Abstract
Snowflake-like structural assembly of isotropic gold nanoparticles (GNPs) is reported. A modified polyamine method has been employed to synthesize positively charged GNPs in presence of a polymeric metaphosphate. This process yields fascinating dendritic self-assembled morphologies. Structural characterization revealed that there was aggregation of crystalline GNPs. The aggregates of GNPs formed in the initial stage of synthesis are assumed to act as the bulging seeds for final growth of complex morphologies at nanometer to micrometer length scale. Self-assembly of GNPs was found to be greatly influenced by the concentration of gold precursor. Diffusion limited aggregation of GNPs is suggested as the plausible mechanism for this nanoparticle self-organization process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fabrication of functional nanostructures with controlled dimensions and novel properties remains a challenging task for those involved in fundamental scientific investigations and in the development of technological applications (Alivisatos 1996; Klabunde 2001; Dahl et al. 2007; Hyeon 2003; Schmid 2004; Murphy et al. 2005; El-Sayed 2001; Burda et al. 2005; Jain et al. 2007). By using controlled synthetic methodologies, molecular self-assembly of desired nanomaterials can be successfully achieved. Two fundamentally different approaches (top-down and bottom-up) have been shown to play important roles in the synthesis and assembly of nanoparticles (NPs). In the top–down approach, a bulk material is systematically broken down to form NPs of desired dimensions. In these cases, particle assembly is directed by a patterned or anisotropic surface or matrix. In contrast, in the bottom-up strategy that is inspired by the principles of molecular recognition, the nanostructures are constructed starting from individual molecular components. The latter approach has received considerable attention owing to its inherent simplicity, speed of production, and ease in generating isotropic and/or anisotropic self assembled morphologies. Hierarchical self-assembly originating from nanoscale building blocks leads to the generation of complex supramolecular structures, whose properties are determined by those of the nanoscale constituents. As a result, a basic understanding of the NP self-assembly process will provide important insights into the fundamental properties of matter at the molecular level and influence practical applications of bottom-up fabrication technologies (Glotzer and Solomon 2007; Tang et al. 2006; Akcora et al. 2009).
In the bottom-up approach, the generation of molecular assemblies is based on equilibrium and non-equilibrium growth phenomena, both of which are facilitated by hydrophobic, electrostatic, and/or van der Waals interactions that take place between the reactants and/or substrate (Dutta and Hofmann 2003; Leite 2004). In the case of non-equilibrium growth, spontaneous, kinetically controlled pattern formation leads to the production of diverse kinds of morphologies (Nittmann and Stanley 1986; Sander 1986; Ben-Jacob and Garik 1990). Occasionally, dendritic structures are generated in non-equilibrium processes due to fractal growth on the molecular scale (Wang et al. 2008; Sun and Hagner 2007; Jin and Dong 2002; Ji et al. 2008; Fang et al. 2007). Most often, dendritic structures are the result of either growth of anisotropic particles or directional interactions of isotropic particles in the medium.
In the past two decades, fractal/dendritic morphologies of metal nanoparticles (MNPs), in particular metal–polymer composites, have attracted great attention owing to their potential applications as advanced materials and for their ability to serve as platforms to gain a fundamental understanding of self assembly methods for the creation of functional building blocks with unique structural properties (Akcora et al. 2009; van Herrikhuyzen et al. 2006; Shenhar et al. 2005).
The major challenge associated with the synthesis of MNPs–polymer composite is the achievement of stable dispersions of NPs in polymer matrices. Frequently, phase separation takes place as a result of the immiscibility of inorganic NPs in organic polymeric phases (Krishnamoorti and Vaia 2007; Bansal et al. 2005; Krishnamoorti 2007; Mackay et al. 2006). Recent reports suggest that charged polymers are more effective in the synthesis and stabilization of MNPs due to either steric hindrance or electrostatic interactions (Tan et al. 2007; Li et al. 2008). The charged polymers can also lead to the self-assembly of the NPs at the molecular level as a consequence of the interactions that occur between particle cores and the polymeric surroundings.
Recent observations made in our investigations of a novel self-assembly method for the construction of snowflake-like arrangements of isotropic gold nanoparticles (GNPs) at the micrometer scale are described below. In this study, the polyamine method (Sun et al. 2004, 2005a, b) for synthesis of gold nanostructures was employed to generate novel and fascinating self-assembled structures. The influence of reactant concentrations on the morphologies of the nanostructures has also been explored. Finally, a plausible mechanism for the self-organization of GNPs with snowflake-like structures is presented.
