Abstract
The solution-combustion synthesis (SCS) method was used to prepare silver nanoparticles using glycine and citric acid as fuels. The different combination of fuel to oxidant ratio was used to prepare Ag nanoparticles and its effect on optical spectra, structure and the morphology explored. The purposed method is rapid, effective, cheap and convenient. Silver nanoparticles with different sizes and shapes were synthesized depending upon the different oxidant/fuel ratios. The nanoparticles were characterized using transmission electron microscopy, X-ray diffraction and ultraviolet–visible absorption spectroscopy. Histograms were drawn to compare the mean particle size of synthesized nanoparticles. It was found that citric acid was better fuel as compared to glycine as it results in the more spherical symmetrical nanoparticles, which are supported by various characteristic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Novel metal nanoparticle synthesis has received considerable attention in recent years as a result of their optical, electronic, magnetic and chemical properties and their potential applications in subsequent technology development (Chrystel et al. 2003). Silver nanoparticles can be used in areas such as integrate circuit (Kotthaus et al. 1997), cell electrode (Klaus et al. 2001), antimicrobial deodorant fibre (Zhang and Wang 2003), catalysis (Claus and Hofmeister 1999), chemical analysis (Compagnini et al. 1997) and surface-enhanced Raman spectroscopy (Shirtcliffe et al. 1999; Bright et al. 1998). It has been demonstrated that, in the case of noble-metal nanocrystals, the electromagnetic, optical and catalytic properties are highly influenced by shape and size (Liz-Marzan 2004; Mulvaney 1996; Burda et al. 2005). This has driven the development of synthesis routes that allow a better control of morphology and size. In most of the related studies reported previously (Bright et al. 1998; Sosa et al. 2003; Jiang et al. 2004; Wang et al. 2005; Kim et al. 2009; Liang et al. 2007), characteristic surface plasmon resonance of silver nanoparticles and nanowires have always been given the maximum importance because of the fact that it has been the first optical response of a nanoscale metal in the visible range of the spectrum. Apart from the optical and electronic properties, the special shape of silver nanoparticles makes them the ideal choice for applications in modern nanotechnology of integrated circuits (Zhou et al. 1999; Xia and Yang 2003; Huang et al. 2001; Gudiksen et al. 2002; Nina et al. 2002). Therefore, it could be highly useful to develop an effective preparation method of silver nanoparticles with well-controlled shape and size. The solution-combustion synthesis (SCS) of metal nanoparticles is being considered to be a promising method to obtain nano-sized metal particles (Fu et al. 2003; Kiminami et al. 2000) as it involves a high level of molecular mixing of the solution components, leading to chemical homogeneity of the synthesized product with high purity in a rapid, inexpensive single step operation. The most important fact about SCS is that it is a short duration process and the various gases formed during the process inhibit particle size growth, which favours the formation of nano-sized powders (Mukasyan et al. 2007). The basis of the SCS technique comes from the thermo-chemical concepts used in the field of propellants and explosives (Aruna and Mukasyan 2008). The SCS method involves the exothermic chemical reaction between metal nitrates and organic fuels, typically like glycine, citric acid, urea, carbohydrazide, etc. (Aruna and Mukasyan 2007; Patil et al. 2002). The SCS reaction, when the elements’ valances are balanced, releases large amount of energy along with gases like N2, H2O and CO2 favouring the formation of fine particles in few minutes. The maximum reaction temperature, generated in this process, depends on the fuel to oxidizer ratio (μ), the initial furnace temperature, the nature of the fuel and the quantity of the initial precursor (Varma et al. 2004). The combustion synthesis can occur in two different ways: first, the self-propagating high-temperature synthesis (SHS) and second, the volume combustion synthesis (VCS); where the samples are heated by external heating source, respectively, locally or uniformly to initiate reaction. In SHS mode, the self-persistent propagation of reaction wave takes place in the heterogeneous mixture of reactants, and the product of desired composition is formed. However, in VCS mode, the entire mixture of reactants is heated uniformly in controlled manner, until the reaction takes place throughout the volume. These modes will lead to the formation of uniform micro, nano-structure and phase composition of product. The VCS mode has been selected for the synthesis of Ag nanoparticles as it is widely used for the weakly exothermic reactions that require preheating before ignition (Varma et al. 2004).
