs@L), introducing more stability in the system. The formation of AuNPs@L with sizes in the range of 20 ± 5 nm was confirmed by the presence of the SPR band between 500 and 600 nm in the UV–Vis spectrum (Figure S1).
The fluorescence study of the composite particle solution of AuNPs@L shows that the luminescence of the fluorescein was totally quenched by the gold metal core. This quenching occurs when the emission spectrum overlaps with the gold SPR band (Sapsford et al. 2006; Gersten and Nitzan 1981; Oh et al. 2005).
In order to keep the emission property of fluorescein units, silver NPs with PVP instead of citrate-like stabilizer were synthesized (Fig. S2). One of the principal advantages of PVP is that it presents higher molecular weight, resulting in the formation of a complete network in solution and is a biocompatible molecule that allows higher biological applications (Zielinska et al. 2009). Although the Zeta potential of AgNPs@PVP was −12.6 mV/cm, the system appears to be very stable by sterical stabilization.
The formation of AgNPs@L with sizes in the range of 11 ± 5 nm was made by exchanging of the PVP molecules located in the NPs surface of AgNPs@PVP for chemosensor L (Fig. 2). After the addition of 5 μL of L to the system AgNPs@PVP, the exchange was totally completed. The absorption spectra shows the SPR band of Ag near 400 nm showing the absorbance of bound fluorescein at ca. 500 nm as a hump near Ag plasmon band (Fig. 2a).
In this acid medium, the fluorescein ring closes and the protonation of the thiol groups takes place. This medium supports the approach of the protonated thiol groups towards the surface of the AgNPs@PVP and the superficial exchange of PVP for L occurs. A fluorescence enhancement is observed due to increased excitation rate of fluorophore by local field enhancement (Kuhn et al. 2006) (Fig. 2b).
With the aim to confirm the influence of the pH value in the absorption behaviour, a pH titration of the AgNPs@L was carried on Fig. 3. TEM images of AgNPs@L in aqueous phase to different pH values were also shown. The initial pH of the AgNPs@L in aqueous phase was 4.2. At this initial value the AgNPs are located very close and only the SPR band of AgNPs was observed at ca. 400 nm. At it is shown in the absorption spectrum of Fig. 3, at pH > 7, an increase in the band centred at ca. 510 nm, assigned to the isobenzofuran-1(3H)-one group in the open form, was observed. This form of the fluorescein in the opened form promoted a negative charge on the surface of the NPs that produces the separation between them and consequently an increase in their stability. Completely opposite behaviour was observed at more acidic conditions (pH 2.01) because at this pH value the isobenzofuran-1(3H)-one group in the closed form promoted the aggregation of the AgNPs.
The sensing of Hg2+ in aqueous solution using the fluorescein-functionalized AuNPs@L and AgNPs@L was carried out. Figure 4a shows the changes in the absorption spectra of the colloidal system AuNPs@L with the addition of increasing amounts of Hg(NO3)2. As a result of this interaction, a new absorption band centred at ca. 635 nm was observed due to the formation of chains in which Hg2+ ions played the role of connectors between the AuNPs@L (see TEM images). The colour changes from red to blue, and indicates the presence of this silent heavy-metal ion in solution (Fig. 4a).
a Spectrophotometric titration of AuNPs@L with the addition of increasing amounts of Hg(NO3)2, naked eye detection and TEM images of AuNPs@L with the addition of 200 μL of [Hg(NO3)2] = 1.00 × 10−3 M. Modifications with time in the absorption spectra and TEM images of an aqueous solution of AuNPs@L with the addition of 25 μL b 50 μL c and 100 μL d of [Hg(NO3)2] = 1.00 × 10−3 M
In the final point of the titration of AuNPs@L with Hg2+, the Zeta potential changes from −35 to −27 mV/cm. This means that a destabilization of the system took place, as can be seen in the TEM images (Fig. 4). The formation of the 1D chains between AuNPs@L modulated for the Hg2+ metal ions was possible by chelation of the Hg2+ ions with the carboxylate groups of chemosensor L located in the AuNPs surface (Fig. 5).
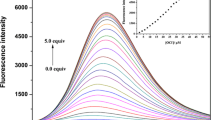
In order to evaluate the detection and the quantification limits of this system, for the detection of Hg2+ in aqueous solution, the evaluation of the SPR band was carried out (Fig. 4b–d). Thus, the minimum amount of mercury detectable at 635 nm was 0.68 ppm and the minimum amount quantified was 1.60 ppm.
