Abstract
This study presents the synthesis of TiO2 doped with different amounts of Co and Ni, starting from a simple metallic titanium powder. A successful electrophoretic deposition of these materials on ITO electrodes was achieved for its potential application as photoanodes. EDX, diffuse reflectance UV–Vis spectroscopy, and XRD measurements gave information on the chemical composition of the material and the location of the Ni or Co within the crystal structure of TiO2. Raman spectroscopy suggests that for a higher content of doping metal above a defined percentage, the formation of metal oxide is promoted. A preliminary study of photoelectrocatalytic orange dye degradation shows higher color removal efficiency as compared to the commercial TiO2 material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
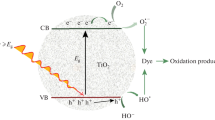
Semiconductor photocatalysis and photoelectrocatalysis have been the subject of many research studies due to the wide range of applications that have been explored for the elimination of hazardous pollutants in air, soil, and water (Han et al. 2009; Zhao et al. 2007; Baea et al. 2008). TiO2 is particularly popular due to its advantageous characteristics such as high photo activity, thermal and chemical stability, low cost and non-toxicity (Castro et al. 2009). On the other hand, the physical and chemical properties of TiO2 can be controlled by its particle size, morphology, and crystalline phase (anatase, rutile, and brookite) (Kim et al. 2008).
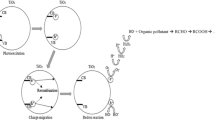
Nevertheless, the practical applications of TiO2 are limited by two main issues. While one of them is the low quantum yield that results from the rapid recombination of photo-generated electrons and holes, the other is related to the semiconductor band gap (3.2 eV) that only absorbs effectively 3–4% of the solar energy spectrum (Tian et al. 2009).
For these reasons, several studies have been carried out in order to improve the photocatalytic properties of TiO2. The main approach consists on doping the TiO2 material with metals such as Fe, Co, Ni, Mn, Au, Ag, and Pt, or with oxides such as WO3 (Li et al. 2008; Gonçalves et al. 2006; Khaleel and Al-Nayli 2008; Tryba 2008; Glaspell and Manivannan 2005; Sobana et al. 2008; Muruganadham et al. 2006; Tiana et al. 2008). Metals either doped or deposited on TiO2 are expected to show several effects on the photocatalytic activity due to different mechanisms. For instance, the metal may (i) enhance the electron–hole separation by acting as electron scavenger and (ii) extend the light absorption of the semiconductor material into the visible range. In this way, it has been suggested that a transition metal with a proper oxidation state replaces some of the Ti4+ centers in the lattice producing an impurity state that reduces the band gap (Kudo et al. 2007; Khan and Kim 2009). In spite of a large quantity of literature dealing with the effect of dopants on the chemical and physical properties of TiO2, there is very little information available on the location of the dopant within the semiconductor structure and its local environment.
In order to obtain TiO2 doped with transition metals, several methods have been developed, being the sol–gel method (Garzella et al. 2003; Tian et al. 2009; Piera et al. 2003; Pacheco et al. 2004; Gonçalves et al. 2006; Facchin et al. 2000; Eshaghi et al. 2011; Kisand et al. 2010) the most frequently employed. However, the synthesis of doped TiO2 starting from metallic titanium mixed with other metals has not been thoroughly explored.
On the other hand, to the best of our knowledge, the preparation of electrophoretically deposited films of TiO2 modified with cobalt or nickel has not been reported. This approach is advantageous because electrophoretic deposition allows the control of the film thickness, porosity, and roughness factor (Esquivel et al. 2009, Manriquez and Godínez 2007) in a short period of preparation time as compared to spin coating, immersion, or impregnation deposit methods (Kim et al. 2007; Bengtsson et al. 2009; Suriye et al. 2007). Additionally, semiconductor films supported on optically transparent electrodes (OTE) avoid the filtration process that is necessary when photocatalytic degradation tests are carried out in suspension (Barakat et al. 2004; Li et al. 2008; Chu et al. 2008, 2009; Esquivel et al. 2009; Selculi et al. 2004).
In this context, the aim of this study is to prepare and characterize TiO2 films on OTE doped with Co or Ni using a non-common method that starts from metallic titanium. The obtained deposits were tested for a preliminary photoelectrocatalytic degradation of a model dye (Orange II) in aqueous medium. Additionally, Raman spectroscopy and X-ray diffraction analysis led us to propose the cation location within the TiO2 lattice.
