Abstract
A convenient method to synthesize uniform, well-dispersed colloidal silver nanoparticles is described. Aldonic acid or α-hydroxy acid compounds of low molecular weight are used instead of polymeric compounds as dispersing agents to prepare silver nanoparticles. The size, conformation, and electrical conductivity of the silver nanoparticles, and the effect and function of the dispersing agents are investigated in detail. Using these low molecular weight compounds as dispersing agents, silver nanoparticles with a diameter of 10 nm or less and high electrical conductivity can be obtained. In addition, this procedure allows silver nanoparticles to be sintered at 150 °C, which is lower than that required for silver nanoparticle formulation using polymeric compounds (200 °C). The silver nanoparticles produced by this process can be used to prepare various inks and to manufacture electronic circuits. It is found that low molecular weight compounds are more effective dispersing agents than polymeric compounds in the formation of silver nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nano-sized metal particles have attracted a great deal of attention in recent years because their optical and chemical properties are different from those of the bulk material (Lewis 1993; Thomas 1988; Schmid 1992). As a result, they find wide application in various fields including catalysis, photonics, electronics, and antibacterial applications. Recently, metal nanoparticles have been used as inkjet inks to produce electronic devices because of advantages such as high electrical conductivity. Silver nanoparticles in particular are used widely in the electronics industry for printed electronic circuits due to their high conductivity (Lee and Chou 2005; Tsuruga and Abe 2008). Apart from electronic applications, silver-based compounds such as colloidal silver have also been used for antibacterial applications (Klueh et al. 2000; Zhao and Steven 1998; Cho and So 2006).
An effective synthetic technique is required to produce nanoparticles with controlled shape and small size. Chemical and physical methods have usually been used to prepare silver nanoparticles. Among them, chemical reduction methods are used extensively because they can be implemented under simple and mild conditions and can be used to prepare nanoparticles on a large scale. It is well known that silver nanoparticles can be produced as a result of chemical reaction at low cost and in high yield (Zielinska et al. 2009; Brust et al. 1994; Zhang et al. 2006; Pathak et al. 2000; Wang et al. 2000; Sondi et al. 2003; Radziuk et al. 2007; Zhang et al. 1996). Although such chemical methods are popular, they possess several problems. For example, organic solvent is needed in many cases; the reduction process requires relatively high temperature; and the complete separation of silver particles from the reaction mixture is difficult. In general, to control the size and morphology of particles, a dispersing agent that can prevent aggregation of the nanoparticles is required. Polymeric compounds have been widely used as dispersing agent in the synthesis of metal nanoparticles (Tan et al. 2003; Hsu and Wu 2007; Chou and Lai 2004; Wang et al. 2005). For example, polymeric compounds such as polyvinylpyrrolidone (PVP) and polyvinyl alcohol (PVA) have been investigated, and their protecting role and mechanism have been demonstrated (Zhang et al. 1996; Wang et al. 2005). However, polymeric compounds have some disadvantages because of their high molecular weight compounds, including high decomposition temperature and low solubility, which limit their use in nanoparticle synthesis.
In the manufacture of electronic circuits, nanoparticles must be sintered to obtain high electric conductivity. It is preferable to perform sintering at the lowest temperature possible. However, the use of polymeric materials as dispersing agents means that a high temperature is required for sintering. For example, silver nanoparticles synthesized with PVP require sintering around 200 °C (Hsu and Wu 2007; Chou and Lai 2004). As dispersing agents, the use of low molecular weight compounds with many hydroxyl groups may be effective in the synthesis of silver nanoparticles and may negate the problems associated with the use of polymeric materials.
