Abstract
Aggregation of glutathione (GSH)-functionalized Au nanoparticles induced by Ni2+ ions were found to be related to pH of the solutions. At pH lower than 9.0, introduction of Ni2+ ions was less effective to induce aggregation of the Au nanoparticles. At pH around 9.8, the Au nanoparticles experienced extensive aggregation upon the addition of Ni2+ ions. When pH was higher than 10.5, Ni2+ ion-induced aggregation of the Au nanoparticles was suppressed gradually with increasing pH. It was identified that such pH-mediated aggregation behaviors are attributed to the different coordination fashions of GSH on the Au nanoparticle surface with Ni2+ ions. At pH lower than 9.0, addition of Ni2+ ions was less effective to induce aggregation of the Au nanoparticles when only the carboxyl group of the glutamyl residue was available for the metal ions. The Au nanoparticles underwent extensive aggregation at pH around 9.8 when both the carboxyl and deprotonated amine groups of the glutamyl residue in GSH could coordinate with the metal ions. At pH higher than 10.5, the coordination was gradually suppressed by the hydroxyl groups in solutions, addition of Ni2+ ions is less effective to cross-link the Au nanoparticles. Such a work is helpful for understanding the sensitivity and selectivity of GSH-functionalized Au nanoparticles to metal ions.
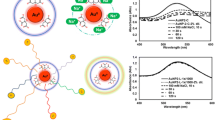
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Au nanoparticles have been demonstrated to be a new kind of probes for colorimetric detection of metal ions (Cao et al. 2005; Elghanian et al. 1997; Hazarika et al. 2004; Ho and Leclerc 2004; Nam et al. 2003; Stojanavic and Landry 2002), which provides new chance to develop assay for quick detection of metal ions in the environmental and biomedical fields (Bal and Kasprzak 2002). After being functionalized by ligands with specificity to metal ions, the coordination between the ligands and metal ions induce the aggregation and thus color change of the Au nanoparticles, which provides the basis for qualitative and quantitative analyses of the metal ions (Darbha et al. 2008; Ghosh and Pal 2007; Hung et al. 2010; Huang et al. 2007; Huang and Chang 2007; Li et al. 2008, 2009, Slocik et al. 2008; Tan et al. 2009; Xue et al. 2008). During the past decade, a wide variety of ligands had been employed to functionalize Au nanoparticles and their performance as probes of metal ions had been evaluated. For examples, Au naoparticles functionalize by DNA-enzyme showed specific response to Pb2+ ions (Liu and Lu 2000, 2003, 2004; Swearingen et al. 2005), Au nanoparticles functionalized by oligo-DNA were sensitive to Hg2+ ions (Lee et al. 2007), histidine-functionalized Au nanoparticles had strong affinity to Fe3+ ions (Guan et al. 2008) etc. Glutathione (γ-Glu–Cys–Gly, GSH) is a tri-peptide with strong affinity to a wide variety of metal ions (Sudeep et al. 2005). However, GSH-functionalized nanoparticles were reported to show selectivity to definite metal ions. For examples, Fang et al. used GSH-functionalized Au nanoparticles to achieve selective detection of Pb2+ ions (Chai et al. 2010). Li et al. (2009) reported that GSH-functionalized Ag nanoparticles were responsive to Ni2+ ions. Kalluri et al. (2009) used GSH-functionalized Au nanoparticles to realize selective detection of As3+ ions in groundwater. Previous works have demonstrated that the coordination sites of GSH are tunable by pH (Bieri and Bürgi 2005). It is deduced that the selectivity may also be related to pH since the change in coordination sites of GSH will affect the affinity of the particles to metal ions. In this study, we investigated the aggregation behaviors of GSH-functionalized Au nanoparticles in presence of Ni2+ ions. It is revealed that such pH-mediated aggregation behaviors are attributed to the different coordination fashions of GSH on the Au nanoparticle surface with Ni2+ ions. At pH lower than 9.0, the Au nanoparticles showed poor sensitivity to Ni2+ due to its weak affinity to the carboxyl group of the glutamyl residue in GSH and better sensitivity to Cu2+ due to its strong affinity to the carboxyl group. At pH around 9.8, both the carboxyl and deprotonated amine groups of the glutamyl residue could coordinate with Ni2+, which is effective to induce the extensive aggregation of Au nanoparticles. At pH higher than 10.5, the coordination is gradually suppressed by the hydroxyl groups in solutions, addition of Ni2+ ions is less effective to cross-link the Au nanoparticles.
