Abstract
Hexagonal close-packed 2D assembly of monodispersed FePt nanoparticles by the co-reduction method have been synthesised with particles size ~3 nm. The X-ray diffraction (XRD) studies reveal a structural transformation from a chemically disordered faced-centred cubic (FCC) phase to a chemically ordered face-centred tetragonal (FCT) phase with heat treatment. The percentage of phase transformation from FCC to FCT has also been investigated by the XRD profile fitting. Annealing at 750 °C gives 95 ± 1 % FCT phase. The structural transformation was further confirmed by the presence of two superlattice rings corresponding to (001) and (110) by the selected area electron diffraction. Transmission electron microscopy (TEM) shows the grain growth with annealing temperature. High resolution TEM shows the presence of the carbonaceous layer over FePt after annealing. This carbonaceous layer has been investigated by the Raman spectroscopy. Magnetic measurement shows the presence of magnetic hard phase with coercivity of 1 Tesla. The soft/hard compositions have also been investigated by the hysteresis measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The alloy Iron-Platinum (FePt) (tetragonal, L10-type structure and space group P4/mmm) with near equiatomic composition is found to be very useful in applications like magnetic recording media and permanent magnet due to its high magneto crystalline anisotropy (7 × 107 J/m3) and chemical stability (Sun et al. 2000; Kim et al. 2007; Nguyen et al. Nguyen et al. 2006; Yao and Coffey 2008; Zeng et al. 2003; Sasaki et al. 2004). This high value of K is due to the spin orbit interaction between Fe and Pt and hybridization between the 3d state of Fe and 5d state of Pt elements (Gutfleish et al. 2005). As-prepared FCC phase FePt nanoparticles have a magnetically soft behaviour which transform to FCT phase with high magnetocrystalline anisotropy (MCA) on annealing. The change in the MCA is a result of distortion from a highly symmetric cubic phase (space group Fm3m) to asymmetric tetragonal phase (space group p4/mmm) (Medwal et al. 2012a, b; Laughlin et al. 2005; Skomski 2003). FePt nanostructures can be prepared by both chemical and physical method. The FePt films prepared by physical techniques are found to be highly crystalline, but with an inhomogeneous size distribution. However, chemically synthesized FePt nanoparticles capped with a surfactant not only results in a very homogeneous distribution of size and shape but also provide chemical stability to the system. Chemical processes, e.g. reduction, co-reduction and seed-mediated growth, etc., have been used to synthesize FePt nanoparticles (Sun et al. 2000; Elkins et al. 2003; Sasaki et al. 2005; Sun et al. 2003). Depending on the preparation methods, different shapes and structures can be formed like nanocubes, nanorod and nanosphere and also core/shell structure with oxide layer as the shell and FePt as a core (Chen et al. 2006; Chen et al. 2004; Hou et al. 2006).
Annealing at higher temperatures results in the process of coalescence to form bigger particles followed by the conversion of the superparamagnetic FCC phase to ferromagnetic FCT phase. Achieving the ordering temperature at which the FCC phase transform to FCT (L10) phase before coalescence process starts is of current research interest. Hence, lowering down the ordering temperature, while retaining the smaller particle size, is an important aspect of L10 system. The decrement in the ordering temperature by adding a third element, viz. silver or gold, during the synthesis of FePt nanoparticles has been reported by several groups (Jeyadevan et al. 2003; Sehdev et al. 2011; Sehdev et al. 2012). An alternative method of controlling the sintering growth of nanoparticles is by annealing the FePt nanoparticles in the nonmagnetic matrix which can sustain high degree of temperature (Rong et al. 2008).
The magnetic properties of FePt nanoparticles strongly depend on the size of the particles and also on the composition of Fe and Pt in FePt alloy, i.e. the value of x in Fe x Pt1−x (Andrew et al. 2004). The most common precursors used in the synthesis of FePt alloy are iron pentacarbonyl (Fe (CO)5) and Pt (acac) 2. The disadvantages associated with Fe (CO)5 are its highly toxic nature and highly flammable nature even at room temperature. The boiling point of Fe (CO)5 is low (103 °C) as compared to the melting point of Pt(acac)2 (249 °C) thereby making it difficult to maintain the composition of the alloy Fe x Pt1−x (Nguyen et al. 2006). The Fe(acac)2 is also used in the synthesis of FePt alloy as an alternative precursor to form monodispersed 2D array.