Materials and methods
Materials
Hydrogen tetrachloroaurate (III) hydrate (HAuCl4·3H2O), and polyethyleneimine (PEI) (mol. wt. 750,000) were obtained from Aldrich, USA and sodium hexametaphosphate, (NaPO3)6 (HMP) were purchased from Alfa Aesar, USA. All the chemicals were used as-received without further purification. Water used throughout this investigation was reagent-grade and purified using a Milli-Q SP ultrapure-water purification system from Nihon Millipore Ltd., Tokyo.
Synthesis of gold nanoparticles and their assembly
The GNPs were synthesized by chemical reduction using PEI in the presence of HMP. In a typical experiment, 7.5 mL of 0.01 M gold precursor solution was added to 1 × 10−4 M PEI solution followed by gentle swirling. To this solution was added 1.5 mL of 0.01 M HMP solution and distilled water to obtain final concentrations of PEI and HMP of 1 × 10−5 and 1.5 × 10−3 M, respectively. The resulting mixture was heated at 70 °C with constant stirring to facilitate the formation of NPs. After the formation of a wine red colored solution (which indicates nanoparticle formation), heating was continued for 7–8 min to ensure the completion of reaction. To study the effect of reaction conditions on the self-assembled morphologies of gold nanostructures, changes in the concentrations of reactants were made and the optimum conditions for generation of dendritic assembly were determined.
Characterization
The GNPs capped with PEI in the presence of HMP were characterized by using various experimental techniques. UV–Vis absorption spectra of the sample solutions were taken on a CARY-100 conc UV–Vis spectrophotometer (Varian, Palo Alto, CA) in the wavelength range of 400–700 nm. X-ray diffraction (XRD) analysis was carried out using RIGAKU-D/MAX-IIIC (3 kW) operated at 40 kV power. Scanning electron microscopy (SEM) analysis was performed using PHILIPS-XL30SFEG microscope, operated at an accelerating voltage of 10 kV. The samples for SEM analysis were prepared by air drying 5 μL of the solution on a silicon wafer. For transmission electron microscopy (TEM), samples were drop casted and dried on a carbon-coated copper grid and imaged at 200 kV (JEM 2010, JEOL). Surface charge of the samples was evaluated using a MALVERN Zetasizer Nano ZS.
Results and discussion
The new methodology we have devised to prepare GNPs employs a modification of the polyamine method involving the use of polymeric hexaphosphate. The typical synthetic procedure for forming GNPs involves the reduction of hydrochloroauric acid (HAuCl4·3H2O) using PEI at 70 °C in presence of sodium HMP. Addition of PEI to gold precursor results in formation of an orange solution whose color changes to wine red, associated with the formation of GNPs, upon heating in presence of HMP. The redox reaction that takes place between the amine groups of PEI and Au+ is responsible for the formation of GNPs.
The UV–Vis absorption spectrum of the resulting solution shows a surface plasmon absorption peak at λ = 518 nm, indicating the formation of colloidal GNPs (Fig. 1a). The diffraction peaks of the sample, observed by using XRD analysis, are similar to those of metallic gold. This demonstrates the crystalline nature of GNPs (Fig. 1b). The XRD pattern shows that the GNPs have been formed by preferential growth along the {111} crystal plane. The results of ζ potential analysis confirm that the resulting GNPs are positively charged, perhaps caused by protonation of the surface amino groups of PEI. The as-synthesized GNPs are found to be quite stable for several months without any sign of agglomeration.
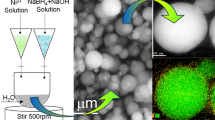
Scanning electron microscopy analysis reveals that an interesting self-organization of the GNPs occurs on the micrometer length scale. Inspection of Fig. 2a clearly shows that snowflake-like assembly has taken place in high yield and with large uniformity. A close view of the high-resolution SEM image reveals the presence of aggregates of very small-sized GNPs (Fig. 2b), the composition of which was further probed using energy dispersive X-ray (EDS) analysis. EDS of a selected area of the SEM image contains a peak associated with gold along with other bands that can be assigned to the silicon wafer substrate and reactants (See supporting information, Fig. S1-d). The absence of peaks for C and N indicates that the dendrites comprised assemblies of GNP aggregates and not the assemblies of coordination complexes of Au+, PEI, and HMP.