Experimental
Materials
GSH (>98 %, C10H17N3O6S, FW 307.33), hydrogen tetrachloroaute(III) trihydrate (99.9+ %, HAuCl4·3H2O, FW 393.8), sodium citrate tribasic dihydrate (≥99.0 %, Na3C6H5O7·2H2O, FW 294.1), nickel(II) sulfate heptahydrate (≥99 %, NiSO4·7H2O, FW 280.86), and other metal ion containing reagents were purchased from Sigma-Aldrich and used as received. All solutions of metal ion containing reagents were prepared freshly before the experiments. Solution of reagent-grade NaOH (0.1 M) were used to adjust the pH value of the colloids. High-purity water (Pall Purelab Plus) with a resistivity of 18 MΩ cm was used in all the experiments.
Preparation of GSH-functionalized Au nanoparticles
Au colloids were prepared by sodium citrate reduction of chloroauric acid according to the modified Frens method (Ji et al. 2007). Typically, 100 mL solution of HAuCl4·3H2O (0.37 mM) was brought to boil under vigorous stirring in a round-bottom flask. A trisodium citrate (1.9 mM) solution was added, resulting in change in solution color from pale yellow to deep red. After the color change, the solution was heated for additional 15 min. After being isolated by centrifugation (8,000 rpm for 30 min), the Au colloid was re-dispersed in pure water and the original pH of the colloid was 6.5. Diameter of the as-prepared Au nanoparticles was determined to be 18 ± 2 nm by transmission electron microscopy (TEM) observations. To prepare glutathione-functionalized Au nanoparticles, 60 μL GSH was added into 1 mL of the as-prepared colloid whose pH value was previously adjusted to 9.0 using 0.1 M NaOH. After addition of glutathione and aging the Au colloid for 2.5 h, the mixture was washed twice with pure water to remove any uncoordinated GSH by centrifugation. The concentration of the GSH modified Au nanoparticles was 3.2 nM. Final pH value of the colloid was about 6.4 and adjusted to different pH values (7.0–12.0) with 0.1 M NaOH before the addition of 50 μM metal ions. The solution was incubated for 5 min after the addition of metal ions before the measurements.
Characterizations
FTIR spectra were recorded with a Perkin-Elmer Spectrum One FTIR spectrometer, performed by placing the CaF2 windows with droplets of the solution in a cabin continuously purged with dry air. UV-visible spectra were collected with a Varian Cary-100 scan UV–Vis spectrophotometer. Dynamic light scattering (DLS) measurements were performed using a particle size analyzer (BI-90 Plus, Brookhaven Instruments) with a scattering angle of 90°. TEM images were observed using a Hitachi H-8100 IV electron microscope at 200 kV using carbon-coated copper grids as substrates. Samples were prepared by dipping a drop of the colloidal solution (the aliquots taken during the course of a reaction) onto the grids. The photographs of the samples were caught by a Konica Minolta DiMAGE Z2 digital camera. All the experiments were carried out at room temperature (25 ± 2 °C).
Results and discussion
In the range of pH 6.4–12.0, the GSH modified Au nanoparticles were red in color, corresponding to a plasmon band at 520 nm. After the addition of 50 μM Ni2+, the nanoparticles presented different absorption spectra under different pH (Fig. 1). For the nanoparticles with pH 6.4, almost no difference in absorption spectra was observable before and after the addition of Ni2+. For the particles with pH 9.8, intensity of the plasmon band at 520 nm decreased evidently accompanied by the appearance of a new band at 610 nm after the addition of 50 μM Ni2+, indicating the aggregation of the particles. At pH 12.0, the plasmon band underwent a slight red-shift and became a little broaden after the addition of 50 μM Ni2+, suggesting the suppressed aggregation of the particles at this high pH. Such a conclusion can also be supported by the TEM observations (Fig. 2). At pH 6.4, the colloid kept the red color and the particles were well dispersed after the addition of Ni2+ ions. The average size of the particles was estimated to be about 28 nm as derived from DLS analyses (Fig. S1A in Supplementary material). At pH 9.8, color of the colloid was changed from red to purple and the particles aggregated extensively after the addition of Ni2+ ions. The average size of the aggregates was estimated to be about 168 nm as derived from the DLS analyses (Fig. S1B in Supplementary material). At pH 12.0, color of the colloid was changed from red to pink and the TEM image was dominated by small clusters of the particles. The average size of the particles was estimated to be about 38 nm as derived from the DLS analyses (Fig. S1C in Supplementary material).