In this paper, an attempt has been made to synthesis monodispersed array of FePt nanoparticles by the co-reduction chemical method in the presence of different concentration of surfactant. The systematic studies of the structural and magnetic transformation by means of the Rietveld and deconvolution of hysteresis, respectively, have been investigated as a function of annealing temperature. The effect of the surfactant on the 2D array has also been investigated.
Experiment
FePt nanoparticles were synthesized by the chemical co-reduction method (Sun et al. 2000; Medwal et al. 2012a). The metal precursors iron(III) acetylacetonate (Fe(acac)3, 97 % pure) and platinum(II) acetylacetonate (Pt(acac)2, 97 % pure) were co-reduced using 1,2 hexadecanediol which serves as a reducing reagent. Phenyl ether was used as a solvent in the presence of oleic acid and oleylamine which act as surfactants. All the above-mentioned chemicals were procured from Sigma Aldrich and used as obtained for the preparation of the FePt nanoparticles.
Fe(acac)3 is a ferric salt which gets easily oxidized in oxygen atmosphere. Hence, an oxygen-free environment is created throughout the experiment. The iron salt is not easily reducible; however, the platinum salt readily reduces to Pt ions and also helps in the reduction of iron salt, thus accelerating the reduction process. A stoichiometric amount of Pt(acac)2 (0.5 mmol) and Fe(acac)3 (0.5 mmol) were mixed in 40 ml phenyl ether in the presence of 0.5 mmol of oleic acid and oleylamine with 4 mmol of 1,2 hexadecanediol. This mixture was stirred for one hour at room temperature under argon atmosphere. The chemical co-reduction of metallic precursors into metal nanoparticles occurs by refluxing the solution at 250 °C for one hour with continuous stirring in the presence of high purity argon gas. During refluxing, the colour of the solution changes from yellowish pink to black indicating the formation of FePt nanoparticles. The solution was allowed to settle down at room temperature under inert atmosphere. On the addition of 40 ml ethanol, a precipitate was obtained which was separated by centrifugation at 12,000 rpm for 15 min and washed with ethanol twice. Finally, the powder obtained was dispersed in hexane and used for further characterization.
X-ray diffractrogram (XRD) in the 2θ range 20°–75° was performed using a Bruker (D8) diffractrometer. The software TOPAS was used for Rietveld refinement to study the formation of the different phases of the alloy provided with Bruker (D8) diffractrometer. A Tecnai T30 Transmission Electron Microscope (TEM) was used to estimate the size and shape of the nanoparticles dispersed on the copper grid. Selected area electron diffraction (SAED) was also carried out for phase investigation. Vibrating Sample Magnetometer (VSM) Micro Sense EV9 with maximum field of ±2.2 Tesla was used for recording the hysteresis loop at room temperature.
Results and discussion
Transmission electron microscopy
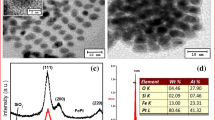
Figure1a, b, c, d shows the as-prepared FePt nanoparticles synthesized using the different ratios of oleic acid to oleylamine, viz. 0.5:0.5, 0.5:1, 1:0.5 and 1:1, respectively. All the TEM images reveal a very well-organized monodispersed FePt array with the particle size ~3 nm. The interparticle distance can be controlled with oleic acid and oleylamine used as a surfactant during the synthesis. The hexagonal close-packed FePt array shown in Fig 1a is observed for the 0.5:0.5 mmol concentration of oleic acid and oleylamine, respectively. The hexagonal close-packed structure can be tuned to honey comb packed array with increasing the surfactant ratio as shown in Fig 1b. On further increasing the concentration of surfactant, the interparticle distance can be increased as shown in Fig 1c, d followed by the disturbance of array. The surfactant not only acts as stabilizing ligands for the FePt nanoparticles but also helps in the formation of array of larger area which is essential for recording media. Typical selected area electron diffraction is shown in Fig 1e obtained for the above array and confirms the formation of FCC phase. It is important to mention that the largest area of the array was observed for the hexagonal close-pack system with 0.5 mmol: 0.5 mmol oleic acid and oleylamine for which further investigations were performed on the system.