Despite the fact, SCS being a very promising method, no work on Ag nanoparticle synthesis using this process, has ever been published. Therefore, here we report the synthesis of the silver nanoparticles through SCS process by using two different fuels and varying their fuel ratio. The effect of different fuels, fuel to oxidant ratio has also been studied on morphology, structure and optical properties of the synthesized Ag nanoparticles.
Experimental
Materials and synthesis
All the chemicals and reagents used were of AR grade. For the preparation of Ag nanoparticles, silver nitrate, glycine and citric acid were used as starting materials. Deionised water was used for preparing solutions. Silver nanoparticles were prepared by SCS method, containing stoichiometric amount of corresponding metal nitrate and a suitable fuel. The stoichiometric composition of solution components (fuels and oxidizer) was calculated according to principle of propellant chemistry, keeping the oxidizer (metal nitrate) to fuel (glycine or citric acid) ratio unity (Aruna and Mukasyan 2008). The stoichiometric amounts of silver nitrate (oxidizer) were dissolved in a minimum amount of distilled water to get clear solution, and then was added aqueous solution of glycine in this solution. This solution after thermal dehydration (preheating the solution to moderate temperature at 80 °C on a hot plate to remove the excess solvent) gave highly viscous liquid. As soon as the viscous liquid was formed, the temperature of the hot plate increased to 250 °C. At this stage, the viscous liquid gets linearly auto-ignited and the burning surface recedes from top to bottom in layers. This reaction is called linear combustion when glycine is taken as fuel. But, however, when citric acid is used as fuel, the volume combustion takes places and the entire reaction mixture ignites to burn with a flame. In both the cases, the rapid evolution of large volume of gases takes place leaving behind product of tailored composition. The as synthesized nano-powder was further calcined at 500 °C for 1 h. This step was done to get crystalline phase, with high purity of silver nanoparticles as the synthesized powder contains unreacted silver nitrate and fuel content. However, this step was avoided in the SCS reaction because it occurs at high temperature. In our case, it is clear from temperature–time profile (Fig. 1) of reaction for glycine and citric, that the maximum temperature arising in both reactions is less than 315 °C. Different combinations of fuels and their oxidant to fuel ratio were used to tailor the powder properties. For Ag nanoparticle synthesis, the three different molar ratios of oxidant to fuel were chosen viz. the stoichiometric ratio, fuel-deficient ratio, fuel-excess ratio, respectively when glycine and citric acid were used as 1:0.5560, 1:0.5445, 1:0.5775 and 1:0.2770, 1:0.2670, 1:0.2900.
Characterization
The temperature changes during the combustion synthesis reaction were measured by a set of thermocouples (76 μm, type C, Omega Engineering Inc.) using a computer equipped with acquisition system. The typical temperature–time profiles for silver nanoparticle synthesis process are shown in Fig. 1a, b, respectively, for citric acid and glycine used as fuels in stoichiometric ratios. The optical characterization of Ag nanoparticles was made by UV–Vis spectroscopy using (UV-Thermoevolution spectrometer). X-ray diffraction was carried out for structural, phase identification and crystallite size estimation using monochromatized Cu-Kα radiation on an X’Pert PRO (PANanalytical, Netherland). For morphology and symmetry of nanoparticles TEM investigation was carried out using (HRTEM, Hitachi H 7500).
