Abstract
A facile way to synthesis ionic solvent-free multi-walled carbon nanotubes (CNTs) (MWNTs) nanofluids has been introduced. Fourier transform infrared spectra and transmission electron microscope (TEM) were employed to study the surface structure of MWNTs in the nanofluids. The thermal property of the nanofluids was characterized by thermogravimetric analysis and differential scanning calorimetry. The stability of the nanofluids in the deionized water was obtained through UV–Vis absorption spectrum. Rotary rheometer was used to test the flow feature of the nanofluids. The results of conductivity indicate that the seepage threshold value of solvent-free nanofluids in water is about 0.408 vol.% (volume fraction). Meanwhile, it is found that the ionic nanofluids dispersed well in epoxy matrix. The mechanical properties, such as bend modulus, strength and impact toughness have been improved at the same time. TEM images can tell the great dispersion of solvent-free CNTs nanofluids in the epoxy matrix. It means that this kind of nanofluids will be excellent nanofiller in the nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs) undoubtedly occupy unique position among advanced materials due to their novel electrical, mechanical, and chemical properties (Dresselhaus et al. 2001; Baughman et al. 2002; Kong et al. 2000; Postma et al. 2001). It has been reported that CNTs are extremely strong and exceptionally stiff, yet remarkably flexible. In addition, CNTs have high length–diameter ratio (>100), but low density. These properties make them excellent additives to improve the mechanical, electronic, tribological, thermal, and rheological properties of the matrix. However, it is known that the CNTs are not compatible with all solvents because of their chemical inertness. Besides, it is also difficult to obtain a homogeneous dispersion in polymer matrix (Chen et al. 2005).
There are mainly two methods to disperse CNTs in base fluids and polymers: mechanical and chemical methods (Hilding et al. 2003; Garg et al. 2009; Bahr and Tour 2002; Chen et al. 1998; Banerjee et al. 2003; Georgakilas et al. 2005). Mechanical methods generally include ultrasonication using a probe or a bath. Chemical methods include the application of surfactants and CNTs-functionalization by using strong acids. The surfactant method changes the wetting or adhesion behavior of CNTs, which helps in reducing their tendency to agglomerate.
In general, the chemical functionalization of CNTs takes place either via covalent grafting to the graphitic surface, structural defects, and end cups of the tubes, through molecular stacking on the walls, or by wrapping the tubes with polymers (Kahn et al. 2002; Holzinger et al. 2003; Huang et al. 2003). The conventional functionalized methods make nanoparticles behave solid-like in the absence of solvent and do not undergo a microscopic solid-to-liquid transition below 150 °C. Recently, we have developed a series of solvent-free nanofluids. (Zhang et al. 2009a, b, c, 2010; Zheng et al. 2010). The solvent-free nanofluids is a liquid, which could flow at room temperature. This kind of nanofluids was first synthesized by Emmanuel’s group in Cornell University (Bourlinos et al. 2005a, b, c). Later, the solvent-free nanoparticle fluids were synthesized by the present authors by attaching a corona of ionic flexible chains onto an inorganic oxide core, such as SiO2, TiO2, Fe3O4, etc. (Yu et al. 2011; Lan et al. 2011). We also prepared nonionic solvent-free multi-walled CNTs (MWNTs) nanofluids through modifying them with amphipathic block copolymers and nanoparticles such as Au (Zhang et al. 2009a, b, c, 2010; Zheng et al. 2010).
In this study, the solvent-free MWNTs nanofluids, which behaved liquid-like at room temperature, were prepared by functionalizing ionic flexible chains onto the surface of carboxylation MWNTs. The content of MWNTs reached up to 18.04 wt% (weight fraction), which was nearly four times higher than that of the traditional nanofluids (Nguyen et al. 2008). It is found that the nanofluids can be dispersed well in deionized water. With the addition into the epoxy matrix, the nanofluids dispersed well in the composites without apparent aggregation. Meanwhile, the mechanical properties had been improved to certain extent.