Ratios of the absorbance intensity at 610 and 520 nm (E610/520), which represented the aggregated and free Au nanoparticles, respectively (Aslan et al. 2004) were calculated from the absorption spectra to further illustrate the effect of pH on aggregation of the Au nanoparticles induced by Ni2+ ions (Fig. 3). In the absence of Ni2+ ions, the ratios were lower than 0.2 in the range of pH 6.4–12.0, indicating the good stability of the particles in this range of pH (Fig. S2 in Supplementary material). In the presence of 50 μM Ni2+ ions, the ratios increased rapidly when pH of the colloid was higher than 9.0. It was likely that the effective aggregation of the particles was related to the deprotonation of the glutamyl amine group (pK = 8.7). At pH 12.0, the aggregation was suppressed greatly, which may be related to the increased concentration of hydroxyl groups (Jiang et al. 2009).
It is known that GSH is a multi-dentate ligand for metal ions. FTIR spectra of pure GSH and GSH loaded on the particle surface are given in Fig. 4. The strong band of –SH group at 2,525 cm−1 and the band of the –COOH group of the glycine residue at 1,713 cm−1 were not observable after loading of GSH onto the particle surface, indicating that GSH is anchored on the Au nanoparticles via its thiol group of the cysteine residue and the carboxylic acid group of the glycine residue (Bieri and Bürgi 2005). After being loaded onto the particle surface, the carboxyl and amine groups of the glutamyl residue are available sites for the metal ions. Protonation/deprotonization of the carboxyl and amine groups may result in different coordination fashions between Ni2+ and GSH (Zhang et al. 2010), thus promoting or suppressing aggregation of the particles. At pH 6.4, after the addition of Ni2+ ions, the symmetric and asymmetric C=O stretches of the glutamyl residue at 1,398 and 1,540 cm−1 shifted to 1,412 and 1,550 cm−1, respectively (Fig. S3A in Supplementary material), indicating the coordination of Ni2+ with the carboxyl group (Bieri and Bürgi 2005; Krezel et al. 2003; Lim et al. 2008). The amine group (pK = 8.7) should not contribute to the coordination since it should exist in the protonated forms at this pH value (Dean 1999). At pH 9.8, GSH loaded on Au nanoparticle surface presented in-plane and out-of-plane deformations of N–H at 1,630 and 1,084 cm−1 from the deprotonated amine group of the glutamic residue. After the addition of Ni2+ ions, the vibrations shifted to 1,652 and 1,128 cm−1, respectively (Fig. S3B in Supplementary material), suggesting that the amine group also participated in the coordination after its deprotonation (Krezel and Bal 2004; Krezel et al. 2003; Lim et al. 2008). It was noted that the symmetric and asymmetric C=O stretches of the glutamyl residue of GSH loaded on the particle surface also shifted from 1,410 and 1,530 cm−1 to 1,420 and 1,540 cm−1 after the addition of Ni2+ ions, indicating the carboxyl group of the glutamic residue still contributed to the coordination at this pH (Krezel and Bal 2004; Lim et al. 2008).