Results and discussion
The emblematic temperature–time profile for silver nanoparticle synthesis is shown in Fig. 1a, b, respectively, for glycine and citric acid as fuels when these are taken in stoichiometric ratio. The stoichiometric amounts of silver nitrate (oxidizer) were dissolved in a minimum amount of distilled water to get clear solution and then was added aqueous solution of glycine in this solution. After the reactants dissolution in sufficient amount of water and thorough mixing, the obtained solution was preheated uniformly (stage I). This was followed by relatively long (~5–7 min) constant temperature stage II, during which the evaporation of water from solution takes place and viscous liquid is formed (gel like). At stage III, the heating rate is higher than the previous stages, and, therefore, it fast reaches the ignition temperature, T i, as well as the reaction temperature abruptly rises to the maximum value at which combustion takes place (i.e., the combustion temperature T c). After this, the linear combustion, in which the viscous liquid gets linearly auto-ignited and the burning surface recedes from top to bottom in layers, takes place in the case of glycine, and the temperature of reaction remains constant, until the combustion is completed; the plateau in the Fig. 1a represents this part. However, in case of citric acid, the volume combustion takes place, in which, the entire reaction mixture ignites to burn with a flame and the reaction temperature rises to ~310 °C. The rate of medium temperature change, dT/dt, is high (stage IV) in both the modes of synthesis, i.e., in VCS and the SHS. The duration of this region varies from ~10 (for SHS mode) to 100 s (for VCS mode) after cooling (stage V). The synthesized products are in the form of fine powders (Varma et al. 2004). The main parameter, varied in experiments, was the fuels and the oxidant to fuel ratio. The choice of fuel for the SCS is the most important factor. The glycine and citric acid have been adopted for the synthesis of Ag nanoparticles because they are widely used for the synthesis of nanomaterials (Jacobsohn et al. 2010; Deganello et al. 2009; Varma et al. 2004; Li et al. 2009) as; first, they both are the source of N, C and H, which on combustion evolve N2, CO2 and H2O gases, favoring the formation of nanoparticles; second, they form complexes with the metal ions facilitating homogenous mixing of the cations in solution. These fuels being water soluble, a good homogenization can be achieved in solution. These factors make them ideal fuels.
UV–Vis analysis
The absorption spectra of the silver nanoparticles are presented in Fig. 2a, b with two different fuels and their different oxidant to fuel ratios. UV–Vis spectrum is quite sensitive to the formation of silver nanoparticles. All samples present the characteristic surface plasmon of silver nanoparticles, in case of glycine and citric acid. At the stoichiometric ratios, the UV–Vis spectra for glycine reveal wide band at low intensity; and for citric acid, narrow band at comparatively high intensity with maxima at 410 nm. Thus citric acid seems to be a better fuel than glycine; this can precisely be because of the fact that, in case of citric acid, the Ag nanoparticles are quite uniform, smaller in size and possess spherical symmetry (see TEM Fig. 5). However, in case of fuel-deficient ratios, the spectra seem to be better and more profound at slightly lower wavelength with high intensity for glycine; this pertains to the fact that, in case of glycine, Ag nanoparticles are more uniform in size and symmetrical vis-a-vis citric acid, which has different morphology and lacks symmetry (TEM Fig. 4). This is supported by the fact that the number and position of surface plasmon resonance (SPR) peaks as well as the effective spectral range for surface-enhanced Raman scattering are strongly dependent on the particle shape, size, symmetry and morphology (Burda et al. 2005; Sosa et al. 2003; Jensen et al. 1999). But in case of fuel-excess ratio, for both fuels, shifting of the band, at higher wavelength and low intensity, was supported by the aggregation in nanoparticles (TEM Figs. 4, 5).