The dispersed arrays were subjected to different annealing temperature and HRTEM and SAED were recorded. At high temperature, the surfactant starts degrading and the particles coalesce. The sintering growth effect dominates, leading to the formation of particles with bigger size. Figure 2 shows the TEM of sample annealed at 600 °C along with HRTEM showing the lattice fringes with interplaner distance of 2.22 Å corresponding to (111). The presence of the superlattice ring in the SAED in inset (Fig 2) reveals the formation of chemically ordered FCT phase. The evolution of superlattice rings is due to the chemically arranged Fe and Pt stacking in the FePt unit cell with annealing. The size of nanoparticles has been estimated with log normal distribution function with average particle size of 5 nm as shown in Fig 2c. On further annealing at 750 °C, the particles are found to attain irregular shape of bigger size as shown in Fig 3a. The indexed SAED pattern shown in Fig 3b. The presence of superlattice ring corresponding to (001) and (110) confirming the formation of ordered L10 phase. The sharpness of the ring indicates the enhanced crystallinity. HRTEM shown in Fig 3c shows the interplaner distance of 2.20 Å which is indentified as the 111 plane. A capping over the FePt nanoparticles has also been observed in High Resolution TEM. It is well known that, with annealing, the organic surfactant-capped nanoparticles convert into the carbonaceous coating on the surface of FePt nanoparticles. This carbonaceous capping was investigated by Raman spectra. The Raman spectra showed the presence of D band and G band peak as shown in Fig 3d due to the presence of graphitic carbon layer over the FePt nanoparticles (Medwal et al. 2012a, b). Figure 4 shows the TEM image for the particles annealed at 750 C, demonstrating coalesce and sintering growth of the nanoparticles. The area under the marked circle shows the inter particle diffusion bridge between two coalesced nanoparticles. The random interparticle diffusion and fast growth rate leads to the formation of the irregular and elongated shapes of the annealed nanoparticles. Further temperature-dependent XRD measurements were performed to confirm the phase transformation and given in detail below.
X-ray diffraction
XRD measurements have been performed on the as-prepared samples and samples annealed at different temperatures. The annealed samples exhibit both FCC and FCT phase. The percentage of FCC and FCT in the annealed samples depends upon the annealing time and temperature. Investigation on the phase composition of FCC and FCT in the system the Rietveld analysis has been performed on the as prepared and annealed samples. The fitting of experimental XRD patterns has been performed using double phase model for Fm3 m and P4/mmm space group. The details of the Wyckoff position of both Fe and Pt in the unit cell used for the analysis are reported elsewhere (Medwal et al. 2012a, b; Hahn 2005). Figure 5 shows the XRD pattern of as prepared nanoparticles with plane corresponding to (111) indicating the presence of disordered face-centred cubic phase (A1). The samples were subjected to heat treatments at 600, 700 and 750 °C for the structural transformation. The samples annealed at 600 °C shows the evolution of FCT with superlattice peaks of L10 phase confirming the onset of FCT phase (L10 Type) shown in Fig 6a, b. Further, annealing at higher temperature (700 and 750 °C) also enhance the intensity of superlattice peaks at 2 theta 240 and 330 corresponding to (001) and (110), respectively. The splitting observed for (200) reveals the transformation from cubic (A1, Fm3m) to tetragonal (L10, P4/mmm) as shown in Figs 7 and 8. The estimated percentage for FCC and FCT phase using the profile fitting at different annealing temperature are tabulated in Table 1. This evolution of super lattice peaks is due to the rearrangement of the Fe and Pt position in the unit cell of FePt alloy giving rise to chemical ordering. The rearrangement of Fe and Pt position depends upon the degree of annealing temperature and time for which sample is exposed. The distortion in the unit cell resulting in the contraction of lattice in c direction is due to the difference in the atomic radii of Fe and Pt. This is also accompanied with shift in 2 theta value for (111) and splitting of (200) peaks.
Lattice parameter has been estimated for both FCC and FCT phase. The estimated c/a ratio for FCC and FCT phase were used for the analysis of degree of ordering while intensity of superlattice peaks are taken into account to investigate the ordering parameter. These are tabulated in Table 2. The performed structural refinement shows the 95 ± 1 % chemical ordering of the Fe and Pt in the FePt alloy unit cell at 750 °C. One of our earlier reports showed the 99 % chemical ordering at 750 °C annealing temperature, but with poor particle size distribution (Medwal et al. 2012a, b).
Magnetic studies
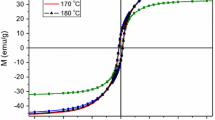
In order to study the effect of annealing on magnetic properties of FePt nanoparticles, M(H) loop of all the sample annealed at different temperature were recorded at room temperature. It is well understood that the as-prepared nanoparticles with FCC phase are soft magnetic and L10 phase nanoparticles with FCT phase are hard magnetic. However, the magnetic transition is not very sharp with annealing temperature. In the intermediate state, the soft and hard phases coexist. Hence, to determine the percentage of the soft and hard composition the obtained M-H loop is deconvoluted. The as-prepared nanoparticles exhibit super paramagnetic behaviour with zero coercivity as shown in Fig 9. The normalized M-H loops corresponding to the annealed samples were deconvoluted by Eq. 1 (Kang et al. 2005).