a A typical SEM image of snowflake-like assembly of GNPs obtained at 5.0 × 10−3 M initial concentration of gold precursor, and b a magnified SEM image of a single snowflake (the inset is a close-up view of the red marked area showing aggregates of small GNPs (inset)). Scale bar = 10 μm (Color figure online)
Additional structural information is provided by TEM analysis along with its related high-resolution TEM (HRTEM) image taken from the edge of the aggregate (Fig. 3). The results of TEM analysis confirm the formation of GNP aggregates (Fig. 3a) with individual particle sizes of ca. 5 nm. Highly ordered, continuous lattice fringes of the individual nanoparticles are clearly observed by using HRTEM analysis (Fig. 3b), a finding that points to the crystalline nature of the particles. The spacing between two adjacent lattice planes is 0.235 nm, which corresponds to the {111} crystal plane. Thus, the observations clearly demonstrate that the GNPs, produced by using the procedure described above, arise by growth along {111} crystal planes. A plausible explanation for this phenomenon invokes electrostatic interactions between positively charged GNPs and anionic phosphate groups from HMP (interplay between dipolar potentials) and short range (van der Waals) interactions between the isotropic GNPs promote the anisotropic self-assembled morphologies (see below).
It is well known that the propensity to produce branched structures depends on the molar ratios and concentrations of reactants used in the synthesis of NPs (Wang et al. 2008; Sun and Hagner 2007). In this effort, the concentrations of reactants were varied and the morphologies of the resulting products were analyzed. The results show that the optimized formation of suitable seeds for favorable growth of branched assemblies takes place when the concentrations of PEI, HMP, and the gold precursor are 1.0 × 10−5, 1.5 × 10−3, and 1.0 × 10−3 M, respectively (see supporting information, Fig. S2). The morphological progression of the GNPs assembly process was explored by using SEM and varying initial metal ion concentrations from 1.0 × 10−3 to 7.5 × 10−3 M. The images displayed in Fig. 4 show that the predominance of snowflake-like structures increases with increasing concentrations of gold precursor.
The assembly of GNPs was found to be highly dependent on the ionic strength of the reaction solution as a consequence of attractive interactions between like charged particles promoted by correlated charge fluctuations in the surrounding ionic clouds. At low concentrations of gold, aggregation of GNPs in the form of clusters takes place to form structures that act as seeds (Fig. 4a). An increase in the gold ion concentration leads to production of greater numbers of GNPs, which add to the ends of growing seeds, thus increasing the size of aggregates to give structures that appear as seeds bursting into blossoms (Fig. 4b). The splitting ends of the GNP aggregate bulging seeds can be clearly seen in the SEM image. A further increase in the gold ion concentration results in the growth of primary and secondary branches, which give the appearance of snowflakes (Fig. 4c). Fractal growth from bulging seeds and primary branches into the snowflake assemblies is observed (see supporting information, Fig. S3). At very high gold concentrations, the growth of fractal pattern becomes denser (Fig. 4d). The SEM observations demonstrate that morphologies of the self-assembled structures are quite dependent upon the starting metal ion concentration. Overall, these nanoparticle assemblies are appeared to be monodispersed and are present in the size range of few micrometers (Figs. 2, 4).
In order to probe the role played by reactants in the growth of these complex dendritic morphologies, reactions were carried out in the absence of HMP and the gold salt. Reduction of gold precursor in the absence of HMP results in formation of a purple colored solution, which contains particle aggregates that do not have snowflake-like structures as seen from the SEM image (see supporting information, Fig. S1). These solutions were found to yield purple precipitates upon standing for a few days. A heated mixture of HMP and PEI not containing gold ions also does not yield any snowflake-like self-assemblies. Thus, it is clear that only solutions containing PEI-capped GNPs in the presence HMP lead to production of well-defined, sub-micrometer-sized snowflake-like structures and HMP plays an important role in the formation of these structures.