For citric acid, for fuel deficient and excess ratio, the UV–Vis spectra show a shift towards the longer wavelength side at low intensity and narrow band, which reveals the bigger nanoparticle size on using citric acid as fuel. It is reported that absorption spectrum of spherical nanoparticles present the maximum between 420 and 450 nm with blue or red shift, respectively, when particle size decreases or increases (Pal et al. 2007; Jana et al. 1999; Manna et al. 2001; So¨nnichsen et al. 2002). The bandwidth of each plasmon is related to the size distribution of the nanoparticles. As the particles become larger in size, the plasmon peak shifts to the longer wavelength side, and broadens, as can be seen in case of silver nanoparticles synthesized with two different fuels, i.e., glycine and citric acid, where peak broadening occurs along with decrease in intensity with shift in the wavelength indicating bigger size of Ag nanoparticles. However, UV–Vis concluded that citric acid is better fuel as it shows strong characteristic surface plasmon resonance as compared to that of glycine.
XRD analysis
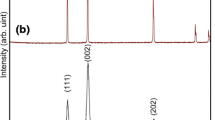
The structure of prepared silver nanoparticles has been investigated by X-ray diffraction (XRD) analysis. Figure 3 shows a typical XRD pattern of the as-prepared silver nanoparticles using citric acid and glycine as fuels in stoichiometric, fuel deficient and fuel-excess ratio. The X-ray diffractogram clearly depicts the peaks corresponding to the (100), (200), (220), (311) planes, which suggest the cubic structure of Ag nanoparticles as per the JCPDS (File no. 03-0931). The lattice constant (a) calculated from these patterns comes out to be 4.067 A°, which is consistent with the standard value (JCPDS File no.03-0931). No impurities are detected from this pattern. This indicates that pure silver metal was obtained under the present synthesis conditions. The broadening of XRD peaks was due to smaller crystallite size; this is since the number of planes available is too small. The crystallite size (D) of the Ag nanoparticles was calculated using the Debye–Scherrer formula from the full-width at half maximum (FWHM), β, of a diffraction peak (Venkatachalam et al. 2005):
where λ is the X-ray wavelength, θ the diffraction angle. To eliminate additional instrumental broadening, the FWHM was corrected, using the FWHM from a large grained Si sample.
Table 1 shows the crystallite size of Ag nanoparticles calculated by Scherrer formula for different oxidant to fuel ratio of citric acid and glycine. It is clear from Table 1 that the crystallite size found in the case of citric acid for different ratios of fuel is less than glycine, which shows that citric acid is more effective fuel as compared to glycine to produce smaller nanoparticles.
TEM analysis
The TEM images in Fig. 4a, b, c show that combustion reaction performed between silver nitrate and glycine as fuel in the stoichiometric ratio (1:0.5560) results in different shapes, i.e., spherical, prismatic and pentagonal shape with mean particle size 5.5423 nm (inset Fig. 4a). However, the particles, obtained in case of fuel-deficient ratio (1:0.5445), were nearly spherical in shape with mean particle size, 40.6518 nm (inset Fig. 4b); and at fuel-excess ratio (1:0.5775) for glycine, the spherical nanoparticles aggregate and attain stability. These spherical particles, obtained in case of glycine at fuel deficient, generate low temperature, whereas at stoichiometric ratio, combustion reaction generates higher temperature, which results in high crystallite size with smaller surface area; similar is the case with fuel-excess ratio, where flame temperature is quite higher. It can be inferred, that higher flame temperatures can affect powder properties adversely. However, in case of citric acid, it has been observed that, for the stoichiometric ratio (1:0.2770), we get the finest spherical nanoparticles (Fig. 5a, b, c) with mean particle size, 1.6489 nm (inset Fig. 5a); and, in case of fuel-deficient ratio (1:0.267), the bigger nanoparticles with mean particle size, 11.26 nm (inset Fig. 4b); and, for fuel-excess ratio (1:0.290), the smaller nanoparticles coalesce to form large size nanoparticles clusters. The Table 1 displays the comparative particle size of Ag nanoparticles for different fuels at different fuel to oxidant ratio. This observation can be attributed to the fact that in case of citric acid, taken in stoichiometric ratio, there is more number of gaseous products, which fragment the product whilst escaping to give finer particles. It appears that the superior powder properties in case of citric acid are due to dominant effect of gas molecules. Thus the TEM studies confirm that the citric acid is considered to be more suitable and effective fuel for the formation of the finer silver nanoparticles as compared to those of the glycine.