Experimental procedures and apparatus
Chemicals and materials
MWNTs (diameter, 10–30 nm; length, 5–15 μm; purity ≥ 95%) were purchased from Shenzhen Nano-Tech Port Co. Ltd. (China). The MWNTs used in our experiment were further treated with mixed acids \( (V_{{{\text{H}}_{ 2} {\text{SO}}_{ 4} }} :V_{{{\text{HNO}}_{ 3} }} = 3 :1) \) to fix the carboxyl groups onto the surface of MWNTs. Then the carboxylation MWNTs were washed by the deionized water, which was prepared by our own lab, until the pH of the water was up to 7.0. In the acid–base titration process of testing, the content of the carboxyl groups, the potassium hydrogen phthalate (C8H5KO4), which needs to be dried in the vacuum dryer at 110–150 °C and hydrochloric acid (HCl), sodium hydroxide (NaOH), sodium hydrogen carbonate (NaHCO3) were used. The reaction mechanism was shown in Scheme 1. First, the carboxylation MWNTs reacted with NaHCO3, the number of moles of which is five times of that of the MWNTs, in deionized water for 3 h. Then HCl, with the same ratio to NaHCO3, was added into the solution, and it will react with the residual NaHCO3 in the solution. The number of moles of the remanent HCl equals to the content of the carboxyl groups in carboxylation MWNTs. (Acid–base titration: First, the concentration of the NaOH solution is titrated by the standard NaHCO3 solution whose concentration is 500 mg/L. Then the concentration of the HCl solution is titrated by the NaOH solution. At last, the residual HCl in the solution has been titrated by the NaOH solution).
N,N-Didecyl-N-methyl-N-(3-trimethoxysilylpropyl) ammonium chloride (C27H60ClNO3Si, 42%, in methanol) was from Gelest, Inc. Poly(ethylene glycol) 4-nonylphenyl 3-sulfopropyl ether and potassium salt (PEGS) were used as-received from Aldrich. The different contents of solvent-free MWNTs nanofluids were directly added into the epoxy resin CYD-128 (Sinopec), which cured with methylhexahydrophthalic anhydride (MTHPA, Guangzhou WeiBo Chemical Co. Ltd.) and 2,4,6-tri(dimethylaminomethyl) phenol (DMP-30 Aladdin Chemistry Co. Ltd.).
Synthesis
The ionic solvent-free CNT nanofluids were synthesized through two steps of ion change reaction (Scheme 2). Before the grafting reaction, the pristine MWNTs were treated with the mixture of acids.
The carboxylation MWNTs were washed with deionized water until the pH of the water was about 7.0. After being dried in the vacuum oven for 2 days, the carboxylation MWNTs and C27H60ClNO3Si were mixed at molar ratio of 1:1 with 50 mL distilled water through the ultrasound dispersion for 20 min. Then the solution was poured into a three-necked flask and the reaction proceeded during magnetic stirring at 70 °C for 24 h. The product of the first procedure was filtrated by the mixed cellulose ester (pore size is 0.22 μm; Xinya Purification Devices Factory, Shanghai, China), then collect the black solid on the film for the second grafting process. In the second step, the black solid was first dispersed in 50 mL deionized water, then PEGS, which was the same molar weight with C27H60ClNO3Si, was added into the solution, and reacted for 24 h through magnetic stirring at 70 °C. The mixture was centrifuged at 6,000 rpm for 30 min, resulting in a homogeneously black solution and the supernatant liquid was collected, concentrated, and dried at 70 °C. We also prepared the epoxy composites with nanofluids. The different contents of nanofluids were added into the epoxy matrix with ultrasound dispersing and cured in the vacuum oven with MTHPA as curing agent and DMP-30 as accelerating agent.
Characterization and measurements
The surface groups on MWNTs were investigated by Fourier transform infrared spectrometer (FTIR WQF-310) using KBr pellets. Transmission electron microscope (TEM) images were obtained at an accelerating voltage of 100 kV with the JEM-2100 instrument. A few drops of an aqueous sample were placed on a copper grid and dried. Thermogravimetric analysis (TGA) measurements were taken under N2 flow by using TGAQ50 TA instrument. Ultraviolet visible spectrophotometer (UV-7800 C, Ningbo Shunyu Instrument Co., Ltd.) was used to test the stability of the nanofluids in water; the range of wavelength is from 190 to 400 nm. The conductivity of the nanofluids in the water was determined by using DDS-307 conductivity meter (ShangHai PuDa Instrument Co. China). Rheological properties were studied by the rheometer of TA instrument (AR-G2). The modulus G′ and G″ were measured at a constant frequency in the temperature range of 20–80 °C. The mechanical properties of the MWNTs–epoxy composite were tested on the universal testing machine (CMT-5105), which gave the value of bend modulus, strength, and impact toughness.