From this UV–Vis spectral study, it was observed that, after the addition of 6.70 ppm of Hg2+ ion, the new SPR band developed at higher wavelength became saturated and the TEM micrograph revealed that the AuNPs were totally assembled into small 1D chains (Fig. 4). Figure 6 shows that a specific interaction between AuNPs@L with Hg2+ was observed in comparison with the other metal ions explored.
On the other hand, a selective interaction of AgNPs@L with Hg2+ was also observed in aqueous phase. As a result, a modification of the AgNPs structure, the formation of 1D chains between them and a colour change from yellow to orange of the aqueous solution of AgNPs@L were observed in the presence of mercury(II) (Fig. 7). TEM images show the aggregation process by the interaction of the AgNPs@L with the other explored metal ions, without an orderly disposition in chains to that observed for Hg2+ (Figure S3).
Spectrophotometric (a) and spectrofluorimetric (b) titration of AgNPs@PVP with compound L in absolute ethanol with the addition of Hg2+ in aqueous solution ([L] = 3.00 × 10−4 M, [Hg2+] = 1.00 × 10−3 M, room temperature, λ exc = 400 nm). Insets show absorption at 400 and 487 nm. Naked eye detection and TEM images of AgNPs@L before (yellow color) and after (orange color) the addition of 200 μL of [Hg(NO3)2] = 1.00 × 10−3 M. (Color figure online)
Similar chelation interaction of the Hg2+ ions with the carboxylate groups of chemosensor L located in the AgNPs surface was predicted to that shown in Fig. 5, being responsible for the formation of chains between the silver NPs. In this case, the minimum amount of mercury detectable at 400 nm was of 0.34 ppm and the minimum amount quantified was 2.05 ppm.
As it is shown in Fig. 7b, an increase in the emission band centred at ca. 520 nm was also observed for the system AgNPs@L in the presence of Hg2+. This result suggests that the interaction of Hg2+ with the free molecular probe does not take place only by the external carboxyl group of the isobenzofuran-1(3H)-one group, because this effect could produce the quenching of the emission for system L. It is important to mention that probably, a fraction of the Hg2+ metal ions added are located in the inner cavity of the ligand anchored to the silver metal surface for the thiol groups and the internal oxygen atoms exert a cooperative effect in the coordination of the metal ion. Probably this could also take place after the titration of AuNPs@L with Hg2+.
Two main reasons support the existence of this binding mode: first, the negative superficial charge of the silver nanoparticle, and, second, the ionic radius of this metal ion is small enough to favour the accommodation of the metal ion in the inner cavity. This effect was also observed in similar recently reported systems (Fernández-Lodeiro et al. 2013).
Moreover, the interaction of the remaining fraction of the Hg2+ ions with the external carboxylate groups of chemosensor L promoted the assembly of the AgNPs into small 1D chains. The formation of these molecular systems introduces more rigidity in the system that at the same time is in agreement with the increase in the emission intensity observed in the AgNPs@L after the interaction with Hg2+ metal ions.
Conclusions
In conclusion, new fluorescein-functionalized AuNPs@L and AgNPs@L have been employed in aqueous solution as colorimetric probes for the detection of mercury ions by 1D-chain formation. Changes in the SPR bands as well as colours due to the network of 1D assemblies of NPs were observed. The AuNPs@L was totally assembled into 1D chains after the addition of 6.70 ppm of Hg2+ in aqueous solution. The chelation interaction of the Hg2+ ions with the carboxylate groups of chemosensor L located in the AuNPs and AgNPs surface is responsible for the selective formation of chains between the NPs modulated for the Hg2+ metal ions.