Materials and methods
M:TiO2 synthesis
The synthesis method is a modification of those reported previously by Martín et al. (2003). As titanium source, metallic titanium powder (99.7%, Aldrich) was used. A precursor solution was obtained by dissolving the titanium powder in hydrochloric acid (HCl, 36.5–38%, J.T. Baker) under stirring conditions for 3 h at the atmospheric boiling point (1 g Ti/20 mL HCl). Once the titanium tetrachloride was obtained in this reaction mixture, nitric acid (10 vol.%) solution was added drop by drop until a color change was visible (from purple to light yellow). This step indicates the complete oxidation from Ti3+ to Ti4+. Then, the solution was cooled and the dopant salt was added, nickel (as Nickel acetate 99%, Ni(CH3COO)2·XH2O, Alfa Aesar) or cobalt (as Cobalt acetate 98%, Co(CH3COO)3·4H2O, Caledon) at different weight percentages (wt%). For both cations, the wt% used were 5, 20, 40, and 50. Ammonium hydroxide (NH3 content 28–30%, Sigma-Aldrich) was used as precipitating agent. The powder was filtered and washed several times with deionized water. The TiO2 modified powder was freeze dried (Free zone 2.5 LABCONCO). The synthetic process with its corresponding chemical equations is presented below (Eqs. 1–3).
Electrophoretic deposition of M:TiO2 powder on ITO electrodes
M:TiO2 films were deposited on ITO glass (Indium Tin Oxide, conductive substrate, 1 cm2 geometric area) using the electrophoretic method (Manriquez and Godínez 2007). In this way, the glass substrate was immersed for 60 s at room temperature in a previously sonicated colloidal suspension (1 g M:TiO2 powder in 10 mL Ethyl Alcohol absolute, J.T. Baker), applying a potential difference of 4 V between a stainless steel sheet and the ITO electrode (Esquivel et al. 2009). The modified substrates were then placed in an oven at 550 °C for 3 h in order to obtain the anatase phase of the modified TiO2 film.
Modified TiO2 characterization and Rietveld Refinement
Surface morphology analysis of the deposited materials was carried out using scanning electron microscopy (SEM) with a JEOL JSM-5400L microscope. The accelerating voltage employed was 15 kV.
Energy dispersive X-ray spectroscopy (EDX) allowed a rough surface elemental analysis and an approximate atomic relationship between Ti4+ and the M2,3+ species (EDX Oxford Inca X-Sight coupled to a JEOL JSM-6060 LV). Band gap values were determined from UV–Vis diffuse reflectance measurements (Ocean Optics Spectrophotometer) applying the Kubelka–Munk theory (Janczarek et al. 2007). X-ray diffraction analysis (XRD) was performed using a Bruker-AXS D8 advanced diffractometer equipped with a Cu seal tube to generate Cu Kα1 radiation (λ = 1.5406 Å) 20 < 2θ < 60° in steps of 0.02°. The collected data were refined by the Rietveld method (Rietveld 1969) using the FULLPROF 05 software, in an attempt to fit the diffraction pattern, in terms of preferred orientation, peak shape, and background. The Thompson-Cox-Hastings pseudo-Voigt function was used to model the peak shape and the preferred orientation. The background was modeled as a polynomial of six refined coefficients. As platform, the Anatase–Rutile template was used. The template was modified by eliminating the rutile phase and varying all the parameters in the next sequence, the scale, background, instrumental, cell and FWHM parameters, preferred orientation, shape, asymmetry, atomic positions, and the overall B-factor (Torres-Martínez et al. 2008). Raman spectroscopy measurements were acquired with LabRAM HR, Horiba Scientific setup equipped with a Nd:YAG laser (λ = 532 nm 80 output power 80 mW). The samples were irradiated with a power of 6 mW over a circular area of 1.5 μm diameter of using a microscope set with a 10× objective.