Results and discussion
Chemical structure
The groups on the hybrid material of MWNTs decorated with ionic flexible chains were studied by FTIR (Fig. 1). The FTIR spectra of MWNTs, which are modified by mixed acid, show that the process of carboxylation had successfully decorated the carboxyl groups (–COOH) onto the surface of CNTs. The results of acid–base titration indicate that the average –COOH content was 2.9604 mmol/g.
The absorption peaks at 3,307 cm−1 is for hydrogen bonding of hydroxyl (–OH). The absorption peaks for the formation of hydrogen bonding between the carbonyl of carboxyl and the hydroxyl of another carboxyl moved to 1,700 cm−1. The absorption peaks of hydroxyl (–OH) in carboxyl also move near to 3,200–2,500 cm−1, which forme a very wide peak and this wide peak is the most important characteristic for the carboxyl groups (–COOH). In the FTIR spectra of Fig. 1e, ionic solvent-free MWNTs nanofluids, the absorption peaks at 3,502 cm−1 is for the free hydroxyl, which may represent the marginalia residual water in the system. The absorption peaks at 824 cm−1 show that it has the same characteristic peaks with curve (c), which represent the group of –Si–O–. The wide absorption at 2,521 cm−1 of curve (c) is for quaternary ammonium salt. It disappears in curve (e) because of the ion exchange reaction between C27H60ClNO3Si and PEGS. The similar peaks both in curves (d) and (e) at 1,456 cm−1 represent the skeleton vibration of phenyl. The FTIR spectra of the hybrid material also show that the characteristic absorption peaks of –CH3, –CH2 (2,932 cm−1), –C–O– (1,101 cm−1), which means the ionic surfactant molecules, are triumphantly grafted or overlapping on the surface of MWNTs.
Physical structure
The microstructure of MWNTs decorated with ionic flexible chains could be clearly observed from the TEM images (Fig. 2). Before carboxylation process, the MWNTs are much longer (Fig. 2a). As shown in Fig. 2b, the carboxylic MWNTs reveal the presence of typical hollow tubes and open ends. In the case of the hybrid material Fig. 2c, ionic flexible chains are found to densely ornament the walls of MWNTs uniformly. The TEM images reveal that the average diameter was 35 nm. Figure 2d is the energy spectrum diagram on the surface of the MWNTs. The existence of –Si– and –O– indicates that the surfactant had been grafted onto the surface of MWNTs. Figure 2e briefly shows the reaction process.
Thermal properties
To verify the content of organic canopy on the surface of nanoparticles in hybrid system, the TGA was carried out to confirm the thermal stability (Fig. 3). The TGA traces indicated that the sample needed to be void of any remaining solvent. The weight loss above 180 °C is due to decomposition of the organic alkyl groups. Figure 3a shows that the pristine MWNTs will not generate weight loss until 600 °C. So the weight of ionic flexible chains and MWNTs are 81.96 and 18.04 wt%, respectively. Such a composition corresponds to a dense surface coverage of one modifying molecule per 50 carbon atoms of the nanotubes. For comparison, other functionalization processes typically lead to a surface coverage of one modifying molecule per 100–200 carbon atoms (Bourlinos et al. 2006).
Rheological behavior
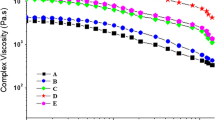
Usually, a dynamic spectrum can be used to understand the structures and properties of material. It is well known that the dynamical storage modulus G′ and loss modulus G″ of rheometrics mechanical spectrometry exhibit the relationship between the molecular motion and rheological behavior of the material (Xu 2003). In general, key factors for liquid-like behavior are related to the organic content and plasticization provided by the organic modifier, the size and density of the nanoparticles, and the surface chemistry of the core (Bourlinos et al. 2005d). Figure 4 shows modulus curve and viscosity curve of ionic solvent-free MWNTs nanofluids under a fixed shear rate (100 rad/s). Figure 4a shows the storage modulus G′ and the loss modulus G″ versus temperature curve. It indicates that the storage modulus G′ and loss modulus G″ decreased with temperature increasing. And the loss modulus G″ is always higher than the storage modulus G′ over the temperature range from room temperature to 80 °C.