Where M s is the saturation magnetization, H c is the coercivity, S is the squareness (ratio of M r to M s) and n is the ferromagnetic components in the system.
The deconvoluted loop along with the curve fitting for sample annealed at of 600, 700 and 750 °C are shown in Figs 10, 11 and 12 respectively. The fitting has been performed with two ferromagnetic components, the hard magnetic FCT (L10) and soft magnetic FCC (A1) phases. The contribution of hard magnetic and soft magnetic part from the total has been determined from the deconvolution of experimental data and tabulated in Table 3.
From the Table 3, for the sample annealed at 600 °C, the net coercivity of the FePt nanoparticles is low due to the presence of high percentage of soft magnetic phase confirming the partial ordering of phase. The increase in coercivity is observed with increase in the annealing temperature with low degree of soft phase which is in conformity with the XRD analysis. The onset of the hard phase is observed at 600 °C with low value of H c 0.27T. This transforms to a hard magnetic phase with large value of H c ~1 Tesla at 750 °C, along with small amount of soft phase, which is in agreement with the XRD measurement.
Conclusion
The formation of monodispersed FePt nanoparticles in the form of 2D array in the presence of oleic acid and oleylamine as a spacer has been demonstrated. It was observed that the interparticle distance in the FePt array can be manipulated by varying the ratio of oleic acid and oleylamine. The 0.5 mM oleic acid and 0.5 mM oleylamine give the completely close-packed hexagonal array which can be tuned to honey comb closed packing with 1 mM oleylamine. The long range positional ordering up to 100 nm has been observed with the as-prepared nanoparticles due to the presence of spacer with superparamagnetic behaviour. The structural transformation in the case of the hexagonal close-packed assembly is found to be 95 ± 1 % by means of XRD profile fitting at 750 °C. At higher temperatures, disorder in the 2D FePt array along with the increase in the particle size is observed which is attributed to coalesce and sintered growth of the nanoparticles. The deconvolution of room temperature hysteresis loop recorded for different annealing temperature was used to estimate the fraction of composition of hard and soft magnetic phase. The theoretical investigation for the various fraction of soft and hard phase is essential to understand the above behaviour and this work is in progress.
References
Andrew CCY, Mizuno M, Sasaki M, Kondo M (2004) Atomic composition effect on the ordering of solution-phase synthesized FePt nanoparticle films. Appl Phys Lett 85:6242–6244. doi:10.1063/1.1835998
Chen M, Liu JP, Sun S (2004) One-step synthesis of FePt NANOPARTICLES with tunable size. J Am Chem Soc 126:8394–8395. doi:10.1021/ja047648m
Chen M, Kim J, Liu JP, Fan H, Sun S (2006) Synthesis of FePt nanocubes and their oriented self-assembly. J Am Chem Soc 128:7132–7133. doi:10.1021/ja061704x
Elkins KE, Vedantam TS, Liu JP, Zeng H, Sun S, Ding Y, Wang ZL (2003) Ultrafine FePt nanoparticles prepared by the chemical reduction method. Nano Lett 3:1647–1649. doi:10.1021/nl034734w
Gutfleish O, Lyubina J, Muller KH, Schultz L (2005) FePt hard magnets. Adv Eng Mat 7:208–212. doi:10.1002/adem.200400183
Hahn T (2005) International table of Crystallography, vol A, 5th edn. Kluwer, Dordrecht
Hou Y, Kondoh H, Che R, Takeguchi M, Ohta T (2006) Ferromagnetic FePt nanowires: solvothermal reduction synthesis and characterization. Small 2:235–238. doi:10.1002/smll.200500328
Jeyadevan B, Hobo A, Urakawa K, Chinnasamy CN, Shinoda K, Tohji K (2003) Towards direct synthesis of fct-FePt nanoparticles by chemical route. J Appl Phys 93:7574–7576. doi:10.1063/1.1558258
Kang K, Lewis LH, Jiang JS, Bader SD (2005) Recoil hysteresis of Sm–Co/Fe exchange-spring bilayers. J Appl Phys 98:113906-1-7. doi:10.1063/1.2138785