Photoelectrocatalysis degradation test
Degradation experiments were carried out using an aqueous solution containing Na2SO4 0.05 M pH 3 as electrolyte and 50 mg L−1 of C16H11N2NaO4S (Orange II dye, ALDRICH) as a model dye. In these tests, a vitreous glassy carbon (2 cm2 geometric area) was used as working, the M:TiO2/ITO (M = 5 and 20 wt% of Ni and/or Co; 2 cm2, geometric area) as counter, and a Hg/Hg2SO4 as reference electrode. The experiments were performed under oxygen atmosphere to promote oxygen reduction via two electrons (Esquivel et al. 2009; Peralta-Hernández et al. 2008). The experiments were carried out under potentiostatic conditions applying −0.9 V versus Hg/Hg2SO4 (VoltaLab potentiostat/galvanostat, PGZ 301). For the photo-assisted process, the system was illuminated using a low-pressure mercury lamp (λ = 365 nm, P = 0.75 μ cm−2). UV–Vis measurements were carried out employing a spectrophotometer (Agilent Technologies) to follow the color disappearing of the model dye species (λ = 487 nm, azo bond).
Results and discussion
Characterization of the Co:TiO2 and Ni:TiO2
Scanning electron microscopy images of the modified materials show that the morphology in both semiconductor films (Co, Ni:TiO2) is characterized by aggregates (clusters) distributed homogeneously over the electrode surface (Fig. 1a, b) (Esquivel et al. 2009; Shieh and Chang 2010). EDX analysis, on the other hand, shows the expected presence of the dopant metal (Co or Ni). An approximation of the mole fraction (X i) was estimated using the values obtained from EDX analysis, showing that, as expected, an increase in the weight percentage of dopant in the preparation method, results in a larger relationship between the cation and the titanium species (Table 1). Elemental analysis experiments also showed that in the final material, only a small percentage of the starting quantities of either Ni or Co used is incorporated in the resulting modified substance.
To confirm the presence of the dopant, diffuse reflectance spectroscopy measurements were carried out. The Kubelka–Munk function (Ananpattarachai et al. 2009) shows a decrease in the band gap value, an observation that supports the doping of the TiO2 material. The band gap value obtained for the undoped TiO2 was 3.1 eV, which is in good agreement with that reported for commercial TiO2 Degussa P25 (3.2 eV). For the nickel-doped materials, the obtained values show slight changes around 0.1 eV. In this way, the obtained values for the TiO2-modified materials with starting Ni amounts of 5, 20, 40, and 50% were 2.5, 2.4, 2.3, and 2.1 eV, respectively. For the cobalt-modified materials, the resulting band gap values were, 2.7, 2.5, 2, and 1.9 eV for 5, 20, 40, and 50 wt% Co, respectively.
A simple inspection of the obtained XRD patterns for all the materials with different weight percentages of Co and Ni did not allow us to establish the presence of these metals. The diffraction angles detected in 2θ (25.3°, 36.96°, 37.82°, 38.6°, 48.04°, 53.92°, and 55.08°) indicates the presence of the crystalline anatase phase (Fig. 2a, b). From the diffraction intensity, a preferential orientation of the plane (101) can be proposed. In the case of samples with a high-amount of dopant (above 40 wt%), new diffraction signals (between 30° and 36° in 2θ) can be associated to the rutile phase of TiO2. This observation is in agreement with previous studies which reported that an increment in the concentration of a doping cation promotes a transformation from the anatase to the rutile phase (Li et al. 2003; Barakat et al. 2005). The fraction of anatase, X A, in the samples of 40 and 50 wt% was calculated using Eq. 4 (Chen et al. 2008):
where K is a constant with a value of 0.79, I A corresponds to the X-ray intensity at the highest peak for anatase, and I R is the intensity at the highest peak for rutile.
The fraction of anatase for samples of 40 wt% of dopant was 0.888 and 0.975 for nickel and cobalt, respectively. For the samples prepared with 50 wt% of dopant, the results were 0.739 and 0.887 for nickel and cobalt, respectively. This information reflects the effect of the increase of the dopant metal amount in the change form anatase to rutile. The XRD measurements were carried out with the material deposited on the ITO glass.
The XRD diffraction pattern of the clean ITO substrate shows signals at 26.48° and 51.56° in 2θ. For the modified materials on the other hand, these signals are present in all the diffraction patterns and with larger intensities in the deposits corresponding to 20 wt% of Ni and Co. This is probably related to the higher porosity of these films as compared to the rest of the samples (SEM images not shown). As it was previously mentioned, the surface is formed by clusters (Fig. 1), and as the weight percentage of the dopant increases, the deposit becomes more compact and uniform. The nanometric dimension of these materials was evaluated employing the Debye–Scherrer equation (Chen et al. 2008), and for all the samples under study, an average size value of 18 nm was obtained.