In Fig. 4a, the curve 1 represents the storage modulus G′, which indicates the capacity to transform the recoverable elastic deformation into energy and stored in the specimens. It is the characterization of elasticity behavior of the material. Curve 2 represents the loss modulus G″, which refers to the energy consumption caused by the irreversible deformation. This parameter will reflect the viscosity extent of material. As the temperature increases, the internal friction of molecules motion will reduce, resulting in the reduction of loss modulus G″. During the heating process, the material mainly exhibits viscous deformation, \( G^{\prime\prime} > G^{\prime}, \) hence, it exhibits liquid behavior (Jiao and Lei 2003). Figure 4b gives the change in viscosity in the temperature range of 20–80 °C. The viscosity lowers with the temperature increasing. With respect to the flow mechanism, it is supposed that the interactions between the surface ionic double layers are mainly dominated by Coulombic force and the ions here have different shapes. The asymmetric paired cation and anion are at their molten state within the testing temperature range in the rheological measurements. Therefore, charge centers of paired cation and anion can move apart a certain distance from the original location, then may recombine with other opposite ions nearby. This effect can cause the continual departing-recombining motion of large organic ions, which results in smooth slipping between the adjacent building blocks and subsequently the flow ability of corresponding nanofluids. The viscosity of the nanofluids at 30 °C is only 9.834 Pa s, which is much lower than that of similar ionic solvent-free nanofluids (\( \eta^{*} \approx 75 \) Pa s), which has been reported (Li et al. 2010) at the same temperature. The inset in Fig. 4c shows the digital image of nanofluids, which indicates the liquid behavior at room temperature.
Stability in deionized water
The carbon atoms of CNTs have sp 2 hybrid orbital, the p electrons of the carbon atoms form a wide range of π bonding, which has a significant conjugated effect. So in the ultraviolet absorption area, there are obvious absorption peaks at the wavelength of 255 nm, which corresponds to B absorption (Zhang 2005) band of π–π* lambda transition. B absorption band is a wide peak within the scope of 230–270 nm, near ultraviolet area. It has a weak absorption band \( (\varepsilon_{\max } \approx 200) \). The polar solvent makes the B peak occur red shift. The solvent used in the experiment is deionized water. As shown in Fig. 5a, the wavelength is located in 255 nm.
The substitute groups of the para-benzene segment in PEGS molecules used in the second step include a power supply group and a suck electricity group. These two substitutes have synergistic effects, making the K absorption band of π–π* lambda transition generate a relative movement. As shown in Fig. 5a, their absorb peak position is 223 nm. Figure 5b is the comparison of fitted curves of Abs% peak value before and after precipitate process for 20 days. It reveals that the concentration of the solution nearly does not change after settlement, indicating that the solution did not generate obvious settlement. The ionic solvent-free MWNTs nanofluids have good dispersion of stability in deionized water.
Conductivity in deionized water
It is well known that the CNTs have excellent electroconductibility for its π bonding of p electrons. Then the conductivity of the hybrid material in deionized water is measured (Fig. 6). It shows that the conductivity increased with the hybrid material concentration increasing. When the concentration is up to 0.408 vol.%, the electrical conductivity increases quickly. This improvement in conductivity suggests that an infinite network of percolated hybrid material started forming. The critical concentration of hybrid material, f c, is proved by the law \( \sigma = \sigma_{0} (f - f_{\text{c}} )^{t} \) (Stauffer and Aharnoy 1991), where f is the volume fraction of filler. The conductivity exponent t generally reflects the dimensionality of the system with values typically around 1.3 and 2.0 for two and three dimensions, respectively. It is easy to prove the value of the seepage threshold through the equation of the slope of the fitted curves, as shown in Fig. 6a, b.
When \( f \le f_{\text{c}} \quad \sigma = \sigma_{0} (f_{\text{c}} - f)^{ - s} \quad \lg \sigma = \lg \sigma_{0} + ( - s)\lg (f_{\text{c}} - f) \)
From the fitted line (a), the value of s is 0.1448.
When \( f \ge f_{\text{c}} \quad \sigma = \sigma_{0} (f - f_{\text{c}} )^{t} \quad \lg \sigma = \lg \sigma_{0} + (t)\lg (f_{\text{c}} - f) \)
The value of t is 0.2448. One can see that s and t is similar. It means that the conductivity increased most quickly when the content reached up to the percolation threshold f c = 0.4 vol.%. Figure 6 presented the slow growth of the conductivity in the area of \( f \ll f_{\text{c}} \) and \( f \gg f_{\text{c}} , \) and the sharp increase at the plot of f c. The low value of t also reflected the decrease in system dimensionality due to the MWNTs’ high aspect ratio. Diffusion processes and particle–particle interaction forces played an important role in the agglomeration and network formation (Sandler et al. 2003). When the hybrid material volume fraction was less than f c, the conductive network was not formed, so the conductivity was low. When the content increased up to f c, the conductive network formed and the conductivity increased rapidly. Figure 6 shows the positive temperature coefficient for conductivity.