Variation in ratios of the absorbance intensity at 610 and 520 nm with concentration of Ni2+ ions was recorded to further understand the Ni2+ induced aggregation processes of the Au nanoparticles (Fig. 5). At pH 6.4, there was almost no change of the ratio when the concentration of Ni2+ was lower than 80 μM, indicating that the added Ni2+ ions were primarily absorbed on surface of the Au nanoparticles. When the concentration of Ni2+ ions was higher than 80 μM, the ratio increased gradually with the increased concentration attributed to the cross-linking of the Au nanoparticles by Ni2+ ions. At pH 9.8, the cross-linking of the Au nanoparticles became dominant when the concentration of Ni2+ was higher than 10 μM. The ratio was proportional to the concentration of Ni2+ in the range of 10–80 μM, indicating that the GSH-modified Au nanoparticles were qualified for ratiometric detection of Ni2+ ions at this pH. When the concentration was higher than 80 μM, the ratio kept almost unchanged with the increased concentration of Ni2+, possibly due to the competition effect of the hydroxyl groups. At pH 12.0, the ratio increased gradually with the increased concentration when the concentration of Ni2+ was lower than 50 μM. However, the slope of the curve at pH 12.0 was much smaller than that at pH 9.8, suggesting the suppressed cross-linking process of the Au nanoparticles by the increased concentration of hydroxyl groups at this high pH. When the concentration of Ni2+ was higher than 50 μM, there was no change of the ratio with the increased concentration of Ni2+ due to the competition effect of hydroxyl groups.
Based on above experimental results and discussion, the coordination fashions between GSH on the particle surface and Ni2+ under different pH were proposed as shown in Scheme 1. After being loaded onto the particle surface, the thiol and the carboxyl group of the glycyl residue are attached to the surface. At pH 6.4, only the carboxyl group of the glutamyl residue is available for Ni2+ ions, which is less effective to induce the cross-linking of the Au nanoparticles due to the relative weak affinity of the carboxyl group to Ni2+ (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 1.81). At pH 9.8, the coordination interactions between the Ni2+ and GSH ligand are effective to induce the aggregation of the Au nanoparticles when GSH acts as a bidentate ligand (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 1.81, Log \( {\text{K}}_{{{\text{NH}}_{2} }} \) = 5.04). At pH 12.0, the coordination between Ni2+ and GSH ligand is less effective to induce the cross-linking of the Au nanoparticles due to increased concentration of the hydroxyl groups, which may compete with the GSH and suppress the cross-linking.
Control experiments were carried out using other metal ions to further understand the aggregation behaviors of the Au nanoparticles. Figure 6 gives the ratio of E610/E520 recorded at different pH upon the addition of 50 μM metal ions. At pH 6.4, the Au nanoparticles were sensitive to the metal ions with strong affinity to carboxyl group, such as Cu2+ ions (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 3.2) and Fe3+ ions (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 3.2) (Dean 1999). The average sizes of the aggregates induced by Cu2+ and Fe3+ were estimated to be about 102 and 38 nm, respectively as derived from the DLS analyses (see Fig. S4A in Supplementary material). Other metal ions such as Ca2+ (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 0.6), Ni2+ (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 1.81) and Zn2+ (Log \( {\text{K}}_{{{\text{COO}}^{ - } }} \) = 1.50) were less effective to induce the aggregation of the Au nanoparticles due to their weak affinity to the carboxyl groups. At pH 9.8, the metal ions with strong affinity to the deprotonated amine group, such as Ni2+ (Log \( {\text{K}}_{{{\text{NH}}_{2} }} \) = 5.04), Cu2+ (Log \( {\text{K}}_{{{\text{NH}}_{2} }} \) = 7.98), and Zn2+ (Log \( {\text{K}}_{{{\text{NH}}_{2} }} \) = 4.81), were more effective to induce the aggregation of the Au nanoparticles than those with weak affinity to the amine group for example Fe3+ (Log \( {\text{K}}_{{{\text{NH}}_{2} }} \) = 2.20) (Dean 1999). Average sizes of the aggregates induced by Ni2+ and Fe3+ were estimated to be about 180 and 45 nm, respectively (see Fig. S4B in Supplementary material). At pH 12.0, all the metal ions became less effective to induce the cross-linking of the Au nanoparticles due to the competition effect of the hydroxyl groups. Average sizes of the aggregates induced by Ni2+, Fe3+, and Cu2+ were smaller than 40 nm (see Fig. S4C in Supplementary material).