Conclusion
In summary, silver nanoparticles were prepared by the solution-combustion reaction by using two different fuels, i.e., glycine and citric acid. The effect of fuel to oxidant ratio on Ag nanoparticles formation, their morphology and size has been studied. The UV–Vis spectra reveal that the number and positions of SPR peaks, the effective spectral range, and the band position are strongly dependent on the particle shape, size, symmetry and the morphology. The XRD studies confirm that for both fuels, the Ag nanoparticles possess cubic structure. The TEM and XRD studies confirm that for the stoichiometric ratios of fuels to oxidant, the Ag nanoparticles are more spherical and symmetrical in shape and size. But, however, in the case of fuel-excess ratio, the smaller nanoparticles coalesce to form large size nanoparticle clusters. Thus, the synthesized nanoparticles have been found to be spherically symmetrical in case of citric acid, whereas, with glycine, they are found to be of different shape and size. Therefore, citric acid has been found to be a better fuel than glycine to synthesize spherical silver nanoparticles using solution-combustion reaction.
References
Aruna ST, Mukasyan AS (2007) Combustion synthesis and nanomaterials. Curr Opinion Solid State Mater Sci 12:44–50
Aruna ST, Mukasyan AS (2008) Combustion synthesis and nanomaterials. Curr Opin Solid State Mater Sci 12:44–50
Bright RM, Musick MD, Natan MJ (1998) Preparation and characterization of Ag colloid monolayers. Langmuir 14:5695–5701
Burda C, Chen X, Narayanan R, El-Sayed MA (2005) Chemistry and properties of nanocrystals of different shapes. Chem Rev (Washington, DC, United States) 105:1025–1102
Chrystel F, Alain D, Wilfrid N (2003) Spontaneous formation of silver nanoparticles in multilamellar vesicles. J Phys Chem B 106:4738–4746
Claus P, Hofmeister H (1999) Electron microscopy and catalytic study of silver catalysts: structure sensitivity of the hydrogenation of crotonaldehyde. J Phys Chem B 103:2766–2775
Compagnini G, Pignataro B, Pelligra B (1997) Nanomorphology and SERS activity in plasma prepared silver surfaces. Chem Phys Lett 272:453–458
Deganello F, Marcì G, Deganello G (2009) Citrate–nitrate auto-combustion synthesis of perovskite-type nanopowders: a systematic approach. J Euro Ceram Soc 29:439–450
Fu Y, Lin C, Pan K (2003) Strontium hexaferrite powders prepared by a microwave-induced combustion process and some of their properties. J Alloys Compd 349:228–231
Gudiksen MS, Lauhon UJ, Wang J, Smith DC, Lieber CM (2002) Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature 415:617–620
Huang Y, Duan XF, Wei Q, Lieber CM (2001) Science directed assembly of one dimensional nanostructures into functional networks. Science 291:630–633
Jacobsohn LG, Tornga SC, Bennett BL, Muenchausen RE, Ugurlu O, Tseng TK, Choi J, Holloway PH (2010) Annealing effects on the photoluminescence yield of Gd2O3:Eu nanoparticles produced by solution combustion synthesis. Radiat Meas 45:611–614
Jana NR, Sau KT, Pal T (1999) Growing small silver particle as redox catalyst. J Phys Chem B 103:115–121
Jensen TR, Kelly L, Lazarides A, Schartz GC (1999) Electrodynamics of noble metal nanoparticles and nanoparticle clusters. J Cluster Sci 10:295–317
Jiang P, Li S, Xie S, Gao Y, Song L (2004) Machinable long PVP-stabilized silver nanowires. Chem Eur J 10:4817–4821
Kim HW, Kebede MA, Kim HS, Lee C (2009) GaN nanowires sputtered with Ag shell layers. Thin Solid Films 517:3908–3911