References
Ajayan PM, Lijima S (1993) Capillarity-induced filling of carbon nanotubes. Nature 361:333–334
Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22:47–52
Aslan K, Lakowicz JR, Szmacinski H, Geddes CD (2004) Metal-enhanced fluorescence solution-based sensing platform. J Fluoresc 14:677–679
Aslan K, Huang J, Wilson GM, Geddes CD (2006) Metal-enhanced fluorescence-based RNA sensing. J Am Chem Soc 128:4206–4207
Aslan K, Wu M, Lakowicz R, Geddes CD (2007) Metal enhanced fluorescence solution-based sensing platform 2: fluorescent core-shell Ag@SiO2 nanoballs. J Fluoresc 17(2):127–131
Chechik V, Crooks RM (1999) Monolayers of thiol-terminated dendrimers on the surface of planar and colloidal gold. Langmuir 15:6364–6369
Cheng D, Xu QH (2007) Separation distance dependent fluorescence enhancement of fluorescein isothiocyanate by silver nanoparticles. Chem Commun 3:248–250
Cölfen H, Qi L (2001) A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer. Chem Eur J 7:106–116
De S, Kundu R (2011) Spectroscopic studies with fluorescein dye-Protonation, aggregation and interaction with nanoparticles. J Photochem Photobiol A 223:71–81
Dulkeith E, Ringler M, Klar TA, Feldmann J (2005) Gold nanoparticles quench fluorescence by phase induced radiative rate suppression. Nano Lett 5:585–589
Enüstün BV, Turkevich J (1963) Coagulation of colloidal gold. J Am Chem Soc 85:3317–3328
Fernández-Lodeiro J, Núñez C, Fernández-Lodeiro A, Oliveira E, Rodríguez-González B, Dos Santos AA, Capelo JL, Lodeiro C (2013) New-coated fluorescent silver nanoparticles with a fluorescein thiol esther derivative: fluorescent enhancement upon interaction with heavy metal ions. (submitted)
Fort E, Ricolleau C, Sau-Pueyo J (2003) Dichroic thin films of silver nanoparticle chain arrays on facetted alumina templates. Nano Lett 3:65–67
Gersten J, Nitzan AJ (1981) Spectroscopic properties of molecules interacting with small dielectric particles. Chem Phys 75:1139–1152
Han WQ, Zettl A (2003) Coating single-walled carbon nanotubes with tin oxide. Nano Lett 3:681–683
Hong B, Kang KA (2006) Biocompatible, nanogold-particle fluorescence enhancer for fluorophore mediated, optical immunosensor. Biosens Bioelectron 21:1333–1338
Kiriy A, Minko S, Gorodyska G, Stamm M, Jaeger W (2002) Palladium wire-shaped nanoparticles from single synthetic polycation molecules. Nano Lett 2:881–885
Kuhn S, Hakanson U, Rogobete L, Sandoghdar V (2006) Enhancement of single-molecule fluorescence using a gold nanoparticle as an optical nanoantenna. Phys Rev Lett 97(017402):1–4
Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, Grycznski I (2002) Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal Biochem 301:261–277
Lee SW, Mao C, Flynn CE, Blecher AM (2002) Ordering of quantum dots using genetically engineered viruses. Science 296:892–895
Lodeiro C, Pina F (2009) Luminescent and chromogenic molecular probes based on polyamines and related compounds. Coord Chem Rev 253:1353–1383
Lodeiro C, Capelo JL, Mejuto JC, Oliveira E, Santos HM, Pedras B, Núñez C (2010) Light and colour as analytical detection tools: a journey into the periodic table using polyamines to bio-inspired systems as chemosensors. Chem Soc Rev 39:2948–2976
Lowe AB, Sumerlin BS, Donovan MS, Mc Cormick CL (2002) Facile preparation of transition metal nanoparticles stabilized by well-defined (Co)polymers synthesized via aqueous reversible addition-fragmentation chain transfer polymerization. J Am Chem Soc 124:11562–11563
Lui J, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643
Maier SA, Brongersma ML, Kik PG, Meltzer S, Requicha AAG, Atwater HA (2001) Plasmonics—a route to nanoscale optical devices. Adv Mater 13:1501–1505
Maier SA, Kik PG, Atwater HA, Meltzer S, Harel E, Koel BE, Requicha AAG (2003) Local detection of electromagnetic energy transport below the diffraction limit in metal nanoparticle plasmon waveguides. Nat Mater 2:229–232
Mann S, Shenton W, Li M, Connolly S, Fitzmaurice D (2000) Biologically programmed nanoparticle assembly. Adv Mater 12:147–150
Martin CR (1996) Membrane-based synthesis of nanomaterials. Chem Mater 8:1739–1746
Minko S, Kiriy A, Gorodyska G, Stamm M (2002) Mineralization of single flexible polyelectrolyte molecules. J Am Chem Soc 124:10192–10197
Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS (2005) Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J Am Chem Soc 127:3270–3271