Additionally, XRD patterns corresponding to Ni and Co oxides were obtained in order to check the presence of these species in the modified materials. The results show their absence in the doped semiconductor films. At this point, it can be stated that the cobalt or nickel is present in the deposit but it was not known if it is inside the crystalline structure substituting the titanium(IV) or just present as an interstitial dopant of the anatase structure. Induced-coupled plasma (ICP) measurements (<0.006 mg L−1), cyclic voltammetry, and polarography showed no presence of nickel or cobalt in solution after electro and photoelectrocatalysis experiments. This fact suggests that the metal is protected inside the TiO2 structure even in acidic media [the Co and Ni are not stable in acidic mediums (Yang et al. 2009)] or in the presence of H2O2.
Despite that the Rietveld refinement method is mainly used for powder samples, we decided to use it as a first approach for the electrophoretic deposit of M:TiO2 in order to propose the possible location of Co2+ or Ni2+ within the structure of the TiO2, (in an interstitial position or as substitution of the Ti4+). In this way, fitting the experimental XRD patterns was carried out assuming that the metal is actually inside the crystalline structure as a substitutional dopant (Li et al. 2005; Djerdj et al. 2006). In Fig. 3a and b, the Rietveld fittings for the samples prepared from 20 wt% of Co and Ni are presented. In these figures, the observed (○) and the calculated patterns (–) are shown along with the difference between them (residual (∆)) and the Bragg positions (∣).The resulting refinement parameters are listed in Table 2a and b. Inspection of this data shows that the Ni or the Co is inserted at the same position of the Ti4+ (0, 0.25, and 0.375, x, y, and z) in the anatase structure. It is also interesting to note that the fitting parameter χ2 is in some cases slightly above 1.3. This difference is probably due to the fact that the collection data were obtained from a diffraction pattern of a deposited film instead of a powder; which can increase the statistical error (Djerdj et al. 2006). When the Ni and/or Co oxide phases were considered on the refinement, no convergence of the method was achieved.
In good agreement with the data reported for the undoped anatase phase (Xu et al. 1999), Raman spectroscopy measurements of the unmodified TiO2 film showed five typical signals (Figs. 4a, 5a), 146(Eg), 199(Eg), 400(B1g), 520(A1g), and 643(Eg) cm−1. When the TiO2 was modified with different amounts of Ni, a slight displacement of the Eg vibration signals (150, 201, and 643 cm−1) was obtained. In the case of Co, a larger displacement was observed due to the lower crystallinity of the relevant vibrations Eg, B1g, and A1g (157, 206, 402, 519, and 634 cm−1). In addition, it is important to note that for weight percentages larger than 40 wt%, it was possible to observe a new signal located at 694.258 cm−1 for Co: TiO2 (Fig. 4b) that can be attributed to the A1g mode of Co3O4 (Hadjiev et al. 1988). Similarly, for the Ni:TiO2 materials, this peak appeared at 706.622 cm−1 (Fig. 5b). In this case, the signal could be attributed to NiO according to the Raman Spectra Database of Minerals and Inorganic Materials (RASMIN). These signals are not seen by XRD due to the lack of crystallinity of the relevant oxides and to the detection limit of the equipment. This observation suggests that the TiO2 structure allows a maximum amount of Ti4+ substitution by the dopant Ni2+ or Co2+. The remaining dopant cations are anchored on the TiO2 surface as another phase, such as Co3O4 and/or NiO.
According to all the physico-chemical characterization discussed, the nickel and cobalt species are part of the TiO2 lattice substituting some of the Ti4+ centers. In this way, as the dopant material wt% increases, some fraction of this species grows as an oxide outside of the TiO2 lattice. Additional support comes from the fact that the phase change from anatase to rutile is related to increasing amounts of nickel/or cobalt in the synthetic reaction mixture.
As it has been reported, the highest photocatalytic activity in degradation tests is presented by the anatase phase of the TiO2 (Yanga et al. 2010; Elsellami et al. 2010; Rauf and Ashraf 2009), and a mixture of phases (anatasa-rutile) should then be characterized by a smaller photocatalytic performance. In this context, it was found convenient to carry out some preliminary experiments on the photoelectrocatalytic degradation of a model dye (azo Orange II) in aqueous solution using the 5 and 20 wt% of the Co and Ni doped materials as photoanodes.