Conclusions
In this study, aggregation of the GSH-modified Au nanoparticles induced by Ni2+ ions was investigated. The difference in aggregation behaviors of the Au nanoparticles under different pH was related to the different coordination fashions between the GSH ligand and Ni2+. At pH lower than 9.0, only the carboxyl group of the glutamyl residue in GSH could act as coordination site for metal ions, the Ni2+ ions are less effective to induce the aggregation of the Au nanoparticles due to their weak affinity to the carboxyl groups. At pH around 9.8, both the carboxyl and deprotonated amine groups of the glutamyl residue could act as coordination sites, thus the Au nanoparticles undergo extensive aggregation upon the addition of Ni2+ ions due to their strong affinity to the amine groups. At pH higher than 10.5, the addition of Ni2+ ions is less effective to induce the aggregation of the Au nanoparticles due to the competition effect of the hydroxyl groups. Such a work should be helpful for understanding the sensitivity and selectivity of GSH-functionalized Au nanoparticles to various metal ions and designing the Au nanoparticles based probes for metal ions with better performance.
References
Aslan K, Luhrs CC, Perez-Luna VH (2004) Controlled and reversible aggregation of biotinylated gold nanoparticles with streptavidin. J Phys Chem B 108:15631–15639. doi:10.1021/jp036089n
Bal W, Kasprzak KS (2002) Induction of oxidative DNA damage by carcinogenic metals. Toxicol Lett 127:55–62. doi:10.1016/S0378-4274(01)00483-0
Bieri M, Bürgi T (2005) l-Glutathione chemisorption on gold and acid/base induced structural changes: a PM-IRRAS and time-resolved in situ ATR-IR spectroscopic study. Langmuir 21:1354–1363. doi:10.1021/la047735s
Cao YC, Jin R, Thaxton CS, Mirkin CA (2005) A two-color-change, nanoparticle-based method for DNA detection. Talanta 67:449–455. doi:10.1016/j.talanta.2005.06.063
Chai F, Wang CG, Wang TT, Su ZM (2010) Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. Appl Mater Interface 2:1466–1470. doi:10.1021am100107k
Darbha GK, Singh AK, Rai US, Yu E, Yu HT, Ray PC (2008) Selective detection of mercury(II) ion using nonlinear optical properties of gold nanoparticles. J Am Chem Soc 130:8038–8043. doi:10.1021/ja801412b
Dean J A (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill Book Co., New York, p 884
Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA (1997) Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277:1078–1080. doi:10.1126/science.277.5329.1078
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862. doi:10.1021/cr0680282
Guan J, Jiang L, Li J, Yang WS (2008) pH-dependent aggregation of histidine-functionalized Au nanoparticles induced by Fe3+ ions. J Phys Chem C 112:3267–3271. doi:10.1021/jp7097763
Hazarika P, Ceyhan B, Niemeyer CM (2004) Reversible switching of DNA–gold nanoparticle aggregation. Angew Chem Int Ed 43:6469–6471. doi:10.1002/anie.200461887
Ho HA, Leclerc M (2004) Optical sensors based on hybrid aptamer/conjugated polymer complexes. J Am Chem Soc 126:1384–1387. doi:10.1021/ja037289f
Huang CC, Chang HT (2007) Parameters for selective colorimetric sensing of mercury(II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem Commun 12:1215–1217. doi:10.1039/b615383f
Huang CC, Yang Z, Lee KH, Chang HT (2007) Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew Chem Int Ed 46:6824–6828. doi:10.1002/anie.200700803
Hung YL, Hsiung TM, Chen YY, Huang CC (2010) A label-free colorimetric detection of lead ions by controlling the ligand shells of gold nanoparticles. Talanta 82:516–522. doi:10.1016/j.talanta.2010.05.004
Ji XH, Song X, Li J, Bai Y, Yang WS, Peng XG (2007) Size control of gold nanocrystals in citrate reduction: the third role of citrate. J Am Chem Soc 129:13939–13948. doi:10.1021/ja074447k