We have previously reported a method to prepare silver nanoparticles with a diameter less than 10 nm using PVP (named Group A here). Group A silver nanoparticles required sintering at 200 °C to remove polymer and exhibit electrical conductivity (Natsuki and Abe 2011). Therefore, we attempted to find low molecular weight compounds that could behave as effective dispersing agents, and to investigate the effect of such compounds on nanoparticle size, morphology and sintering temperature. Sodium gluconate and dl-malic acid disodium salt, which have low molecular weight, were used instead of polymeric compounds to synthesize silver nanoparticles (named Group B). In addition, Group B silver nanoparticles can be sintered at 150 °C, which is lower than that required for Group A (200 °C). The colloidal silver nanoparticles formed are stable in an aqueous medium. These advantages not only make the silver nanoparticles suitable for preparing inks for manufacturing electronic circuits with screen or inkjet printing, but also suggest a wide range of potential biomedical applications. It is expected that electronic circuits can be manufactured by sintering at relatively low temperature using the silver nanoparticles prepared by this method.
Experimental
Materials
Silver nitrate (AgNO3), trisodium 2-hydroxypropane-1,2,3-tricarboxylate (Na3Ct), dimethylaminoethanol (DMAE), triethylamine (Et3N), imidazole, dl-malic acid disodium salt and sodium gluconate (sodium (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate) were obtained from Wako Pure Chemicals Co., Ltd. (Osaka, Japan).
Preparation of silver nanoparticles
Group A silver nanoparticles were prepared using PVP as a dispersing agent, which was reported in our previous work (Natsuki and Abe 2011).
By the similar procedure, Group B was prepared as follows: sodium gluconate (1.0 g, 4.58 mmol, the molar ratio of sodium gluconate to AgNO3 is 1.5) was dissolved in de-ionized water (20 ml) by stirring for 10 min at room temperature. Into this solution, AgNO3 (0.50 g, 2.94 mmol) was added and the solution was kept stirring for 10 min to dissolve the AgNO3 completely, then an aqueous solution of sodium citrate hydrate (0.88 g, 2.94 mmol) in de-ionized water (20 ml) was added drop-wise. After all of the solution was added, an aqueous solution of dimethylaminoethanol (0.027 g, 0.294 mmol) was added to the reaction mixture. Then, the mixture was kept stirring for 1 h at room temperature.
After the reaction, the silver particles were separated from the solution by centrifugation at 5,000 rpm for 1 min, and washed with 20 ml de-ionized water twice, then dispersed again into de-ionized water (10 ml).
The same reaction procedure described above was used to prepare other silver nanoparticles by varying the kind of reactants (as shown in Table 1).
Volume resistivity measurements
To test the behavior of Group B silver nanoparticles, samples for volume resistivity measurements were prepared by coating a suspension of silver nanoparticles on a polyimide film surface, then the samples were heated at 150 °C for 1 h and then soaked in water or 0.1 M KOH solution for 1 h, then dried at room temperature. Finally, the volume resistivity of these samples was measured.
Characterization
UV–Vis spectra of silver suspensions were obtained with a Hitachi U-4100 UV–Vis spectrophotometer (Hitachi, Japan). Transmission electron microscopy (TEM) images of silver nanoparticles were obtained with a JEOL JEM2010 microscope operating at 200 kV. The samples were prepared by placing a drop of the silver suspension on a carbon-coated formvar film on copper grids, and dried at room temperature. The size distribution of silver particles was measured with a Zetasizer Nano Series analyzer (Malvern Instruments). Energy dispersive X-ray spectroscopy (EDS) measurements were done with an emission scanning electron microscope (SEM, Hitachi S-5000) equipped with an EDS instrument. X-ray diffraction (XRD) experiments were carried out with a Rigaku D/MAX-IIIV X-ray diffractometer using Cu Kα radiation. The volume resistivity was measured with Loresta-GP MCP-T610 resistivity meter (Mitsubishi Chemical Analytech Co., Ltd.). A four-probe method was used to measure the volume resistivity of silver nanoparticles.