In principle, HMP acts as an anionic polymeric stabilizer during the synthesis of GNPs. When the PEI-capped positively charged GNPs are formed in the solution, negatively charged phosphate groups from HMP covers the synthesized nanoparticles due to the strong electrostatic interaction. The coverage of negatively charged HMP causes electrostatic repulsion between the nanoparticles along with the steric hindrance due to the bulky nature of HMP, which consequently results in stabilization of GNPs suspension against agglomeration. Further, the short range (van der Waals) directional interactions between the HMP-stabilized GNPs moving randomly in the solution encourage the formation of anisotropic self-assembled morphologies (see below). In the absence of HMP during synthesis, on contrary, a purple colored solution of nanoparticles is formed, which fails to generate the snowflake-like assembly of GNPs. Thus, it is reasonable to hypothesize that the presence of HMP is critical for the fractal growth process. On the basis of these observations and the results of previous investigations of related self-assembled nanostructures, a plausible mechanism for development of microscale fractal patterns can be formulated. As shown in Fig. 5, the snowflake-like fractal and dendritic growth is expected to be kinetically controlled in diffusion limited regimes, when systems are not at equilibrium. Particularly, the fabrication of snowflake-like hybrid materials appears to follow a diffusion limited aggregation (DLA) model.
Initially, GNPs form when the reaction mixture is heated as a consequence of the presence of PEI. Previous efforts (Sun et al. 2004, 2005a, b) have shown that PEI acts as a reducing agent in the high-temperature reaction leading to formation of GNPs. The short range (van der Waals) interactions between the initially formed isotropic GNPs cause aggregation and then the formation of clusters that act as seeds. The abundant seeds undergo random collisions in solution, thus facilitating their growth, which occurs faster at the ends rather than interior parts. Thus, the growing clusters begin to branch and finally adopt a more open and feathery, micrometer lengthed, snowflake-like shape. We assume that the rate-limiting step for particle aggregation and fractal formation is diffusion and hence the entire process can be viewed as being kinetically controlled by using the DLA mechanism. Finally, rapid nucleation and growth of polymer-capped GNPs in addition to the directional nature of inter-particle interactions facilitated by DLA appear to be the major factors responsible for the formation of the snowflake-like fractal assembly.
In order to utilize the GNPs synthesized in the present studies for various clinical applications, it is essential to study their in vitro cell viability according to an exposure to the nanoparticles during different clinical applications. Figure S4 shows the cell viability results for MCF-7 (human breast adenocarcinoma) cell line in the presence of GNPs. The cytotoxicity studies revealed that there is no obvious change in cell viability in the studied metal concentration range, although 50 μM concentration is considered to be far higher than the normal use. Thus, the GNPs synthesized herein were found to be quite compatible against the cancerous cell lines demonstrating their possible utility for different clinical applications in future.
Conclusions
In summary, the observations made in this investigation demonstrate that isotropic GNPs, capped with polymer, self-assemble into anisotropic superstructures at the micrometer scale in the presence of an anionic polymeric metaphosphate. Structural analysis confirms that the dendritic structures are aggregates of NPs that are crystalline in nature. The concentrations of reactants influence the formation of branched structures. Rapid nucleation and growth of polymer-capped GNPs in addition to the directional nature of inter-particle interactions facilitated by DLA appear to be the major factors responsible for the formation of the snowflake-like fractal assembly. This study also demonstrates the importance of anisotropic inter-particle interactions at the microscale level. Equally significant is the fact that the results provide a foundation for understanding the role of electrostatic interactions in the generation of complex morphologies under non-equilibrium conditions. Further studies are underway to uncover potential applications of these self-assembled structures.
References
Akcora P, Liu H, Kumar SK, Moll J, Li Y, Benicewicz BC, Schadler LS, Acehan D, Panagiotopoulos AZ, Pryamitsyn V, Ganesan V, Ilavsky J, Thiyagarajan P, Colby RH, Douglas JF (2009) Anisotropic self-assembly of spherical polymer-grafted nanoparticles. Nat Mater 8:354–359
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937