Kim C, Loedding T, Jang S, Zeng H, Li Z, Sui Y, Sellmyer DJ (2007) FePt nanodot arrays with perpendicular easy axis, large coercivity and extremely high density. Appl Phys Lett 91:172508-1-3. doi:10.1063/1.2802038
Laughlin DE, Srinivasan K, Tanase M, Wang L (2005) Crystallographic aspects of L10 magnetic materials. Scripta materialie 53:383–388. doi:10.1016/j.scriptamat.2005.04.039
Medwal R, Sehdev N, Annapoorni S (2012a) Order–disorder investigation of hard magnetic nanostructured FePt alloy. J Phys D 45(5):055001-1–055001-6. doi:10.1088/0022-3727/45/5/055001
Medwal R, Sehdev N, Annapoorni S (2012b) Electronic states of self stabilized L10 FePt alloy nanoparticles. Appl Phys A 109(2):403–408
Nguyen HL, Howard LEM, Stinton GW, Giblin SR, Tanner BK, Terry I, Hughes AK, Ross IM, Serres A, Evans JSO (2006) Synthesis of size-controlled fcc and fct FePt nanoparticles. J Chem Mater 18:6414–6424. doi:10.1021/cm062127e
Rong CB, Poudyal N, Chaubey GS, Nandwana V, Liu Y, Wu YQ, Kramer MJ, Kozlov ME, Baughman RH, Liu JP (2008) High thermal stability of carbon-coated L10-FePt nanoparticles prepared by salt-matrix annealing. J Appl Phys 103:07E131-1–07E131-3. doi:10.1063/1.2832506
Sasaki Y, Mizuno M, Yu ACC, Inoue M, Yazawa K, Ohta I, Takahashi M, Jeyadevan B, Tohji K (2004) Crystallographic structures and magnetic properties of L10-type FePt nanoparticle monolayered films stabilized on functionalized surfaces. J Magn Magn Mater 282:122–126. doi:10.1016/j.jmmm.2004.04.029
Sasaki Y, Mizuno M, Yu ACC, Miyauchi T, Hasegawa D, Ogawa T, Takahashi M, Jeyadevan B, Tohji K, Sato K, Hisano S (2005) Chemically synthesized L10-type FePt nanoparticles and nanoparticle arrays via template-assisted self-assembly. IEEE Magn 41:660–664. doi:10.1109/TMAG.2004.838040
Sehdev N, Medwal R, Annapoorni S (2011) Enhanced phase stabilization of CoPt in the presence of Ag. J Appl Phys 110:033901-1-7. doi:10.1063/1.3615939
Sehdev N, Medwal R, Annapoorni S (2012) Ag assisted evolution of ordered L10CoPt alloy nanoparticles. J Alloys compd 522:85–89. doi:10.1016/j.jallcom.2012.01.090
Skomski R (2003) Nanomagnetics. J Phys 15:R841–R896. doi:10.1088/0953-8984/15/20/202
Sun S, Murray CB, Weller D, Folks L, Moser A (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 287:1989–1991. doi:10.1126/science.287.5460.1989
Sun S, Anders S, Thomson T, Baglin JEE, Toney MF, Hamann HF, Murray CB, Terris BD (2003) Controlled synthesis and assembly of FePt nanoparticles. J Phys Chem B 107:5419–5425. doi:10.1021/jp027314o
Yao B, Coffey KR (2008) The influence of periodicity on the structures and properties of annealed [Fe/Pt]n multilayer films. J Magn Magn Mater 320:559–564. doi:10.1016/j.jmmm.2007.07.030
Zeng H, Sun S, Sandstrom RL, Murray CB (2003) Chemical ordering of FePt nanoparticle self-assemblies by rapid thermal annealing. J Magn Magn Mater 266:227–232. doi:10.1016/S0304-8853(03)00482-7
Acknowledgments
The work is supported by DST project SR/NM/NS-45/2007 under nanomission Department of Science and Technology, India. Authors would like to acknowledge the help extended by Mr. Manish Kumar, Mr. Rahul Bharadwaj, Mr. Rahul Chauhan and Mr. Harsh Kumar of the University Science Instrumentation Centre (USIC), University of Delhi. Authors would also like to thanks Dr. Alok Banerjee, CSR-DAE, Indore, for magnetic measurements. RM and NS would like to acknowledge CSIR, India, for giving financial support in the form of fellowship under the CSIR-SRF scheme.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Medwal, R., Sehdev, N. & Annapoorni, S. Temperature-dependent magnetic and structural ordering of self-assembled magnetic array of FePt nanoparticles. J Nanopart Res 15, 1423 (2013). https://doi.org/10.1007/s11051-013-1423-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1423-x