Results and discussion
Optical properties of Group B silver nanoparticles
The structure of PVP and sodium gluconate, which has a characteristic structure with many hydroxyl groups, is shown in Fig. 1a and b, respectively. An aldonic acid is any of a family of sugar acids obtained by oxidation of the aldehyde functional group of an aldose to form a carboxylic acid functional group, and gluconic acid is one sort of aldonic acid; α-hydroxy acids are a class of chemical compounds that consist of a carboxylic acid substituted with a hydroxyl group on the adjacent carbon, and dl-Malic acid is one of them. These compounds can be used as dispersing agents, and play an important role in stabilizing the nanoparticles. The sodium gluconate and the dl-Malic acid disodium salt were used in this work.
Similar to Group A silver nanoparticles, those in Group B show a characteristic absorption band around 400 nm in their UV–Vis absorption spectrum (shown in Fig. 2) caused by the silver nanoparticles.
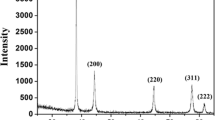
Figure 3 shows the results of EDS analysis for the synthesized nanoparticles. The intense peak around 3 keV confirms that the presence of nanoparticles and they are highly pure. No impurities are observed besides small amounts of carbon and oxygen, which indicates that the reagents used have not remained. Moreover, because the chemicals used in this work are water–solution, the final product with high purity that is insoluble in water can be separated easily from the reaction mixture. Their XRD pattern shown in Fig. 4 indicates that they are highly crystalline. Figure 5 shows TEM images of some of Group B silver nanoparticles. It is observed that silver nanoparticles have a spherical shape with a diameter of 10 nm or less.
Possible protection and reaction mechanism
In this study, we also demonstrated the formation of [Ag+–(C5H11O5COOH)] complexes and revealed the protective mechanism of sodium gluconate (C5H11O5COONa) using (Fourier transform infrared absorption spectroscopy) FT–IR analysis. Figure 6 shows the FT–IR spectra of some samples. For sodium gluconate, the peaks in the range of 3,000–3,520 cm−1 can be assigned to the hydroxyl groups, while the peaks at 1,631 cm−1 can be assigned to C=O bond vibration. For silver nitrate, a very strong peak is observed at 1,313 cm−1, which results from the vibration of nitrate ions. It was found that the FT–IR spectrum of sodium gluconate +AgNO3 (mixing in water with stirring for 10 min, then water is removed before measurement of IR) is nearly identical to pure sodium gluconate. The peaks in the range of 3,000–3,520 cm−1 are very broad and can be assigned to the hydroxyl groups either from gluconic acid, from adsorbed moisture or both, and the absorption peaks at 1,631 cm−1 shifted slightly. These changes may result from coordination of sodium gluconate with silver ions, resulting in the formation of [Ag+–(C5H11O5COOH)] complex (pH is 5.6 before the addition of amine). Furthermore, when amine was added (pH is 9.0 at this time), the reaction advanced, then silver nanoparticles formed. For silver nanoparticles, the peaks in the range of 3,000–3,520 cm−1 became weak and the absorption peaks at 1,631 cm−1 were not observed; instead, peaks appear at 1,566, 1,531, and 1,514 cm−1. These spectra show that the silver particles coordinate with O of hydroxyl groups in C5H11O5COO−, generating a cover on the surface of the metal particles. The layer can prevent the silver particles from agglomerating, leading to small spherical colloidal particles.
The proposed reaction mechanism can be written as follows, which is similar to that of PVP (Natsuki and Abe 2011).