Kiminami RHGA, Morelli MR, Folz DC (2000) Microwave synthesis of alumina powders. Am Ceram Soc Bull 79:63–67
Klaus T, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory: metal accumulating bacteria and their potential for materials science. Trends Biotechnol 19:15–20
Kotthaus S, Gunther BH, Hang R, Schafer H (1997) Study of isotropically conductive bondings filled with aggregates of nano-sited Ag-particles. IEEE Trans Compon Packag Technol 20(1):15–20
Kovtyukhova NI, Mallouk TE (2002) Nanowires as building blocks for self-assembling logic and memory circuits. Chem Eur J 8:4354–4363
Li Y, Xue L, Fan L, Yan Y (2009) The effect of citric acid to metal nitrates molar ratio on sol–gel combustion synthesis of nanocrystalline LaMnO3 powders. J Alloy Compd 478:493–497
Liang C, Terabe K, Tsuruoka T, Osada M, Hasegawa Aono M (2007) AgI/Ag Heterojunction Nanowires: facile electrochemical synthesis, photoluminescence, and enhanced ionic conductivity. Adv Funct Mater 17:1466–1472
Liz-Marzan LM (2004) Nanometals: formation and color. Mater Today 7:26–31
Manna A, Imae T, Aoi K, Okada M, Yogo T (2001) Synthesis of dendrimer-passivated noble metal nanoparticles in a polar medium: comparison of size between silver and gold particles. Chem Mater 13:1674–1681
Mukasyan AS, Epstein P, Dinka P (2007) Solution combustion synthesis of nanomaterials. Proc Combust Inst 31:1789–1795
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800
Pal S, Tak KY, Song MJ (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720
Patil KC, Aruna ST, Mimani T (2002) Combustion synthesis: an update. Curr Opinion Solid State Mater Sci 6:507–512
Shirtcliffe N, Nickel U, Schneider S (1999) Reproducible preparation of silver sols with small particle size using borohydride reduction: for use as nuclei for preparation of larger particles. J Colloid Interface Sci 211:122–129
So¨nnichsen C, Franzl T, Wilk T, von Plessen G, Feldmann J (2002) Plasmon resonances in large noble-metal clusters. New J Phys 4:931–938
Sosa JO, Noguez C, Barrere RG (2003) Optical properties of metal nanoparticles with arbitrary shapes. J Phys Chem B 107:6269–6275
Varma A, Mukasyan AS (2004) Combustion synthesis of advanced materials: fundamentals and applications. Korean J Chem Eng 21:527–536
Varma A, Mukasyan AS, Deshpande KT, Pranda P, Erri PR (2004) Combustion synthesis of nanoscale oxide powders mechanism, characterization and properties. Mat Res Soc Symp Proc 800:AA4.1.1–AA4.1.12
Venkatachalam S, Mangalaraj D, Narayandass SK, Kim K, Yi J (2005) Structure, optical and electrical properties of ZnSe thin films. Physica B 358:27–35
Wang Z, Liu J, Chen X, Wan J, Qian Y (2005) A simple hydrothermal route to large-scale synthesis of uniform silver nanowires. Chem Eur J 11:160–163
Xia Y, Yang P (2003) Chemistry and physics of nanowires. Adv Mater 15:351–352
Zhang W, Wang G (2003) Research and development for antibacterial materials of silver nanoparticles. New Chem Mater 31:42–44
Zhou Y, Yu SH, Cui XP, Wang CY, Chen ZY (1999) Formation of silver nanowires by a novel solid–liquid phase arc discharge method. Chem Mater 11:545–546
Acknowledgments
One of the authors—Gurmeet Singh—gratefully acknowledges Department of Science and Technology, India for the INSPIRE fellowship enabling him to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Lotey, G.S., Singh, S. et al. Solution-combustion: the versatile route to synthesize silver nanoparticles. J Nanopart Res 13, 2553–2561 (2011). https://doi.org/10.1007/s11051-010-0148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0148-3