Degradation test using the Co:TiO2 and Ni:TiO2 supported on ITO as photoanodes
In the photoelectrochemical experiments for both modified materials (Co and Ni), the color in solution was totally removed after 15 min of running the test (Fig. 6). For the non-modified TiO2, the photoelectrocatalytic activity was substantially lower since the color removal percentage was barely above 50% after 20 min of reaction time. This color removal percentage was achieved with the two modified materials (20 wt% Co and Ni) after 5 min. For 5 wt% of Co and Ni:TiO2 on the other hand, the same level of color removal was achieved in 10 and 40 min, respectively. These preliminary experiments suggest that the presence of Ni and Co within the anatase lattice increases the effectiveness of the electrophotocatalytic process as evidenced by a faster color removal rate of the dye Orange II when it is compared to the undoped TiO2. This observation not only suggests a potential application of these modified semiconductor films as photoanodes in water treatment reactors, but also supports the interpretation of the location of the dopant metals within the semiconductor structure.
Absorbance relationships (color removal of orange dye) using TiO2 and M:TiO2 materials modified with different wt% of the metallic doping agent (5 and 20 wt% of Co and Ni). [OG II] 50 mg L−1; electrolyte pH 3; 0.05 M Na2SO4 solution; degradation time 60 min; UV lamp: λ = 365 nm, P = 0.75 μW/cm2; E = −0.9 V versus Hg/Hg2SO4
Conclusion
It was possible to modify TiO2, obtained from metallic titanium, with Co and Ni. Also, it was possible to obtain a deposit of the modified materials on a conductive surface by the electrophoretic method. Using the Rietveld refinement, it can be proposed that the Co and Ni are located inside the TiO2 crystalline anatase structure, substituting some of the titanium (Ti4+) centers. Raman spectroscopy experiments, on the other hand, showed the presence of the oxides Co3O4 and NiO when the starting amount of doping cation was above 40 wt%. In this way, it is possible to suggest that there is some point where Ti4+ ions cannot be substituted by Ni or Co within the lattice. Since in preliminary photoelectrocatalytic tests, a better performance was observed with these modified materials as compared to the case of undoped TiO2, this study also suggests that TiO2 doped with different amounts of Co and Ni can be used in films that constitute a new promising type of photoanodes.
References
Ananpattarachai J, Kajitvichyanukul P, Seraphin S (2009) Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J Hazard Mater doi:10.1016/j.jhazmat.2009.02.036
Baea S, Shimb E, Yoonc J, Joo H (2008) Enzymatic hydrogen production by light-sensitized anodized tubular TiO2 photoanode. Solar Energy Mater Solar Cells 92:402–409
Barakat MA, Schaeffer H, Hayes G, Ismat SS (2004) Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl Catal B 57:23–30
Barakat MA, Hayes G, Ismat SS (2005) Effect of cobalt doping on the phase transformation of TiO2 nanoparticles. J Nanosci Nanotechnol 10:1–7
Bengtsson N, Castellote M, Lopez-Muñoz MJ, Cerro L (2009) Preparation of Co-doped TiO2 for photocatalytic degradation of NO x in air under visible light. J Adv Oxid Technol 12(1):55–64
Castro AL, Nunes MR, Carvalho MD, Ferreira LP, Jumas JC, Costa FM, Florencio MH (2009) Doped titanium dioxide nanocrystalline powders with high photocatalytic activity. J Solid State Chem. doi:10.1016/j.jssc.2009.04.020
Chen J, Yao M, Wang X (2008) Investigation of transition metal ion doping behaviours on TiO2 nanoparticles. J Nanopart Res 10:163–171
Chen L-C, Ho Y-C, Guo W-S, Huang C-M, Pan T-C (2009) Enhanced visible light-induced photoelectrocatalytic degradation of phenol by carbon nanotube-doped TiO2 electrodes. Electrochim Acta 54:3884–3891