Jiang L, Guan J, Zhao L, Li J, Yang WS (2009) pH-dependent aggregation of citrate-capped au nanoparticles induced by Cu2+ ions: the competition effect of hydroxyl groups with the carboxyl groups. Colloids Surf A 346:216–220. doi:10.1016/j.colsurfa.2009.06.023
Kalluri JR, Arbneshi T, Khan SA, Neely A, Candice P, Varisli B, Washington M, McAfee S, Robinson B, Banerjee S, Singh AK, Senapati D, Ray PC (2009) Use of gold nanoparticles in a simple colorimetric and ultrasensitive dynamic light scattering assay: selective detection of arsenic in groundwater. Angew Chem Int Ed 48:9668–9671. doi:10.1002/anie.200903958
Krezel A, Bal W (2004) Studies of zinc(II) and nickel(II) complexes of GSH, GSSG and their analogs shed more light on their biological relevance. Bioinorg Chem Appl 2:293–305. doi:10.1155/s1565363304000172
Krezel A, Szczepanik W, Sokolowska M, Jezowska-Bojczuk M, Bal W (2003) Correlations between complexation modes and redox activities of Ni(II)–GSH complexes. Chem Res Toxicol 16:855–864. doi:10.1021/tx034012k
Lee JS, Han MS, Mirkin CA (2007) Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles. Angew Chem Int Ed 46:4093–4096. doi:10.1002/anie.200700269
Li D, Wieckowska A, Willner I (2008) Optical analysis of Hg2+ ions by oligonucleotide-Au nanoparticles hybrids and DNA-based machines. Angew Chem Int Ed 47:3927–3931. doi:10.1002/anie.200705991
Li HB, Cui ZM, Han CP (2009) Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni2+ ion. Sens Actuators B 143:87–92. doi:10.1016/j.snb.2009.09.013
Lim IS, Mott D, Ip W, Njoki PN, Pan Y, Qin S (2008) Interparticle interactions in glutathione mediated assembly of gold nanoparticles. Langmuir 24:8857–8863. doi:10.1021la800970p
Liu J, Lu Y (2000) A highly sensitive and selective catalytic DNA biosensor for lead ions. J Am Chem Soc 122:10466–10467. doi:10.1021ja0021316
Liu J, Lu Y (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125(22): 6642–6643. doi:10.1021/ja034775u
Liu J, Lu Y (2004) Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J Am Chem Soc 126:12298–12305. doi:10.1021ja046628h
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109:1948–1998. doi:10.1021cr030183i
Nam JM, Thaxton CS, Mirkin CA (2003) Nanoparticle-based biobarcodes for the ultrasensitive detection of proteins. Science 301:1884–1886. doi:10.1126science.1088755
Slocik JM, Zabinski JS, Phillips DM, Naik RR (2008) Colorimetric response of peptide-functionalized gold nanoparticles to metal ions. Small 4:548–551. doi:10.1002smll.200700920
Stojanavic MN, Landry DW (2002) Aptamer-based colorimetric probe for cocaine. J Am Chem Soc 124:9678–9679. doi:10.1021/ja0259483
Sudeep PK, Joseph STS, Thomas KG (2005) Selective detection of cysteine and glutathione using gold nanorods. J Am Chem Soc 127:6516–6517. doi:10.1021ja051145e
Swearingen CB, Wernette DP, Cropek DM, Lu Y, Sweelder JV, Bohn PW (2005) Immobilization of a catalytic DNA molecular beacon on Au for Pb(II) detection. Anal Chem 77:442–448. doi:10.1021ac0401016
Tan ZQ, Liu JF, Liu R, Yin YG, Jiang GB (2009) Visual and colorimetric detection of Hg(2+) by Cloud point extraction with functionalized gold nanoparticles as a probe. Chem Commun 45:7030–7032. doi:10.1039b915237g
Xue XJ, Wang F, Liu XG (2008) One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J Am Chem Soc 130:3244–3245. doi:10.1021ja076716c
Zhang J, Li J, Zhang JX, Xie RG, Yang WS (2010) Aqueous synthesis of ZnSe nanocrystals by using glutathione As ligand: the pH-mediated coordination of Zn2+ with glutathione. J Phys Chem C 114:11087–11091. doi:10.1021jp102540w
Acknowledgments
This study was supported by the National Research Fund for Fundamental Key Project (No. 2009CB939701, 2011CB935800) and the National Nature Science Foundation of China (21073078, 50825202).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, R., Li, J. & Yang, W. Aggregation of glutathione-functionalized Au nanoparticles induced by Ni2+ ions. J Nanopart Res 14, 929 (2012). https://doi.org/10.1007/s11051-012-0929-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0929-y