As shown in Eq. (1), the amine withdraws a hydrogen ion and leaves a hydroxyl ion in solution when it is dissolved in water. The hydrogen ions oxidize the OH group in Na3Ct and an electron is released in the process. It suggests that the amine acts as catalyst that can accelerate the reaction. From Table 1, we can see that the particle size of Group B silver nanoparticles changes with various combinations of reagents. Small and relatively uniform particles with a diameter of 10 nm or less can be obtained under different experimental conditions. Compared with Group A silver nanoparticles formed using PVP as the dispersing agent, however, there is no great influence on the particle size in Group B silver nanoparticles whether sodium gluconate or dl-Malic acid disodium salt is used as the dispersing agent. Furthermore, when the mass of dispersing agent is increased to 2.0 g (9.16 mmol, the molar ratio of sodium gluconate to AgNO3 is 3.1), the size of the nanoparticles show little change, which is very different from the case of Group A silver nanoparticles. This is because the surface of Group B nanoparticles are covered with anion resulting from dispersing agents, forming a layer of charges and silver nanoparticles are repulsed by electrostatic repulsive force each other, which can prevent aggregation of silver nanoparticles. It seems that the charges on the surface of nanoparticles do not increase with the amount of dispersing agents. This is different from the neutral polymeric compounds, in which particle size is affected easily by the amount of dispersing agents since the nanoparticles are dispersed by steric hindrance of PVP.
Conductivity of Group B silver nanoparticles
Figure 7 shows the change in the volume resistivity of Group A silver nanoparticles with temperature. It is found that the nanoparticles indicate poor electrical conductivity when they are sintered at less than 200 °C. The volume resistivity of the samples decreases significantly with rising temperature and shows a high electrical conductivity because the covered polymer has been removed when sintered at 250 °C. However, Group B samples show high electrical conductivity in this study when they are treated with water or alkaline aqueous solution after sintering even at a relatively low temperature of 150 °C. In particular, when treated with water followed by sintering at 150 °C, the samples exhibit almost the same level of conductivity as that of Group A silver nanoparticles that are sintered at 200 °C. The SEM images of Group B silver nanoparticles are shown in Fig. 8, it is clear that the silver nanoparticles have coalesced with each other after sintering and treating with water, which improves electrical conductivity.
Figure 9 shows an example of the change in volume resistivity of Group B silver nanoparticles that are sintered at 150 °C for 1 h and then soaked in 0.1 M aqueous KOH for 1 h. The volume resistivity decreases with increasing soaking time up to 1 h, showing a volume resistivity of 3.7 × 10−3 Ω cm at this point. There is no significant change in volume resistivity when the soaking time is extended past 1 h.
Table 2 shows the change in the volume resistivity of Group B silver nanoparticles with soaking time for samples that are sintered at 150 °C for 1 h. When the samples are soaked in water for less than 10 min, the volume resistivity is very high, meaning the electrical conductivity is low. However, the volume resistivity decreases significantly after the samples are soaked in water for over 45 min. The volume resistivity does not change significantly when the soaking period is extended past 1 h. The result shows that the nanoparticles can be purified simply and in an environmentally friendly manner, which are both important factors from an industrial manufacturing point of view. The comparison of volume resistivity between Group A and B silver nanoparticles is shown in Table 3. Compared with Group A silver nanoparticles, those in Group B are smaller and more uniform in particle size, and exhibit almost the same level of electrical conductivity when sintered at 150 °C as those in Group A sintered at 200 °C.
Conclusions
In our previous method (Natsuki and Abe 2011), polymeric compound PVP was used as a dispersing agent in the synthesis of silver nanoparticles. However, polymeric compounds with high molecular weight and low solubility resulted in a high temperature (>200 °C) being required for sintering. In contrast, low molecular weight compounds such as aldonic acid or α-hydroxy acid have good protecting function similar to polymeric compounds, which can produce uniform, small silver nanoparticles with high conductivity and also allow them to be sintered at a relatively low temperature of 150 °C. The lowering of the sintering temperature in 50° is very important and useful from the perspective of industrial manufacturing. In addition, these compounds are water soluble, cheap, easy to deal with and environmentally benign, which is useful for the preparation of silver nanoparticles. These results suggest that such low molecular weight compounds are better at preventing aggregation of silver nanoparticles than polymeric compounds.