Bansal A, Yang HC, Li CZ, Cho KW, Benicewicz BC, Kumar SK, Schadler LS (2005) Quantitative equivalence between polymer nanocomposites and thin polymer films. Nat Mater 4:693–698
Ben-Jacob E, Garik P (1990) The formation of patterns in non-equilibrium growth. Nature 343:523–530
Burda C, Chen XB, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev 105:1025–1102
Dahl JA, Maddux BLS, Hutchison JE (2007) Toward greener nanosynthesis. Chem Rev 107:2228–2269
Dutta J, Hofmann H (2003) In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology, vol 10. American Scientific Publishers, Stevenson Ranch, CA, pp 1–23
El-Sayed MA (2001) Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res 34:257–264
Fang JX, You HJ, Kong P, Yi Y, Song XP, Ding BJ (2007) Dendritic silver nanostructure growth and evolution in replacement reaction. Cryst Growth Des 7:864–867
Glotzer SC, Solomon MJ (2007) Anisotropy of building blocks and their assembly into complex structures. Nat Mater 6:557–562
Hyeon T (2003) Chemical synthesis of magnetic nanoparticles. Chem Commun 927–934
Jain PK, Huang X, El-Sayed IH, El-Sayad MA (2007) Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2:107–118
Ji ZY, Li HX, Liu YL, Hu WP, Liu YQ (2008) The replacement reaction controlling the fractal assembly of copper nanoparticles. Nanotechnology 19: 135602 (5 pp)
Jin YD, Dong SJ (2002) Diffusion-limited, aggregation-based, mesoscopic assembly of roughened core shell bimetallic nanoparticles into fractal networks at the air-water interface. Angew Chem Int Ed 41:1040–1044
Klabunde KJ (2001) Nanoscale materials in chemistry. John Wiley & Sons Inc, New York
Krishnamoorti R (2007) Strategies for dispersing nanoparticles in polymers. MRS Bull 32:341–347
Krishnamoorti R, Vaia RA (2007) Polymer nanocomposites. J Polym Sci B Polym Phys 45:3252–3256
Leite ER (2004) In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology, vol 6. American Scientific Publishers, Stevenson Ranch, CA, pp 537–554
Li T, Du Y, Wang EK (2008) Polyethyleneimine-functionalized platinum nanoparticles with high electrochemiluminescence activity and their applications to amplified analysis of biomolecules. Chem Asian J 3:1942–1948
Mackay ME, Tuteja A, Duxbury PM, Hawker CJ, Van Horn B, Guan ZB, Chen GH, Krishnan RS (2006) General strategies for nanoparticle dispersion. Science 311:1740–1743
Murphy CJ, San TK, Gole AM, Orendorff CJ, Gao JX, Gou L, Hunyadi SE, Li T (2005) Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B 109:13857–13870
Nittmann J, Stanley HE (1986) Tip splitting without interfacial tension and dendritic growth patterns arising from molecular anisotropy. Nature 321:663–668
Sander LM (1986) Fractal growth processes. Nature 322:789–793
Schmid G (2004) Nanoparticles: from theory to application. Wiley-VCH, Verlag GmbH, Weinheim
Shenhar R, Norsten TB, Rotello VM (2005) Polymer-mediated nanoparticle assembly: structural control and applications. Adv Mater 17:657–669
Sun XP, Hagner M (2007) Novel preparation of snowflake-like dendritic nanostructures of Ag or Au at room temperature via a wet-chemical route. Langmuir 23:9147–9150
Sun XP, Dong SJ, Wang EK (2004) One-step synthesis and characterization of polyelectrolyte-protected gold nanoparticles through a thermal process. Polymer 45:2181–2184
Sun XP, Dong SJ, Wang EK (2005a) High-yield synthesis of large single-crystalline gold nanoplates through a polyamine process. Langmuir 21:4710–4712
Sun XP, Dong SJ, Wang EK (2005b) One-step preparation of highly concentrated well-stable gold colloids by direct mix of polyelectrolyte and HAuCl4 aqueous solutions at room temperature. J Colloid Interface Sci 288:301–303
Tan S, Erol M, Attygalle A, Du H, Sukhishvili S (2007) Synthesis of positively charged silver nanoparticles via photoreduction of AgNO3 in branched polyethyleneimine/HEPES solutions. Langmuir 23:9836–9843
Tang ZY, Zhang ZL, Wang Y, Glotzer SC, Kotov NA (2006) Self-assembly of CdTe nanocrystals into free-floating sheets. Science 314:274–278
van Herrikhuyzen J, Janssen RA, Meijer EW, Meskers SCJ, Schenning A (2006) Fractal-like self-assembly of oligo(p-phenylene vinylene) capped gold nanoparticles. J Am Chem Soc 128:686–687
Wang YL, Camargo PHC, Skrabalak SE, Gu HC, Xia YN (2008) A facile, water-based synthesis of highly branched nanostructures of silver. Langmuir 24:12042–12046
Acknowledgments
Financial support for this investigation was provided by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MOST, No. 2009-008-0602 & No. R01-2007-000-11851-0) and the Brain Korea 21 (BK21) program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parab, H., Jung, C., Woo, MA. et al. An anisotropic snowflake-like structural assembly of polymer-capped gold nanoparticles. J Nanopart Res 13, 2173–2180 (2011). https://doi.org/10.1007/s11051-010-9975-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-9975-5