Saha A, Basiruddin SK, Sarkar R, Pradhan N, Jana NR (2009) Functionalized plasmonic-fluorescent nanoparticles for imaging and detection. J Phys Chem C 113:18492–18498
Sánchez-Iglesias A, Pastoriza-Santos I, Pérez-Juste J, Rodríguez-González B, García de Abajo F, Liz-Marzán L (2006) Synthesis and optical properties of gold nanodecahedra with size control. Adv Mater 18:2529–2534
Sapsford KE, Berti L, Medintz IL (2006) Materials for fluorescence resonance energy transfer analysis: beyond traditional donor-acceptor combinations. Angew Chem Int Ed 45:4562–4589
Schneider G, Decher G (2006) Distance-dependent fluorescence quenching on gold nanoparticles ensheathed with layer-by-layer assembled polyelectrolytes. Nano Lett 6:530–536
Si S, Kotal A, Mandal TK (2007a) One-dimensional assembly of peptide-functionalized gold nanoparticles: an approach toward mercury ion sensing. J Phys Chem C 111:1248–1255
Si S, Dinda E, Mandal TK (2007b) In situ synthesis of gold and silver nanoparticles by using redox-active amphiphiles and their phase transfer to organic solvents. Chem Eur J 13:9850
Tang Z, Kotov NA (2005) One-dimensional assemblies of nanoparticles: preparation, properties, and promise. Adv Mater 17:951–962
Turkevich J (1985a) Colloidal gold. Part 1: historical and preparative aspects, morphology and structure. Gold Bull 18:86–91
Turkevich J (1985b) Colloidal gold. Part 2: colour, coagulation, adhesion, alloying and catalytic properties. Gold Bull 18:125–131
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. J Discuss Faraday Soc 11:55
Ugarte D, Stoeckli T, Bonard JM, Chatelain A, De Heer WA (1998) Filling carbon nanotubes. Appl Phys A 67:101–105
Walker MM, Dennis TE, Kirschvink JL (2002) The magnetic sense and its use in long-distance navigation by animals. Curr Opin Neurobiol 12:735–744
Walsh D, Arcelli L, Ikoma T, Tanaka J, Mann S (2003) Dextran templating for the synthesis of metallic and metal oxide sponges. Nat Mater 2:386–390
Wang Y, Tang Z, Liang X, Liz-Marzán LM, Kotov NA (2004) SiO2-coated CdTe nanowires: bristled nano centipedes. Nano Lett 4:225–231
Warner MG, Hutchinson JE (2003) Linear assemblies of nanoparticles electrostatically organized on DNA scaffolds. Nat Mater 2:272–277
Xu S, Cao Y, Zhou J, Wang X, Wang X, Xu W (2011) Plasmonic enhancement of fluorescence on silver nanoparticle films. Nanotechnology 27571:1–7
Yin LW, Bando Y, Zhu YC, Golberg D, Li MS (2004) A two-stage route to coaxial cubic-aluminum-nitride–boron-nitride composite nanotubes. Adv Mater 16:929–933
Yu SH, Cölfen H (2004) Bio-inspired crystal morphogenesis by hydrophilic polymers. J Mater Chem 14:2124–2147
Yu SH, Antonietti M, Cölfen H, Giersig M (2002) Synthesis of very thin 1D and 2D CdWO4 nanoparticles with improved fluorescence behavior by polymer-controlled crystallization. Angew Chem Int Ed 41:2356–2360
Yu SH, Cölfen H, Antonietti M (2003) The combination of colloid-controlled heterogeneous nucleation and polymer-controlled crystallization: facile synthesis of separated, uniform high-aspect-ratio single-crystalline BaCrO4 nanofibers. Adv Mater 15:133–136
Zhang J, Lakowicz JR (2006) A model for DNA detection by metal-enhanced fluorescence from immobilized silver nanoparticles on solid substrate. J Phys Chem B 110:2387–2392
Zhang D, Qi L, Ma J, Cheng H (2001) Formation of silver nanowires in aqueous solutions of a double-hydrophilic block vopolymer. Chem Mater 13:2753–2755
Zielinska A, Skwarekb E, Zaleska A, Gazdac M, Hupkaa J (2009) Preparation of silver nanoparticles with controlled particle size. Procedia Chem 1:1560–1566
Acknowledgments
We are grateful to the Scientific Association ProteoMass (Portugal) for financial support. J.F.L. thanks Xunta de Galicia for a research contract under project 09CSA043383PR (Biomedicine). C.N. thanks Xunta de Galicia (Spain) for her postdoctoral contract (I2C programme). E.O. acknowledges the post-doctoral grant SFRH/BPD/72557/2010 provided by Fundação para a Ciência e a Tecnologia–Ministério da Educação e Ciência, FCT–MEC, Portugal.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fernández-Lodeiro, J., Núñez, C., Oliveira, E. et al. 1D chain fluorescein-functionalized gold and silver nanoparticles as new optical mercury chemosensor in aqueous media. J Nanopart Res 15, 1828 (2013). https://doi.org/10.1007/s11051-013-1828-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1828-6