Chu D, Yuan X, Qin G, Xu M, Zheng P, Lu J, Zha L (2008) Efficient carbon-doped nanostructured TiO2 (anatase) film for photoelectrochemical solar cells. J Nanopart Res 10:357–363
Djerdj I, Tonejc AM, Tonejc A (2006) Structural refinement of nanocrystalline TiO2 samples. Electron Crystallogr 497–501
Elsellami L, Vocanson F, Dappozze F, Puzenat E, Païsse O, Houas A, Guillard C (2010) Kinetic of adsorption and of photocatalytic degradation of phenylalanine effect of pH and light intensity. Appl Catal A 380:142–148
Eshaghi A, Mozaffarinia R, Pakshir M, Eshaghi A (2011) Photocatalytic properties of TiO2 sol–gel modified nanocomposite films. Ceram Int 37:327–331
Esquivel K, Arriaga LG, Rodríguez FJ, Martínez L, Godínez LA (2009) Development of a TiO2 modified optical fiber electrode and its incorporation into a photoelectrochemical reactor for wastewater treatment. Water Res 43:3593–3603
Facchin G, Carturan G, Campostrini R, Gialanella S, Lutterotti L, Armelao L, Marcí G, Palmisano L, Sclafani A (2000) Sol-gel synthesis and characterisation of TiO2-anatase powders containing nanometric platinum particles employed as catalysts for 4-nitrophenol photodegradation. J Sol-Gel Sci Technol 18:29–59
Garzella C, Bontempi E, Depero LE, Vomiero A, Della Mea G, Sberveglieri G (2003) Novel selective ethanol sensors: W/TiO2 thin films by sol–gel spin-coating. Sens Actuators B 93:495–502
Glaspell G, Manivannan A (2005) Sol–gel synthesis and magnetic studies of titanium dioxide doped with 10% M (M = Fe, Mn and Ni). J Clust Sci 16(4):501–513
Gonçalves G, Lenzi MK, Santos OAA, Jorge LMM (2006) Preparation and characterization of nickel based catalysts on silica, alumina and titania obtained by sol–gel method. J Non-Cryst Solids 352:3697–3704
Hadjiev VG, Iliev MN, Vergilov IV (1988) The Raman spectra of Co3O4. J Phys C 21:L199–L201
Han F, Subba Rao KV, Srinivasan M, Dharmarajan R, Naidu R (2009) Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: a review. Appl Catal A 359:25–40
Janczarek M, Kisch H, Hupka J (2007) Photoelectrochemical characterization of nitrogen-modified TiO2. Physicochem Probl Miner Process 41:159–166
Khaleel A, Al-Nayli A (2008) Supported and mixed oxide catalysts based on iron and titanium for the oxidative decomposition of chlorobenzene. Appl Catal B 80:176–184
Khan R, Kim T-J (2009) Preparation and application of visible-light-responsive Ni-doped and SnO2-coupled TiO2 nanocomposite photocatalysts. J Hazard Mater 163:1179–1184
Kim HR, Choi KY, Shul Y-G (2007) Preparation of TiO2/SiO2 hollow spheres and their activity in methylene blue photodecomposition. Korean J Chem Eng 24(4):596–599
Kim DH, Choi D-K, Kim S-J, Lee KS (2008) The effect of phase type on photocatalytic activity in transition metal doped TiO2 nanoparticles. Catal Commun 9:654–657
Kisand V, Joost U, Reedo V, Pärna R, Tätte T, Shulga J, Saar A, Matisen L, Kikas A, Kink I (2010) Influence of the heating temperature on the properties of nickel doped TiO2 films prepared by sol–gel method. Appl Surf Sci 256:4538–4542
Kudo A, Niishiro R, Iwase A, Kato H (2007) Effects of doping of metal cations on morphology, activity, and visible light response of photocatalysts. Chem Phys 339:104–110
Li X, Yue P-L, Kutal C (2003) Synthesis and photocatalytic oxidation properties of iron doped titanium dioxide nanosemiconductor particles. New J Chem 27:1264–1269
Li W, Frenkel AI, Woicik JC, Ni C, Ismat SS (2005) Dopant location identification in Nd3+-doped TiO2 nanoparticles. Phys Rev B 72:155315-1–155315-6
Li J, Liu S, He Y, Wang J (2008) Adsorption and degradation of the cationic dyes over Co doped amorphous mesoporous titania–silica catalyst under UV and visible light irradiation. Microporous Mesoporous Mater 115:416–425
Manriquez J, Godínez LA (2007) Tuning the structural, electrical and optical properties of Ti(III)-doped nanocrystalline TiO2 films by electrophoretic deposition time. Thin Solid Films 515:3402–3413