Abbreviations
- XRD:
-
X-ray diffraction
- TEM:
-
Transmission electron microscopy
- EDS:
-
Energy dispersive X-ray spectroscopy
- SEM:
-
Scanning electron microscopy
- FT–IR:
-
Fourier transform infrared absorption spectroscopy
- C5H11O5COONa:
-
(2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanoate (also called sodium gluconate)
- AgNO3 :
-
Silver nitrate
- Na3Ct:
-
Trisodium 2-hydroxypropane-1, 2, 3-tricarboxylate
- DMAE:
-
2-(Dimethylamino) ethanol
- PVP:
-
Polyvinylpyrrolidone
- PVA:
-
Polyvinyl alcohol
References
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid dystem. J Chem Soc Chem Commun 1:801–802
Cho JW, So JH (2006) Polyurethane–silver fibers prepared by infiltration and reduction of silver nitrate. Mater Lett 60:2653–2656
Chou KS, Lai YS (2004) Effect of polyvinyl pyrrolidone molecular weights on the formation of nanosized silver colloids mater. Chem Phys 83:82–88
Hsu SLC, Wu RT (2007) Synthesis of contamination-free silver nanoparticle suspensions for micro-interconnects. Mater Lett 61:3719–3722
Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD, Biomed J (2000) Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. Mater Res 53:621–631
Lee H, Chou KS (2005) Inkjet printing of nanosized silver colloids. Nanotechnology 16:2411–2436
Lewis LN (1993) Chemical catalysis by colloids and clusters. Chem Rev 93:2693–2730
Natsuki J, Abe T (2011) Synthesis of pure colloidal silver nanoparticles with high electroconductivity for printed electronic circuits: the effect of amines on their formation in aqueous media. J Colloid Interface Sci 359:19–23
Pathak S, Greci MT, Kwong RC, Mercado K, Prakash OGA, Thompson ME (2000) Synthesis and applications of palladium-coated poly(vinylpyridine) nanospheres. Chem Mater 12:1689–1985
Radziuk D, Skirtach A, Sukhorukov GB, Shchukin DG, Möhwald H (2007) Stabilization of silver nanoparticles by polyelectrolytes and polyethylene glycol. Rapid Commun 28:848–855
Schmid G (1992) Large clusters and colloids. Metals in the embryonic state. Chem Rev 92:1709–1727
Sondi I, Goia DV, Matijevic E (2003) Preparation of highly concentrated stable dispersions of monodispersed silver nanoparticles. J Colloid Interface Sci 260:75–81
Tan Y, Dai X, Li Y, Zhu D (2003) Preparation of gold, platinum, palladium and silver nanoparticles by the reduction of their salts with a weak reductant—potassium bitartrate. J Mater Chem 13:1069–1075
Thomas JM (1988) Colloidal metals: past, present and future. Pure Appl Chem 60:1517–1528
Tsuruga S, Abe T (2008) Proceedings of the Pan-Pacific imaging conference, preparation of electro-conductive inkjet inks through silver halide photographic emulsion, Tokyo, Japan, p 56–59
Wang Y, Ren J, Deng K, Gui L, Tang Y (2000) Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem Mater 12:1622–1627
Wang H, Qiao X, Chen J, Wang X, Ding S (2005) Mechanisms of PVP in the preparation of silver nanoparticles. Mater Chem Phys 94:449–453
Zhang Z, Zhao B, Hu L (1996) PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J Solid State Chem 121:105–110
Zhang W, Qiao X, Chen J, Wang H (2006) Preparation of silver nanoparticles in water-in-oil AOT reverse micelles. J Colloid Interface Sci 302:370–373
Zhao G, Steven SE (1998) Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 11:27–32
Zielinska A, Skwarek E, Zaleska A, Gazda M, Hupka J (2009) Preparation of silver nanoparticles with controlled particle size. Procedia Chemistry 1:1560–1566
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natsuki, J., Natsuki, T. & Abe, T. Low molecular weight compounds as effective dispersing agents in the formation of colloidal silver nanoparticles. J Nanopart Res 15, 1483 (2013). https://doi.org/10.1007/s11051-013-1483-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1483-y