We also found that the quality of the hybrid material dispersion had a pronounced effect on the conductivity in experiment.
Dispersity in the epoxy matrix
In this article, not only the dispersion stability of the nanofluids in water has been tested but also the dispersion in epoxy matrix has been studied through the TEM images (Fig. 8).
Composites with different weight fraction of nanofluids have been prepared through cast molding method in the vacuum drying oven. When the content of nanofluids is up to 1 wt%, the flexural strength and impact toughness is 9.6 and 73.3%, higher than those of the pure epoxy matrix, respectively (Fig. 7). As shown in Fig. 8, when the weight fraction is more than 1 wt%, the mechanical property reduces. In order to analyze the reforcement reason, we focus on the images, which show that MWNTs is monodispersed in the polymer matrix. In this experiment, there is nearly no chemical interactions between MWNTs and epoxy matrix. So the dispersion of nanometer-sized particles in the polymer matrix is reported to have a significant impact on the mechanical properties of nanocomposites (Zheng et al. 2003). Good dispersion will improve the bondage at interfaces. It is mainly caused by the physical interactions between the nanoparticles and the matrix. Meanwhile, the modification surfactant used in the experiment will improve the compatibilization between nanoparticles and matrix (Zheng and Wang 2002; Shang et al. 2000). It will result in the increase of strength and toughness.
Conclusion
In summary, the capped hybrid nanomaterials consisting of MWNTs and ionic flexible chains were produced by a facile method. The content of MWNTs reached up to 18.04 wt% in the capped hybrid material. The hybrid nanomaterial can flow at ambient temperature. It can be stable when dispersed in water. The conductivity of the hybrid material exhibits that the percolation threshold is about 0.408 vol.%. The ionic solvent-free MWNTs nanofluids dispersed well in epoxy matrix. Meanwhile, the tiny amount addition of nanofluids will improve the mechanical properties of the MWNTs–epoxy composites to some extent.
References
Bahr JL, Tour JM (2002) Covalent chemistry of single-wall carbon nanotubes. J Mater Chem 12:1952–1958
Banerjee S, Kahn MGC, Wong SS (2003) Rational chemical strategies for carbon nanotube functionalization. Chem Eur J 9:1898–1908
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297:787–792
Bourlinos AB, Chowdhury SR, Herrera R, Jiang DD, Zhang Q, Archer LA, Giannelis EP (2005a) Functionalized nanostructures with liquid-like behavior. Adv Funct Mater 15:1285–1290
Bourlinos AB, Chowdhury SR, Jiang DD et al (2005b) Solvent-free fluids based on rhombohedral nanoparticles of calcium carbonate. J Mater Sci 40:5095–5097
Bourlinos AB, Chowdhury SR, Jiang DD et al (2005c) Layered organosilicate nanoparticles with liquidlike behavior. Adv Funct Mater Small 1:80–82
Bourlinos AB, Herrera R, Chalkias N et al (2005d) Surface-functionalized nanoparticles with liquid-like behavior. Adv Mater 17:234–237
Bourlinos AB, Georgakilas V, Tzitzios V, Boukos N, Herrera R, Giannelis EP (2006) Functionalized carbon nanotubes: synthesis of meltable and amphiphilic derivatives. Small 2:1188–1191
Chen J, Hamon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC (1998) Solution properties of single-walled carbon nanotubes. Science 282:95–98
Chen CS, Chen XH, Xu LS, Li WH (2005) Modification of multiwalled carbon nanotubes with fatty acid and their tribological properties as lubricant additive. Carbon 43:1660–1666
Dresselhaus MS, Dresselhaus G, Avouris P (2001) Carbon nanotubes in topics in applied physics. Springer, Berlin, pp 5–10
Garg P, Alvarado JL, Marsh C, Carlson TA, Kessler DA, Annamalai K (2009) An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int J Heat Mass Transfer 52:5090–5101
Georgakilas V, Tzitzios V, Gournis D, Petridis D (2005) Attachment of magnetic nanoparticles on carbon nanotubes and their soluble derivatives. Chem Mater 17:1613–1617