Martín C, Solana G, Malet P, Rives V (2003) Nb2O5-supported WO3: a comparative study with WO3/Al2O3. Catal Today 78:365–376
Muruganadham M, Swaminathan M (2006) Nano-Ag particles doped TiO2 for efficient photodegradation of direct azo dyes. J Mol Catal A 258:124–132
Pacheco F, González M, Medina A, Velumani S, Ascencio JA (2004) Structural analysis of cobalt titanate nanoparticles obtained by sol–gel process. Appl Phys A 78:531–536
Peralta-Hernández JM, Meas-Vong Y, Rodríguez FJ, Chapman TW, Maldonado MI, Godínez LA (2008) Comparison of hydrogen peroxide-based processes for treating dye-containing wastewater: decolorization and destruction of Orange II azo dye in dilute water. Dyes Pigments 76:656–662
Piera E, Tejedor-Tejedor MI, Zorn ME, Anderson MA (2003) Relationship concerning the nature and concentration of Fe(III) species on the surface of TiO2 particles and photocatalytic activity of the catalyst. Appl Catal B 46:671–685
Raman Spectra Database of Minerals and Inorganic Materials (RASMIN) at RIO-DB of AIST (http://www.aist.go.jp/RIODB/rasmin/E_index.htm)
Rauf MA, Ashraf SS (2009) Review: fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem Eng J 151:10–18
Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2:65–71
Selculi H, Zaltner W, Sene JJ, Bekbolet M, Anderson MA (2004) Photocatalytic and photoelectrocatalytic performance of 1% Pt doped TiO2 for the detoxification of water. J Appl Electrochem 34:653–658
Shieh Y-N, Chang Y-Y (2010) Influence of cobalt ion implantation on optical properties of titanium dioxide thin films. Thin Solid Films 518:7464–7467
Sobana SJ, Qiao L, Sun S, Wang G (2008) Photocatalytic degradation of Orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J Hazard Mater 155:312–319
Suriye K, Praserthdam P, Jongsomjit B (2007) Effect of surface sites of TiO2 support on the formation of cobalt-support compound in Co/TiO2 catalysts. Catal Commun 8:1772–1780
Tian B, Li C, Gu F, Jiang H (2009) Synergetic effects of nitrogen doping and Au loading on enhancing the visible-light photocatalytic activity of nano-TiO2. Catal Commun 10:925–929
Tiana H, Maa J, Li K, Li J (2008) Photocatalytic degradation of methyl orange with W-doped TiO2 synthesized by a hydrothermal method. Mater Chem Phys 112:47–51
Torres-Martínez LM, Juárez-Ramírez I, Del Ángel-Sánchez K, Garza-Tovar L, Cruz-López A, Del Ángel G (2008) Rietveld refinement of sol–gel Na2Ti6O13 and its photocatalytic performance on the degradation of methylene blue. J Sol-Gel Sci Technol 47:158–164
Tryba B (2008) Immobilization of TiO2 and Fe–C–TiO2 photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water. J Hazard Mater 151:623–627
Xu W-X, Zhu S, Fu X-C, Chen Q (1999) The structure of TiO x thin film studied by Raman spectroscopy and XRD. Appl Surf Sci 148:253–262
Yang Q, Choi H, Al-Abed SR, Dionysiou DD (2009) Iron–cobalt mixed oxide nanocatalysts: heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl Catal B 88:462–469
Yanga H, Ana T, Li G, Song W, Cooper WJ, Luo H, Guo X (2010) Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: a case of β blockers. J Hazard Mater 179:834–839
Zhao H, Jiang D, Zhang S, Wen W (2007) Photoelectrocatalytic oxidation of organic compounds at nanoporous TiO2 electrodes in a thin-layer photoelectrochemical cell. J Catal 250:102–109
Acknowledgments
The authors thank the Mexican Council for Science and Technology (CONACyT, Grant SEP-CONACYT 83894) for financial support of this work. K.E.E. also acknowledges CONACyT for a graduate fellowship and to Eng. Gregorio Solorioc for his support on the EDX analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esquivel, K., García J., M.G., Rodríguez, F.J. et al. Titanium dioxide doped with transition metals (M x Ti1−x O2, M: Ni, Co): synthesis and characterization for its potential application as photoanode. J Nanopart Res 13, 3313–3325 (2011). https://doi.org/10.1007/s11051-011-0245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0245-y