Hilding J, Grulke EA, Zhang ZG, Lockwood F (2003) Dispersion of carbon nanotubes in liquids. Dispers Sci Technol 24:1–41
Holzinger M, Abraham J, Whelan P, Graupner R, Ley L, Hennrich F, Kappes M, Hirsch A (2003) Functionalization of single-walled carbon nanotubes with (R-)oxycarbonyl nitrenes. J Am Chem Soc 125:8566–8580
Huang W, Fernando S, Allard LF, Sun YP (2003) Solubilization of single-walled carbon nanotubes with diamine-terminated oligomeric poly(ethylene glycol) in different functionalization reactions. Nano Lett 3:565–568
Jiao J, Lei WY (2003) Polymer structure, performance and testing. Chemical Industry Press, Beijing, pp 361–364
Kahn MGC, Banerjee S, Wong SS (2002) Solubilization of oxidized single-walled carbon nanotubes in organic and aqueous solvents through organic derivatization. Nano Lett 2:1215–1218
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Science 287:622–625
Lan L, Zheng YP, Zhang JX, Yu PY, Shi W, Yang XD, Li JH (2011) The preparation of solvent-free SiO2 nanofluids and the study of their conductivity. J Funct Mater Devices 3:279–285
Li Q, Dong LJ, Fang JF, Xiong CX (2010) Property–structure relationship of nanoscale ionic materials based on multiwalled carbon nanotubes. ACS Nano 10:5797–5806
Nguyen CT, Desgranges F, Galanis N, Roy G, Maré T, Boucher S, Mintsa HA (2008) Viscosity data for Al2O3–water nanofluid—hysteresis: Is heat transfer enhancement using nanofluids reliable? Int J Therm Sci 47:103–111
Postma HWC, Teepen T, Yao Z, Grifoni M, Dekker C (2001) Carbon nanotube single-electron transistors at room temperature. Science 293:76–79
Sandler JKW, Kirk JE, Shaffer MSP, Kinloch IA, Windle AH (2003) Ultra-low electrical percolation threshold in carbon-nanotube–epoxy composites. Polymer 44:5893–5899
Shang XY, Zhu ZK, Yin J, Ma XD (2000) Influence of coupling agents on the morphology and properties of PI/SiO2 nanocomposites. Acta Mater Compos Sin 4:15–19
Stauffer D, Aharnoy A (1991) Introduction to the percolation theory. Taylor and Francis, London, pp 73–86
Xu PX (2003) Polymer rheology and their application. Chemical Industry Press, Beijing, pp 3–13
Yu PY, Zheng YP, Lan L (2011) The synthesis of solvent-free TiO2 nanofluids through surface modification. Soft Nanosci Lett 1:45–49
Zhang H (2005) Modern organic spectrum analysis. Chemical Industry Press, Beijing, pp 190–196
Zhang JX, Zheng YP, Lan L, Wang RM (2009a) The direct synthesis of solvent-free multiwall carbon nanotubes/silica nonionic nanofluid hybrid material. ACS Nano 3:2185–2190
Zhang JX, Zheng YP, Yang XD, Wang RM (2009b) Modified carbon nanotubes with liquid-like behavior at 45°C. Carbon 47:2776–2781
Zhang JX, Zheng YP, Yu PY, Wang RM (2009c) The synthesis of functionalized carbon nanotubes by hyperbranched poly(amine-ester) with liquid-like behavior at room temperature. Polymer 50:2953–2957
Zhang JX, Zheng YP, Yu PY, Xu L, Wang RM (2010) The synthesis of gel-like hybrid nanomaterials based on carbon nanotube decorated with metal nanoparticles at 45°C. Soft Mater 8:39–48
Zheng YP, Wang B (2002) Study on the properties of TiO2/epoxy nanocomposites. Acta Mater Compos Sin 4:11–13
Zheng YP, Zheng Y, Ning RC (2003) Effects of nanoparticles SiO2 on the performance of nanocomposites. Mater Lett 57:2940–2944
Zheng YP, Zhang JX, Lan L, Yu PY, Rodriguez R (2010) The preparation of solvent-free Au nanofluids with facile self-assemble technique. ChemPhysChem 11:61–64
Acknowledgment
We greatly acknowledge the fund supported by National Science Foundation (51073129 and 50971104), Aeronautical Science Foundation of China (2010ZF53060), and NWPU Graduate student Entrepreneurship Seed Fund (ZX2010083).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lan, L., Zheng, Y.P., Zhang, A.B. et al. Study of ionic solvent-free carbon nanotube nanofluids and its composites with epoxy matrix. J Nanopart Res 14, 753 (2012). https://doi.org/10.1007/s11051-012-0753-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-012-0753-4